Abstract
The biosensing of bacterial pathogens is of a high priority. Electrochemical biosensors are an important future tool for rapid bacteria detection. A monolayer of bacterial-binding peptides can serve as a recognition layer in such detection devices. Here, we explore the potential of random peptide mixtures (RPMs) composed of phenylalanine and lysine in random sequences and of controlled length, to form a monolayer that can be utilized for sensing. RPMs were found to assemble in a thin and diluted layer that attracts various bacteria. Faradaic electrochemical impedance spectroscopy was used with modified gold electrodes to measure the charge-transfer resistance (RCT) caused due to the binding of bacteria to RPMs. Pseudomonas aeruginosa was found to cause the most prominent increase in RCT compared to other model bacteria. We show that the combination of highly accessible antimicrobial RPMs and electrochemical analysis can be used to generate a new promising line of bacterial biosensors.
1. Introduction
Bacterial infections pose a public health crisis worldwide [1]. A crucial step in detecting the pathogenic status of a patient, as well as of food, surfaces, and medicinal equipment, is the identification of the bacterial infection source [2]. There is an urgent need for the development of rapid bacterial identification methods to improve treatment and minimize contamination and resistance-occurrence risks [3].
Electrochemical biosensors are integrated devices that convert specific biological interactions into electrical output that provides analytical information and are, hence, suitable for the detection of bacteria [4]. They can be fabricated at a relatively low cost and can provide a rapid and accurate analysis. Electrochemical impedance spectroscopy (EIS) is a powerful technique used for the analysis of the interfacial properties related to the bio-recognition events occurring at the electrode surface. Faradaic impedance measurement is performed in the presence of redox species. The changes in the charge-transfer resistance (RCT) translate the molecular-recognition event on the monolayer to a measurable signal [5]. Thus, EIS enables the label-free detection of biomolecules and biochemical interactions.
Monolayers composed of aptamers, antibodies, polymers, and peptides are used for bacterial detection [4,5,6]. Among the variety of recognition molecules, peptides have great potential for being utilized in biosensing. Peptides, which are short chains of amino acids, are biocompatible and possess stability and selectivity by adopting a proper conformation toward the analyte. They are easily obtained and modified by chemical methods, allowing them to be tailored for specific surface chemistry applications [7]. Thiol–Au is the simplest and most common strategy for assembling peptides monolayers [8,9]. The process of self-assembled monolayer (SAM) organization includes an initial stage of fast S-Au bond formation. The second stage is a slow organization process where the immobilized molecules assemble to maximize their intermolecular interactions [9,10]. While interactions between the peptides might stabilize the monolayer and lead to a high density, repulsion due to electrostatic and/or steric hindrance can hinder monolayer assembly [11]. Antimicrobial peptides (AMPs) are short natural or synthetic peptides that eradicate bacteria through interactions with their membrane. While there is a high diversity in the sequences, lengths, and structures of AMPs, the different variants have two features in common: cationic charges and a high degree of hydrophobicity. Random peptide mixtures (RPMs) are peptides that optimize the advantage of AMPs’ common features—their 20 amino acids chain length combines hydrophobic and cationic amino acid residues in random sequences [12]. RPMs are easy to synthesize in large quantities (Figure 1), and an analysis showed that their synthesis is reproducible [13]. When equal ratios were used for coupling during the synthesis, this resulted in a ratio close to 1:1, in line with the features of the amino acids used with a minor favor towards hydrophobic amino acids [12]. Our previous studies revealed that RPMs have broad-spectrum antimicrobial activity [12,13,14,15,16,17]. This activity was preserved at varying pH values and physiological media, as well as in mice models with high efficacy and safety [18,19,20]. Of the multiple possible combinations of hydrophobic and cationic amino acids, those containing phenylalanine and lysine were among the combinations with the highest antimicrobial activity. Furthermore, we explored their possible mechanism of action and found that it involves electrostatic and hydrophobic interactions with the negatively charged bacterial membrane, via either pore-formation or non-selective distribution on the membrane [14]. Lastly, RPMs immobilized on polystyrene beads bind and eliminate bacterial cells very efficiently [18]. Since RPMs have a demonstrated ability to bind bacteria based on their intrinsic features of hydrophobicity and a positive charge, they have the potential to be used as recognition molecules for the electrochemical sensing of bacteria, with the considerable advantage of eliminating the need to synthesize a peptide with a specific sequence, which is time-consuming and expensive. The straight-forward and cost-effective production process, which does not involve tedious purification protocols, using RPMs as recognition molecules has many advantages compared to using standard peptides.
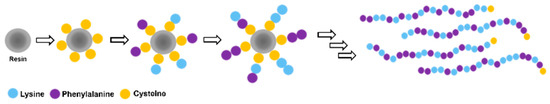
Figure 1.
Solid-phase peptide synthesis of phenylalanine–lysine random peptide mixtures with cysteine at the C’ terminus (first coupling step: yellow circle). After the first coupling of cysteine, a 1:1 mixture of Fmoc–phenylalanine and Fmoc–lysine-(Boc) was coupled at each of the following 20 coupling steps to generate a 21-mer of Cys-[(Phe)0.4-(Lys)0.6]n=20, abbreviated as CFK.
However, unlike the assembly of homogeneous peptides on gold, which is expected to self-organize and to form strong intermolecular interactions, the assembly of RPMs has not been explored yet. RPMs contain mixtures of entities with different sequences and a distribution of positive charge/hydrophobic residues along the chain length that may affect the assembly. Here, we studied the ability of RPMs to form a monolayer on a gold surface and their potential use for bacterial sensing using EIS (Figure 2).
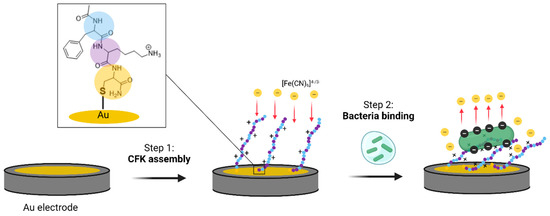
Figure 2.
A graphical presentation of the study: CFK assembly on Au surfaces (step 1) and its rearrangement after bacterial binding (step 2). The charge-transfer resistance (RCT) increases in response to the bacteria adhesion due to its electrostatic repulsion of the redox active species [Fe(CN)6]3−/4−.
2. Experimental Section
2.1. Materials
All solutions were prepared with triple-deionized water (TDW, 18.3 MΩ∙cm, Millipore Milli-Q, Bedford, MA, USA). The buffer solution was PBS in low ionic strength (PBS-L) containing 10 mM Na2HPO4·7H2O, 1.8 mM KH2PO4, 1.37 mM NaCl, and 0.027 mM KCl (pH 7.4). Electrochemical analyses were conducted with Metrohm-Autolab PGSTAT-12 digital potentiostat (EcoChemie BV, Utrecht, The Netherlands), operated with Nova software for utilizing electrochemical impedance spectroscopy (EIS). A three-electrodes cell was used for the measurements: Ag/AgCl (in 3 M KCl) as reference electrode (RE) and a Pt wire as a counter electrode (CE). Polycrystalline bulk gold electrodes with a 2 mm diameter were used as working electrodes (WE) (CH instruments, USA). WE were manually polished on micro-cloth pads (Buehler, Lake Bluff, IL, USA) with deagglomerated 0.05 μm alumina suspension (Buehler). After polishing, the electrodes were washed with TDW. Bacterial strains used in the research are E. coli K12 MG1655 (rp), Pseudomonas aeruginosa PAO1, Staphylococcus aureus Newmann (kindly received from Prof. Roni Shapira’s lab, HUJI), Methicillin-resistant Staphylococcus aureus (MRSA), Bacillus subtilis 168, E. coli K12 BW25113 (kindly received from Prof. Shimshon Belkin’s lab, HUJI). All bacteria were grown in Luria broth (LB) at 37 °C.
2.2. Methods
2.2.1. Synthesis of CFK Random Peptide Mixtures (RPMs)
CFK random peptide mixtures were synthesized using standard Fmoc-based solid-phase peptide synthesis (SPPS) with a Liberty Blue™ automated microwave peptide synthesizer (CEM Corp., Matthews, NC, USA) as was published previously [12]. Cys-[(Phe)0.4-(Lys)0.6]n=20 (abbrev. CFK) was synthetized on 0.25 mmol scale on rink amide resin (loading 0.53 mmol/g (ChemImpex, IL, USA)) as the solid support. After swelling (5 min) of the resin, Fmoc removal (deprotection) was completed with 20% v/v piperidine/DMF. Standard couplings of amino acids were carried out at 0.1 M in N,N-Dimethylformamide (DMF) using DIC/OxymaPure® activation (0.2 and 0.1 M, respectively). Fmoc–Cysteine–Trt was coupled first at 50 °C, followed by deprotection. The next 20 coupling steps were carried out by using 1:1 molar ratio mixture of protected α-amino acids: Fmoc-L-Phenylalanine with Fmoc-L-Lysine-Boc. At the end of the synthesis, the resin was washed with DMF and DCM and dried with methanol and diethyl ether. The peptides were cleaved from the resin by mixing the resin with a 4 mL cleavage cocktail of 92.5% Trifluoroacetic acid (TFA), 2.5% 1,2-Ethanedithiol, 2.5% triisopropylsilane (TIPS), and 2.5% TDW for 3 h with agitation. After filtration, the peptides were precipitated from the TFA solution by the addition of cold diethyl ether and collected by centrifugation (Eppendorf R5810 8000 rpm for 10 min). The ether was then removed, and the process repeated once more. The peptides were dried under a stream of nitrogen, dissolved in 20% ACN/TDW, and lyophilized. The product was analyzed by MALDI TOF/TOF AutoFlex Speed (Bruker Daltonics Inc., Bremen, Germany) to evaluate the success of synthesis.
2.2.2. CFK Assembly on a Gold Surface
Gold substrates for surface characterization were prepared by evaporation of 10 nm chrome as adhesion layer followed by evaporation of 100 nm Au on top of highly doped n-type Silicon wafer. The surfaces were washed with ethanol, dried under a mild stream of nitrogen gas, and cleaned using UVOCS (ultraviolet ozone cleaning system) for 20 min.
Solution of 1.4 mg/mL (0.5 mM) CFK was prepared in PBS-L buffer solution (pH = 7.4). A variety of buffers was examined. Finally, the adsorption process was completed in PBS buffer with low ionic strength (PBS-L) in order to enable the optimal organization and interaction of the peptides chains. Chemisorption was performed by drop casting CFK solution on bare gold substrates for an overnight incubation ( h) at 37 °C. The surfaces were washed after incubation with PBS-L buffer and TDW and dried under nitrogen stream. Characterization of Au-CFK surfaces is described at the next section.
2.2.3. Surface Characterization
Variable angle spectroscopic ellipsometry (VASE) measurements were carried with a VB-400 ellipsometer (J.A. Woollam Co., Inc., Lincoln, NE, USA). The optical thickness of the random peptides layer on the gold surface was measured by fitting the results to the CAUCHY model, when the values are an average with standard deviation of surfaces from different batches.
Kelvin-probe-derived contact potential difference (CPD) measurements were performed with a Kelvin probe S vibrating gold grid reference electrode (WF—4.8 eV), operated by the Kelvin control 07 unit (DeltaPhi Besocke, Julich, Germany), in a specially built Faraday cage under inert argon atmosphere. Ohmic back contacts were made with eutectic Ga-In (99.99%, Sigma-Aldrich, Darmstadt, Germany), and the CPD signal was recorded using Keithley 2450 SMU. The measurements were taken after the few minutes needed for the signal to stabilize and were performed with respect to a reference electrode.
X-ray photoelectron spectroscopy (XPS) spectra were recorded with a Kratos Axis Supra+ spectrometer (Kratos Analytical Ltd., Manchester, UK) using an Al Kα monochromatic radiation X-ray source (1486.7 eV). Data were collected and analyzed by a Casa XPS (Casa Software Ltd., Devon, UK) and Vision data-processing program (Kratos Analytical Ltd.). The high-resolution XPS spectra were collected with a take-off angle of 90° (normal to analyzer); vacuum conditions in the chamber were 1.9 nTorr, for the C 1s, O 1s, N 1s, S 2p, and Au 4f levels, with pass energy of 20 and 0.1 eV step size. The binding energies were calibrated using C 1s peak energy as 285.0 eV.
Reductive desorption analysis conducted by cyclic voltammetry (CV) was performed to determine the surface coverage of the peptides mixture, termed FK, on Au electrodes, by scanning over the potential range of −0.5 to −1.4 V at the scan rate of 150 mV/s in 0.1 M KOH solution. The electrolyte solution was degassed with nitrogen stream prior to CV scans.
Atomic force microscopy (AFM) analysis on ultra-flat gold substrates was performed using a Dimension Icon XR probe microscope (Bruker) in tapping mode. Ultra-flat gold substrates were prepared as previously described [21]. Au (100 nm) was evaporated on 3 × 3 cm2 pieces of highly doped n-type Silicon wafer. An optical adhesive (NOA 88, Norland products, NJ, USA) was used for attaching a glass slide to the surface of the Au. The OA was cured by exposing UV LED (λmax = 365 nm) for 20 min. The glass/OA/Au composite was carefully separated from the silicon template to expose the smooth surface at the Au/silicon interface. The assembly of CFK was performed as described in Section 2.2.2.
2.2.4. Florescence Microscopy
Fluorescence microscopy was used to evaluate the binding ability of bacteria to CFK-modified Au surfaces. The bacteria cells were grown overnight in LB at 37 °C. The bacterial cells’ suspension was diluted 1/50 in fresh LB and grown for additional 3–4 h. Bacteria pellets were washed twice with PBS-L by centrifugation (8000 rpm for 2 min). The bacteria pellet was diluted to OD600 nm = 0.5 and stained with Syto9 (live, green; Life Technologies Corp., Carlsbad, CA, USA). The suspension was dropped on the immobilized peptide surfaces and incubated for 40 min at room temperature. The surfaces were washed with PBS-L and TDW (three times) to remove unbound bacteria and observed with a fully motorized IX81 fluorescent microscope (Olympus, Tokyo, Japan). Images were captured at ×20 magnification in at least three distinct areas of the sample. PMT emission was 490–530 nm.
2.2.5. Gold Electrodes’ Surface Modifications and Impedimetric Sensing
WE were dipped in 0.5 mM (FK)20-Cys in PBS in low ionic strength (PBS-L) (pH 7.4) for 16 h at 37 °C, rinsed by dipping in PBS-L, and then measured. Subsequently, the electrodes were exposed to 108 CFU/mL bacterial cells’ suspension in PBS-L for 40 min under gentle agitation. The bacteria cells were grown overnight in LB at 37 °C. The bacterial cells’ suspension was diluted 1/50 in fresh LB and grown for additional 3–4 h. Bacteria pellets were washed twice with PBS-L by centrifugation (8000 rpm for 2 min) and diluted to an optical density of 0.5 at 600 nm. Following incubation with bacteria, the electrodes were washed and measured. The EIS characterization was prepared in an EIS solution, containing 1 mM K3[Fe(CN)6] and 1 mM K4[Fe(CN)6] (RedOx species) in PBS-L. Spectra were recorded by applying a single sine AC potential of 10 mV amplitude superimposed with 0.21 V DC potential (vs. RE) and scanning over the frequency range of 100 kHz to 0.1 Hz. The data were analyzed as Nyquist plots and fitted to a Randles-like equivalent circuit of where RS is the solution resistance, RCT is the interface charge-transfer resistance, W is the Warburg diffusion element, and Q is the constant phase element of non-ideal capacitance. All experiments were completed on at least 3 samples with at least 3 repetitions.
3. Results and Discussion
RPMs were synthetized with a 1:1 mixture of phenylalanine and lysine in each coupling step of the solid-phase peptide synthesis. The product is a mixture of 2n possible different sequences composed of only phenylalanine and lysine (Figure 1). The peptides mixture, termed FK, was previously shown to have significant antimicrobial activity against a broad spectrum of bacteria, by binding their membrane [13,14,15,16,17]. Here, we equipped the FK RPMs with a cysteine moiety at the C terminal to allow anchoring to gold surfaces, termed Cys-[(Phe)0.4-(Lys)0.6]n=20 (CFK). CFK was analyzed by MALDI in order to verify the length of peptides chains and the ratio between the amino acids. MALDI analysis revealed several peaks around 2.8 kDa, which correlates with a 21-mer peptide composed of one cysteine and a mixture of phenylalanine and lysine (Figure S1). The most common peptide combinations were found to be composed of sequences with a 3:2 F:K ratio. The higher content of hydrophobic amino acid observed for CFK can be attributed to the relative coupling reactivity of the two amino acids [12,13,14].
FK terminated with cysteine at the C-terminal (CFK) was incubated on gold electrodes or surfaces. The thiol chemisorbs onto the gold via the formation of a S–Au bond, and the peptides self-organized to form a monolayer [6]. The resulting layers could then be characterized through various methods for surface-chemistry analysis. To characterize the monolayer, CFKs were assembled on Au-coated Si wafers (Au-CFK). VASE analysis showed an additional thickness of 15.3 (±0.8) Å (MSE = 5.45). The deviation of the measured thickness from the theoretical length of the peptides (~47 Å) indicates that CFK forms a monolayer and that either the RPMs are tilted or the assembly did not reach full coverage. The surface number density (NS) of the CFK monolayer was extracted from reductive desorption analysis [22]. The concentration of CFK was calculated by the characteristic peak at −1.1 V (Figure S2), which yielded NS = 1.4 × 10−10 mole CFK/cm2. A typical NS of alkanethiol SAM is 9.3 × 10−10 mole RSH/cm2 [23]. Our results show that the CFK surface coverage is lower than that of typical alkanethiols. This may be due to repulsion between the peptide chains. Consistent with CFK RPMs being positively charged molecules with substantial intermolecular electrostatic repulsion, we found our NS comparable to other charged alkanethiol monolayers [24]. Our reductive desorption studies support these VASE results, confirming that CFK assembly results in about 10% coverage.
AFM analysis was performed to evaluate the topography of the CFK peptide monolayer on the gold surface. The results show that while the average surface roughness (Ra) of bare gold is 0.26 nm, the FK monolayer assembly results in only a slight increase to 0.31 nm (Figure 3A,B). This moderate increase in roughness further hints at the assembly of a peptides monolayer on the surface [25].
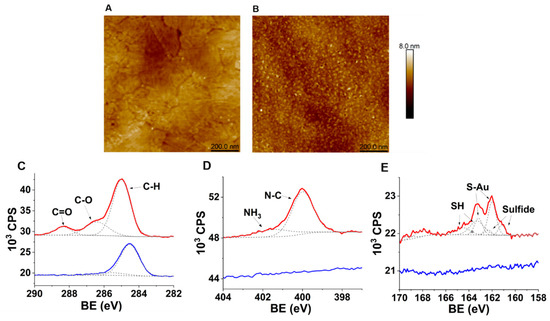
Figure 3.
Characterization of Au-CFK surfaces. Upper panel: AFM analysis of the modified gold substrates. (A) Bare gold (Ra = 0.26 nm); (B) after-adsorption CFK random peptide mixtures (Ra = 0.31 nm). Lower panel: XPS spectra of CFK-Au surface: (C) C 1s BE region; (D) N 1s BE region; (E) S 2p BE region (red lines) and bare Au surface (blue lines). Recorded data are shown with lines, and the Gaussian fit is shown with dashed lines.
The surface potential caused by adsorption of the CFK monolayer on Au was measured using CPD. The CFK assembly yields a ΔCPD of −0.94 (±0.08) V with respect to the bare gold substrate. The decrease in the surface potential indicates that the dipole is directed toward the surface. This proves that the surface is covered with positively charged molecules and confirms the presence of lysine-rich CFK peptides.
The assembly of CFK peptides on the gold surface was further characterized using XPS analysis, to trace the peptides at the atomic level features: sulfur, nitrogen, and carbon atoms. A binding energy (BE) peak that corresponds with the C=O bond of carbonyl in peptide chains was observed at 288.3 eV (Figure 3C) [26]. The two peaks at the nitrogen-related binding energy spectral region, 400 eV and 401.5 eV, correspond with the primary amine and the quaternary amine, respectively (Figure 3D) [27]. BE peaks at 162.1, and 163.2 eV corresponds with the 2p electrons of the sulfur attached to the gold (Figure 3E). The peaks at 163.4 and 164.6 eV are related to the 2p electrons of the unbound thiols (S-H) [28]. The ratio between the S-Au-related peaks and the SH ones (1:1.35) suggests that most of the CFK is chemisorbed, while part of it is physisorbed as π–π interactions between peptide chains, or that interactions of amine side chains with gold may be contributing to the monolayer [29]. Atomic percentages of Au, C, and N were 32%, 47%, and 7%, respectively. The C/Au ratio is 1.4, indicating that most of the Au surface is covered by the peptide monolayer.
The CFK monolayers binding with the bacteria were evaluated. The interaction of FK with bacteria in solution was demonstrated previously [13,14]; however, when peptides are anchored to a surface, their interaction with the bacteria might decrease, as their flexibility and their folding into secondary structures can be limited [18,30]. To study the ability of the CFK layer to attract bacterial cells, various Gram-positive and Gram-negative bacterial cells were stained with a syto9 fluorescent dye and incubated on the CFK-modified gold surfaces. Strains included Pseudomonas aeruginosa (PAO1), E. coli, methicillin-resistant Staphylococcus aureus (MRSA), and Bacillus subtills. The binding of all bacterial cells to Au-CFK surfaces was observed at different microbial loads, with maximal coverage gained at 108 CFU/mL (Figure 4 and Figure S3). High coverage of the surface was obtained for PAO1, MRSA, and E. coli; less coverage was observed in B. subtilis. In a control experiment, the binding of bacteria to a bare Au surface was minor (Figure S4). Au-CFK analyses demonstrated that the anchored peptides maintain their bacterial-binding properties.
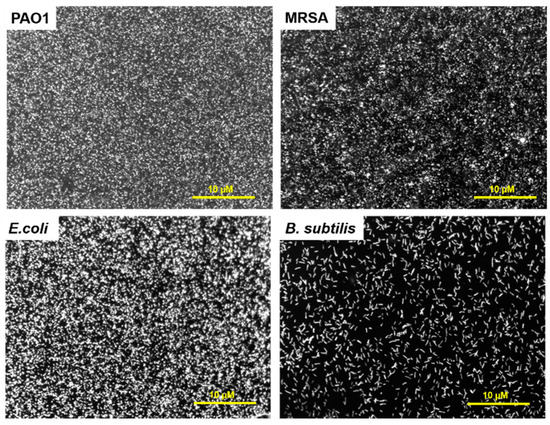
Figure 4.
Binding of various bacterial cells to Au-CFK. Bacteria were stained with syto9 and incubated on FK-modified surface. The unbound bacteria were washed, and the surfaces were observed via a fluorescence microscope (×20 magnification).
We next developed a sensing platform for detecting bacteria using electrochemical sensing. EIS was performed before and after adsorption of CFK to the electrodes, forming the peptide monolayer on the gold electrode (AuE-CFK). To quantify the impedimetric response, data were fit to a Randles circuit [31]. The resistance and capacitance components of the system were extracted. A change in the ratio between the resistance and the capacitance was observed, depending on the bacteria species. However, since the dominant change was in the resistance parameter, the response to the binding bacteria was analyzed according to the changes in the resistance for redox-active [Fe(CN)6]3−/[Fe(CN)6]4− mobility. The charge-transfer resistance (RCT) values for AuE-CFK were in the range of 600–2000 Ω compared to 80 Ω for the bare Au electrode. Subsequently, EIS measurements of AuE-CFK were performed in response to incubation with different bacteria (at the same microbial load).
After incubation with bacteria or buffer (negative control), the electrodes were washed with buffer to remove unbound bacteria and measured again. The incubation with 108 CFU/mL E. coli resulted in an RCT increase of ~40,000 Ω (Figure 5A). AuE-CFK was incubated with three other bacteria (Figure 5B and Figure S5). The increase in RCT was bacteria-dependent. PAO1 had the strongest response, showing a 69-fold increase. E. coli presented a 16-fold increase in RCT, while MRSA and B. subtilis both yielded a rather weak response. Incubation of the layer in buffer is expected to also cause an increase in RCT, due to the re-organization of the layer resulting in a change in the impedimetric signal [32,33]. Specifically, since the CFK monolayer is composed of different sequences, due to the intramolecular interactions between the positively charged lysine residues and the bulky phosphate anion of the buffer, the monolayer is densified and exposes a negatively charged interface that repels the negatively charged redox active couple, ferri-/ferro-cyanide. However, the response of CFK with PAO1 is an order of magnitude larger than the response of the buffer only, showing a specific and tangible detectability of the bacteria.
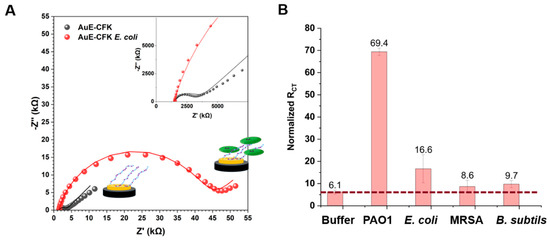
Figure 5.
Impedimetric response of AuE-CFK to exposure to bacteria. (A) A Nyquist plot of AuE-CFK before (black) and after (red) a 40 min incubation with E. coli. Raw data are presented as circles and as lines when fit to a Randles circuit. (B) Normalized RCT values of AuE-CFK after exposure to bacteria. Results are Avg + SD of 3 different electrodes with 3 exposure repetitions.
The binding of bacteria to CFK surfaces that was observed by fluorescent microscopy did not entirely translate to an EIS signal, which requires a deeper evaluation of the observed effect. Since the peptides have antimicrobial activity when in solution, we examined whether the signal related to the CFK surfaces was caused by either dead bacteria or the redox active species effect itself. Our control studies showed that dead bacterial cells do not lead to an EIS response and that the redox species do not affect the bacteria’s viability nor their removal from the surface (Figures S6–S8).
EIS response results from the permeation of the redox active species through the monolayer, which, hence, depends on the changes in the monolayer charge, dipole, density, and morphology; this might be affected by the nature of the analyte attached to the substrates. The CFK monolayer is positively charged and can interact with [Fe(CN)6]3−/4− via electrostatic interactions. While bacteria are attached, the positive charge of CFK can be shielded, and the changes in the redox species permeation could result in an increase in RCT (Figure 2). Gram-negative bacteria have more negatively charged surface density than Gram-positive bacteria [34]. Therefore, interactions of [Fe(CN)6]3−/4− with PAO1 and E. coli may result in larger electrostatic repulsion in comparison to MRSA and B. subtilis and in a higher EIS response. The differences between the responses to PAO1 and E. coli are due to the nature of their membranes and of the other components on the membrane, such as receptors, saccharides, etc. It is known that the Zeta potentials of PAO1 and E. coli have different values [35]. The PAO membrane contains a few components that carry a dominant weight in its surface total charge. Although the main phospholipid of PAO and E. coli is phosphatidylethanolamine, which is zwitterionic [36], PAO has an unusually high phosphorous content, contributing to its overall negative surface charge [37]. The LPS of PAO contains special acidic saccharides such as 2-keto-3-deoxyoctulosonic acid (KDO), 2-amino-2-deoxyuronic acids, 2,3-diamino-2,3-dideoxyuronic acids, and 5,7-diamino-3,5,7,9-tetradeoxynonulosnic acids. The PAO capsule also consists of negatively charged o-capsular and alginate polysaccharides [37,38]. All those components may contribute to the negative charge of PAO and to the repulsion effect on the redox active species and, subsequently, provide the basis for the high EIS signal.
4. Conclusions
We demonstrate a new strategy for bacterial detection, which relies on readily accessible RPMs as recognition monolayers. Among antimicrobial peptides, the random peptide mixture, CFK was demonstrated to target a broad spectrum of bacteria. Here, for the first time, the co-assembly of random peptides was utilized to form the active layer of an electrochemical bacterial biosensor. We showed that those accessible RPMs can be assembled on a gold surface while maintaining bacteria-binding properties. In their immobilized state, CFK mixtures can bind various bacterial cells to the surface. The translation of the binding to an electrochemical signal was achieved by EIS methodology, resulting in a high signal for PAO1, due to its negatively charged extra-cellular components. This study proves that RPM-based biosensors can be used for bacteria detection. Their advantages from the applicability point of view are a straight-forward synthesis protocol that does not require purification, a low cost of production, stability in different environments, and an affinity to a wide range of bacteria. The different responses of AuE-CFK to the various bacteria species observed in this study require further analysis. Applying alternative electrochemical methods can be used to further utilize the AuE-CFK for bacteria biosensing. Further optimization of the device architecture can also be applied to increase the sensitivity, thus offering a new and acutely needed application for this very intriguing family of synthetic peptides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s23020561/s1.
Author Contributions
Conceptualization, Z.H.; Methodology, T.S.B., M.H., S.Y. and Z.H.; Formal analysis, T.S.B. and M.H.; Investigation, T.S.B. and R.Y.; Data curation, T.S.B., M.H. and S.Y.; Project administration, Z.H.; Funding acquisition, M.H. and Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Chief Scientist of the Israel Ministry of Agriculture (grant number 12-23-0001) and by the Racah Nano Venture Fund from the Yissum Research Development Company. T.S.B. was supported by a Ph.D. student scholarship from the Ministry of Science and Technology of Israel. The graphical abstract and Figure 2 were created using BioRender.com.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of Multi-Drug Resistant Bacteria on Economic and Clinical Outcomes of Healthcare-Associated Infections in Adults: Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Rello, J.; Blasi, F.; Goossens, H.; Sotgiu, G.; Tavoschi, L.; Zasowski, E.J.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. Systematic Review of the Impact of Appropriate versus Inappropriate Initial Antibiotic Therapy on Outcomes of Patients with Severe Bacterial Infections. Int. J. Antimicrob. Agents 2020, 56, 106184. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A Review of Methods for the Detection of Pathogenic Microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Riu, J.; Giussani, B. Electrochemical Biosensors for the Detection of Pathogenic Bacteria in Food. TrAC-Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2018, 119, 700–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Z.; Ying, Y. New Trends in Impedimetric Biosensors for the Detection of Foodborne Pathogenic Bacteria. Sensors 2012, 12, 3449–3471. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The Role of Peptides in the Design of Electrochemical Biosensors for Clinical Diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F. Self-Assembled Alkanethiol Monolayers on Gold Surfaces: Resolving the Complex Structure at the Interface. Phys. Chem. Chem. Phys. 2014, 16, 19074–19090. [Google Scholar] [CrossRef]
- Mandler, D.; Kraus-Ophir, S. Self-Assembled Monolayers (SAMs) for Electrochemical Sensing. J. Solid State Electrochem. 2011, 15, 1535–1558. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Venanzi, M. Self-Assembled Monolayers Formed by Helical Peptide Building Blocks: A New Tool for Bioinspired Nanotechnology. Polym. J. 2013, 45, 468–480. [Google Scholar] [CrossRef]
- Hayouka, Z.; Chakraborty, S.; Liu, R.; Boersma, M.D.; Weisblum, B.; Gellman, S.H. Interplay among Subunit Identity, Subunit Proportion, Chain Length, and Stereochemistry in the Activity Profile of Sequence-Random Peptide Mixtures. J. Am. Chem. Soc. 2013, 135, 11748–11751. [Google Scholar] [CrossRef] [PubMed]
- Stern, T.; Zelinger, E.; Hayouka, Z.; Boersma, M.D.; Weisblum, B.; Gellman, S.H.; Welch, R.A.; Weisblum, B.; Masters, K.S.; Gellman, S.H.; et al. Random Peptide Mixtures Inhibit and Eradicate Methicillin-Resistant Staphylococcus Aureus Biofilms. Chem. Commun. 2016, 52, 7102–7105. [Google Scholar] [CrossRef] [PubMed]
- Hayouka, Z.; Bella, A.; Stern, T.; Ray, S.; Jiang, H.; Grovenor, C.R.M.M.; Ryadnov, M.G. Binary Encoding of Random Peptide Sequences for Selective and Differential Antimicrobial Mechanisms. Angew. Chem. 2017, 129, 8211–8215. [Google Scholar] [CrossRef]
- Topman, S.; Tamir-ariel, D.; Bochnic-, H.; Bauer, T.S.; Sha, S. Random Peptide Mixtures as New Crop Protection Agents. Microb. Biotechnol. 2018, 11, 1027–1036. [Google Scholar] [CrossRef]
- Topman-Rakover, S.; Malach, E.; Burdman, S.; Hayouka, Z. Antibacterial Lipo-Random Peptide Mixtures Exhibit High Selectivity and Synergistic Interactions. Chem. Commun. 2020, 56, 12053–12056. [Google Scholar] [CrossRef]
- Bauer Stern, T.; Hayouka, Z. Random Mixtures of Antimicrobial Peptides Inhibit Bacteria Associated with Pasteurized Bovine Milk. J. Pept. Sci. 2018, 24, e3088. [Google Scholar] [CrossRef]
- Cheriker, H.; Stern Bauer, T.; Oren, Y.; Nir, S.; Hayouka, Z. Immobilized Random Peptide Mixtures Exhibit Broad Antimicrobial Activity with High Selectivity. Chem. Commun. 2020, 56, 11022–11025. [Google Scholar] [CrossRef]
- Bennett, R.C.; Oh, M.W.; Kuo, S.H.; Belo, Y.; Maron, B.; Malach, E.; Lin, J.; Hayouka, Z.; Lau, G.W. Random Peptide Mixtures as Safe and Effective Antimicrobials against Pseudomonas Aeruginosa and MRSA in Mouse Models of Bacteremia and Pneumonia. ACS Infect. Dis. 2021, 7, 672–680. [Google Scholar] [CrossRef]
- Caraway, H.E.; Lau, J.Z.; Maron, B.; Oh, M.W.; Belo, Y.; Brill, A.; Malach, E.; Ismail, N.; Hayouka, Z.; Lau, G.W. Antimicrobial Random Peptide Mixtures Eradicate Acinetobacter Baumannii Biofilms and Inhibit Mouse Models of Infection. Antibiotics 2022, 11, 413. [Google Scholar] [CrossRef]
- Weiss, E.A.; Kaufman, G.K.; Kriebel, J.K.; Li, Z.; Schalek, R.; Whitesides, G.M. Si/SiO2-Templated Formation of Ultraflat Metal Surfaces on Glass, Polymer, and Solder Supports: Their Use as Substrates for Self-Assembled Monolayers. Langmuir 2007, 23, 9686–9694. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.M.; Popenoe, D.D.; Deinhammer, R.S.; Lamp, B.D.; Chung, C.; Porter, M.D. Reductive Desorption of Alkanethiolate Monolayers at Gold: A Measure of Surface Coverage. Langmuir 1991, 7, 2687–2693. [Google Scholar] [CrossRef]
- Widrig, C.A.; Chung, C.; Porter, M.D. The Electrochemical Desorption of N-Alkanethiol Monolayers from Polycrystalline Au and Ag Electrodes. J. Electroanal. Chem. 1991, 310, 335–359. [Google Scholar] [CrossRef]
- Imabayashi, S.I.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive Desorption of Carboxylic-Acid-Terminated Alkanethiol Monolayers from Au(111) Surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar] [CrossRef]
- Joshi, P.N.; Mervinetsky, E.; Solomon, O.; Chen, Y.J.; Yitzchaik, S.; Friedler, A. Electrochemical Biosensors Based on Peptide-Kinase Interactions at the Kinase Docking Site. Biosens. Bioelectron. 2022, 207, 114177. [Google Scholar] [CrossRef]
- Shamsi, F.; Coster, H.; Jolliffe, K.A. Characterization of Peptide Immobilization on an Acetylene Terminated Surface via Click Chemistry. Surf. Sci. 2011, 605, 1763–1770. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-Ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-Ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 7463, 5083–5086. [Google Scholar] [CrossRef]
- de la Llave, E.; Clarenc, R.; Schiffrin, D.J.; Williams, F.J. Organization of alkane amines on a gold surface: Structure, surface dipole, and electron transfer. J. Phys. Chem. C 2014, 118, 468–475. [Google Scholar] [CrossRef]
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization Reduces the Activity of Surface-Bound Cationic Antimicrobial Peptides with No Influence upon the Activity Spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Kanyong, P.; Davis, J.J. Homogeneous Functional Self-Assembled Monolayers: Faradaic Impedance Baseline Signal Drift Suppression for High-Sensitivity Immunosensing of C-Reactive Protein. J. Electroanal. Chem. 2020, 856, 113675. [Google Scholar] [CrossRef]
- Xu, X.; Makaraviciute, A.; Kumar, S.; Wen, C.; Sjödin, M.; Abdurakhmanov, E. Zhang, Z. Structural Changes of Mercaptohexanol Self-Assembled Monolayers on Gold and Their Influence on Impedimetric Aptamer Sensors. Anal. Chem. 2019, 91, 14697–14704. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia Coli, Pseudomonas Aeruginosa, and Staphylococcus Aureus Attachment Patterns on Glass Surfaces with Nanoscale Roughness. Curr. Microbiol. 2009, 58, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in Surface Properties between Escherichia Coli and Staphylococcus Aureus as Revealed by Electrophoretic Mobility Measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.G. Composition and Structure of Lipopolysaccharides from Pseudomonas Aeruginosa. Rev. Infect. Dis. 1983, 5, S941–S949. [Google Scholar] [CrossRef]
- Epand, R.F.; Savage, P.B.; Epand, R.M. Bacterial Lipid Composition and the Antimicrobial Efficacy of Cationic Steroid Compounds (Ceragenins). Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2500–2509. [Google Scholar] [CrossRef]
- McGroarty, E.J. Lipopolysaccharide and Capsular Polysaccharide. In Pseudomonas. Biotechnology Handbooks; Monties, T.C., Ed.; Springer: Boston, MA, USA, 1998; pp. 73–110. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).