A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
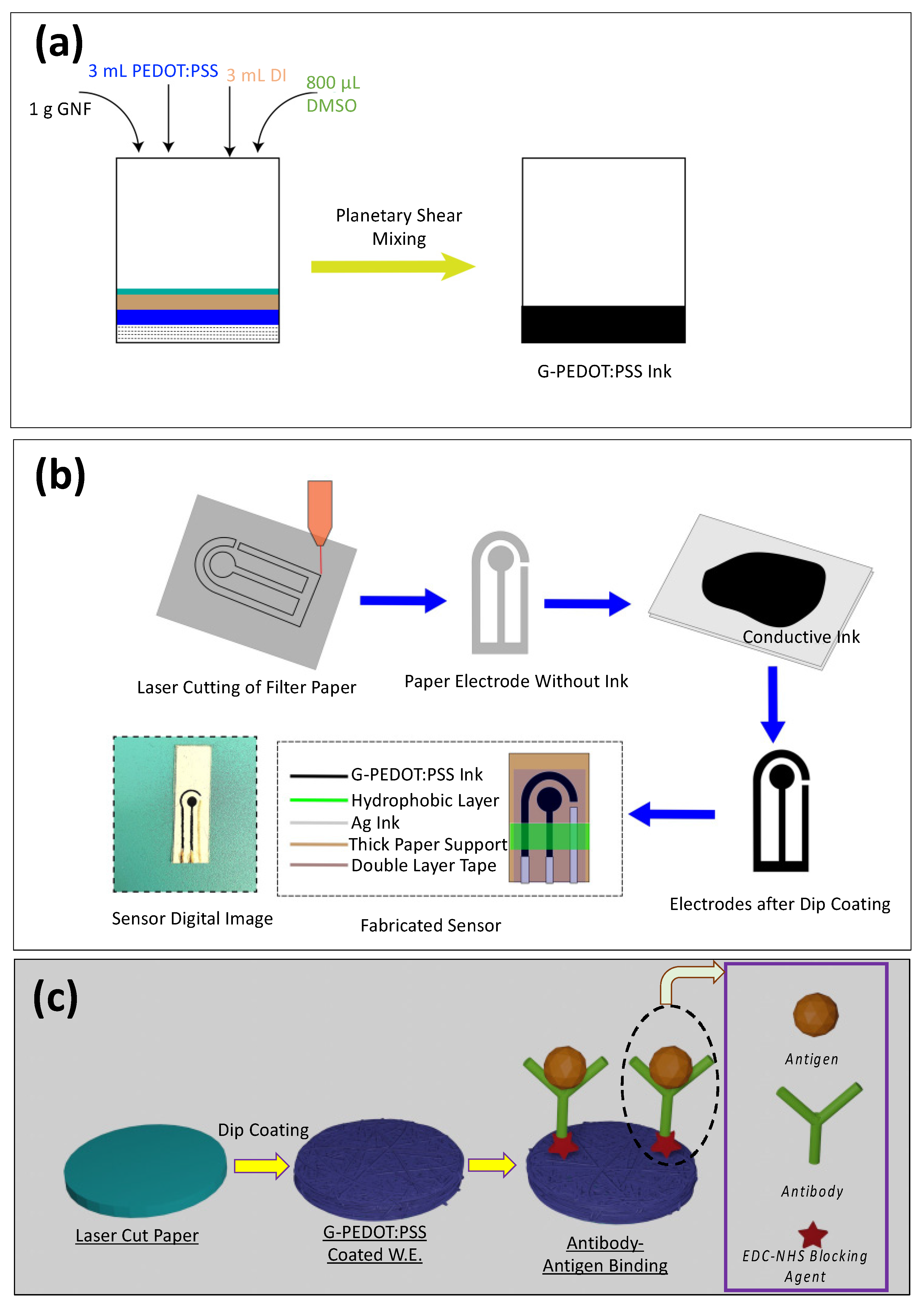
2.2. Ink Preparation
2.3. Laser Cutting of Filter Paper and Electrode Dip Coating
2.4. Dopamine Solution Preparation
2.5. Surface Modification, EDC/NHS Conjugation, TNF-α Antigen, and IL-6 Antigen Solution Preparation
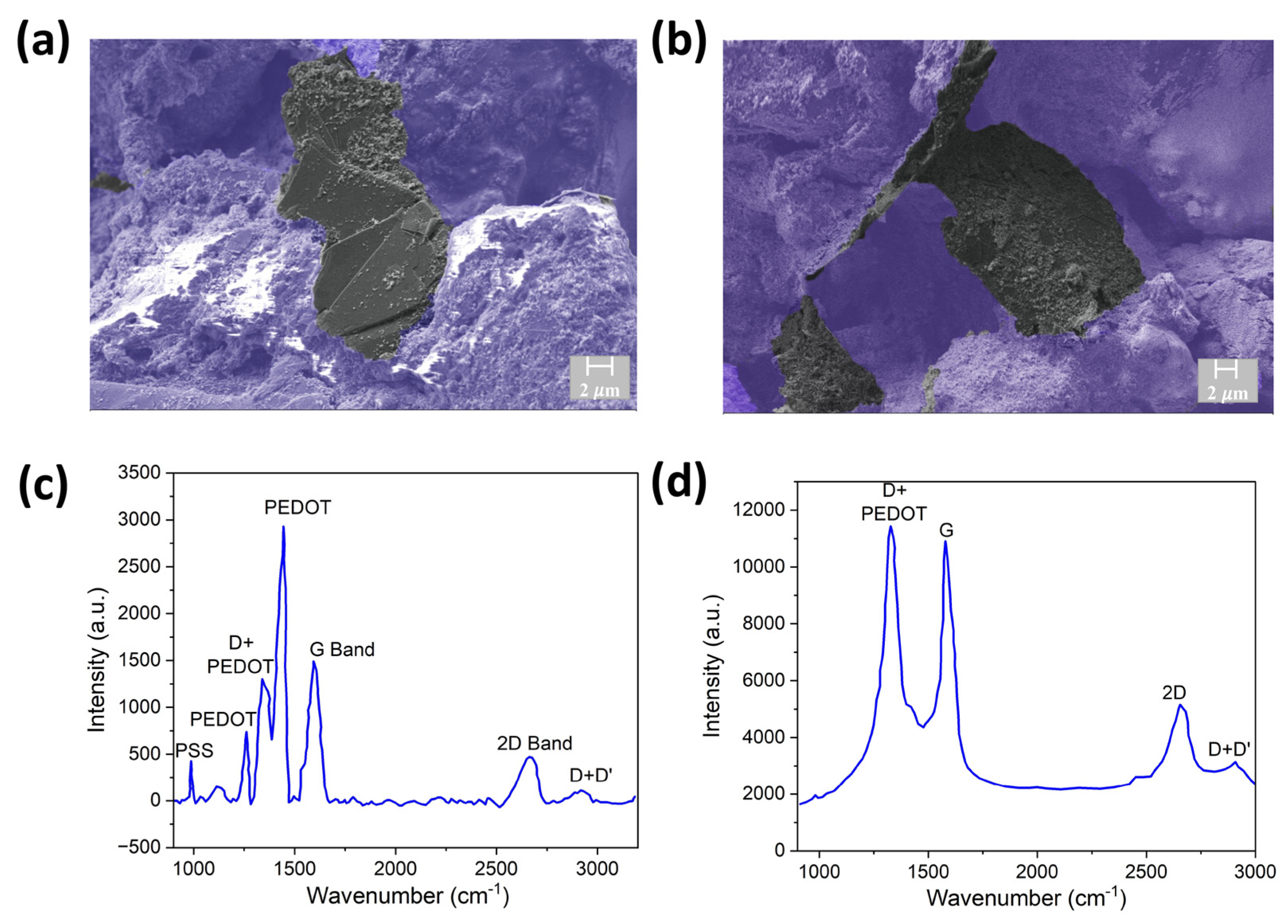
2.6. Scanning Electron Microscopy (SEM) and Raman Characterization
2.7. Electrochemical Analysis of Dopamine/TNF-α/IL-6
3. Results and Discussion
3.1. Characterization
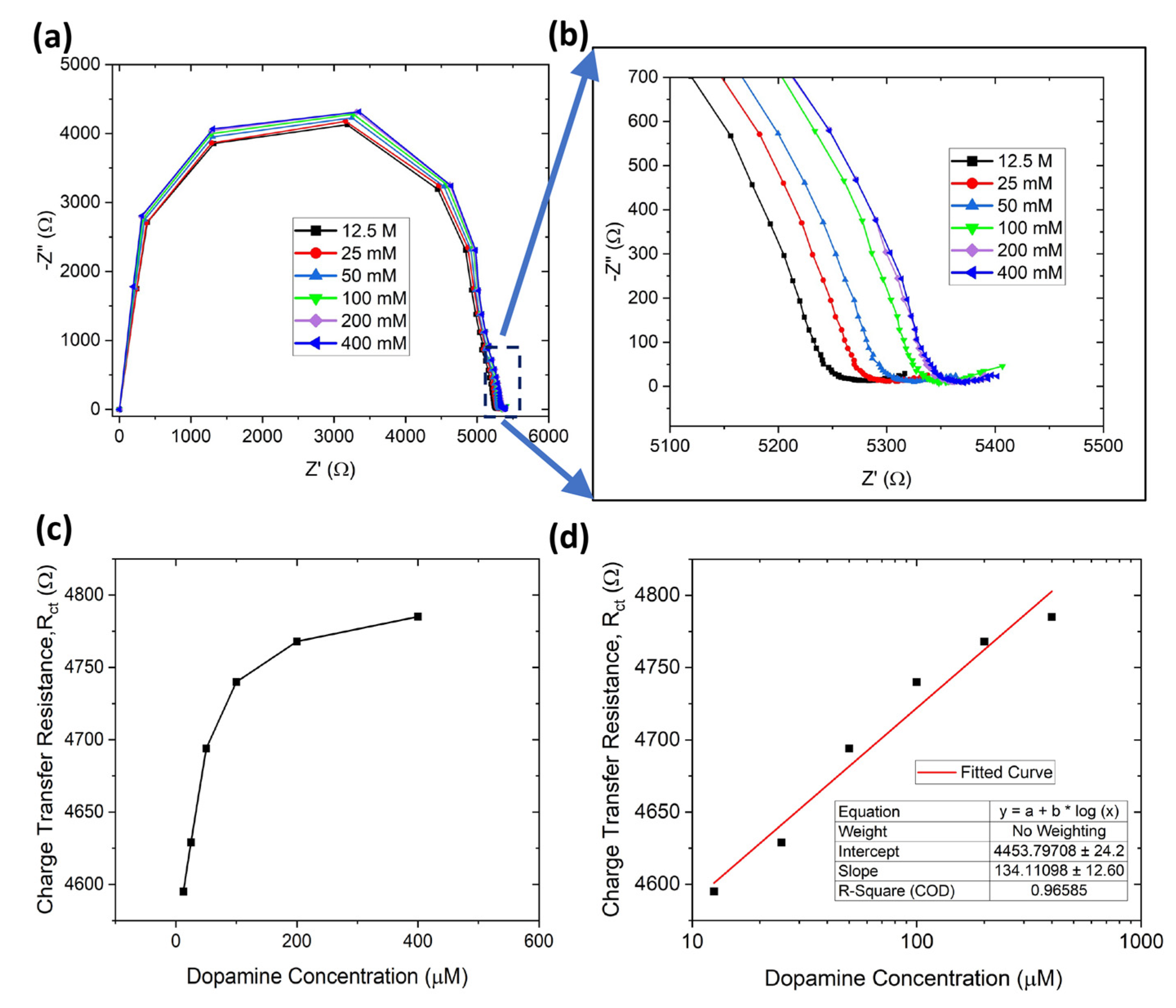
3.2. Dopamine Detection
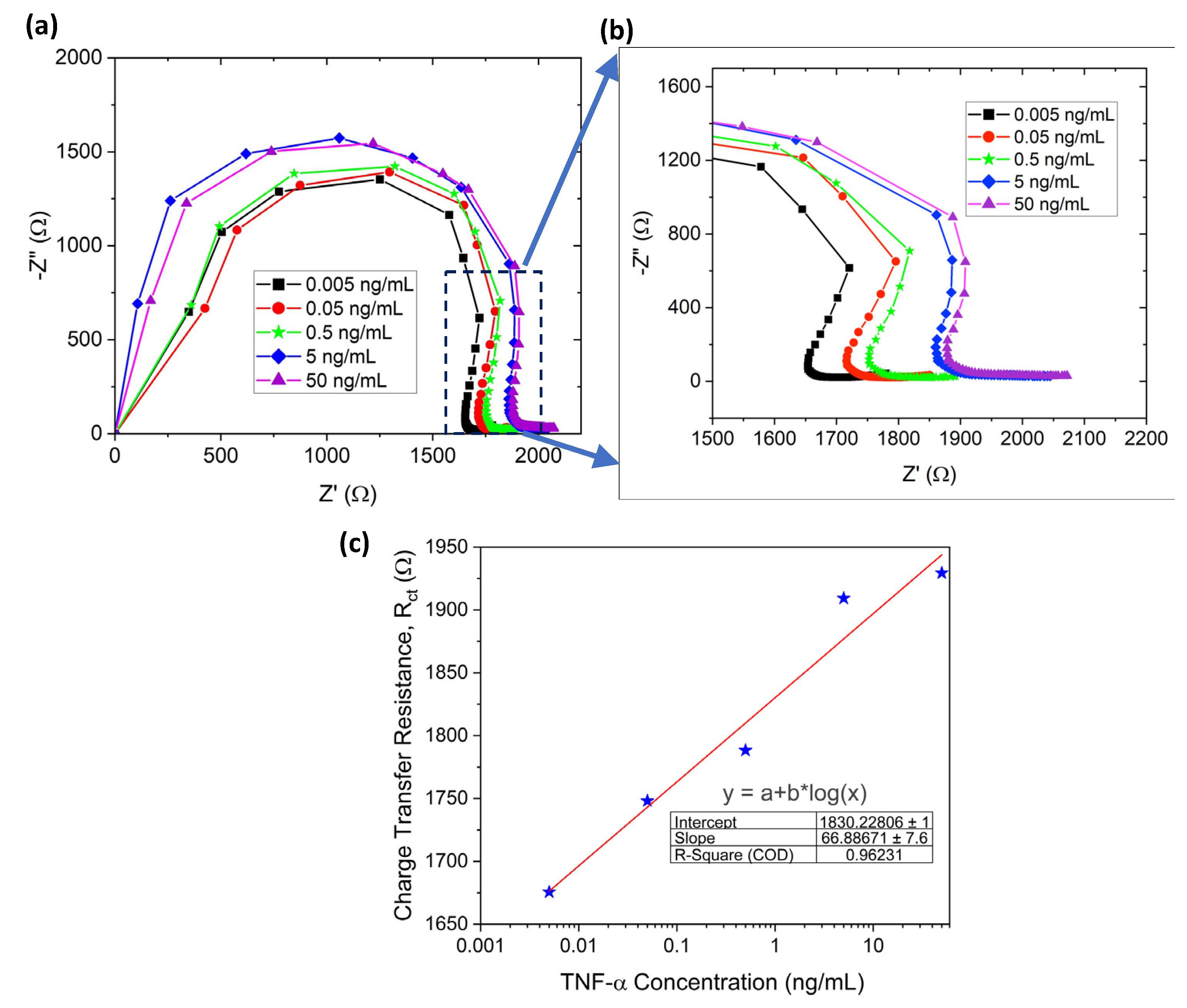
3.3. TNF-α Detection
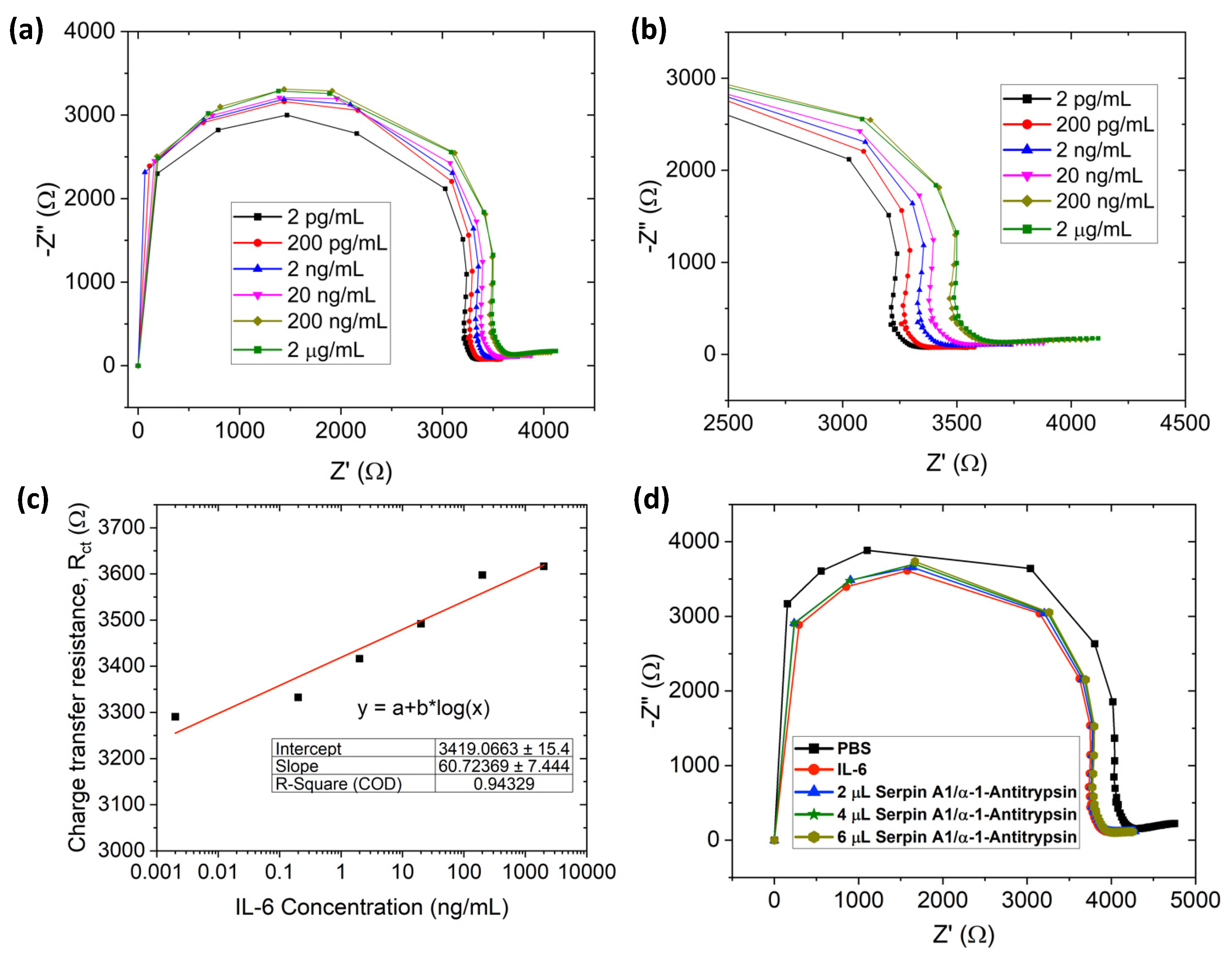
3.4. IL-6 Detection
3.5. Selective Detection of IL-6
3.6. IL-6 Detection with Human Serum
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Rye, D.; Hochman, S. Restless legs syndrome: Revisiting the dopamine hypothesis from the spinal cord perspective. Neurology 2006, 67, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.-Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Caporali, R.; Pallavicini, F.B.; Filippini, M.; Gorla, R.; Marchesoni, A.; Favalli, E.G.; Sarzi-Puttini, P.; Atzeni, F.; Montecucco, C. Treatment of rheumatoid arthritis with anti-TNF-alpha agents: A reappraisal. Autoimmun. Rev. 2009, 8, 274–280. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Anti-TNF (alpha) therapy or rheumatoid arthritis: What have we learned? Annu. Rev. Immunol. 2001, 19, 163. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; Van Riel, P.L.; Van Der Meer, J.W.; Stalenhoef, A.F. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Rojo, L.E.; Fernandez, J.A.; Kuljis, R.O. The role of neuroimmunomodulation in Alzheimer’s disease. Ann. New York Acad. Sci. 2009, 1153, 240–246. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Cohen, J.D.; Bournerias, I.; Buffard, V.; Paufler, A.; Chevalier, X.; Bagot, M.; Claudepierre, P. Psoriasis induced by tumor necrosis factor-alpha antagonist therapy: A case series. J. Rheumatol. 2007, 34, 380–385. [Google Scholar] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, Ø.; Sandanger, Ø.; Ryan, L.; Otterdal, K.; Damaas, J.K.; Pukstad, B.; Espevik, T. Cellular sources and inducers of cytokines present in acute wound fluid. Wound Repair Regen. 2011, 19, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.M.; Alba-Patiño, A.; Barón, E.; Borges, M.; Gonzalez-Freire, M.; De La Rica, R. Biosensors for managing the COVID-19 cytokine storm: Challenges ahead. ACS Sens. 2020, 5, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Grayson, L.; Hansbrough, J.; Zapata-Sirvent, R.; Dore, C.; Morgan, J.; Nicolson, M. Quantitation of cytokine levels in skin graft donor site wound fluid. Burns 1993, 19, 401–405. [Google Scholar] [CrossRef]
- Baker, E.A.; Leaper, D.J. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen. 2000, 8, 392–398. [Google Scholar] [CrossRef]
- Barton, B.E. IL-6-like cytokines and cancer cachexia. Immunol. Res. 2001, 23, 41–58. [Google Scholar] [CrossRef]
- Knüpfer, H.; Preiß, R. Significance of interleukin-6 (IL-6) in breast cancer. Breast Cancer Res. Treat. 2007, 102, 129–135. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Pascual, M.; Nieto, A.; Mataran, L.; Balsa, A.; Pascual-Salcedo, D.; Martin, J. IL-6 promoter polymorphisms in rheumatoid arthritis. Genes Immun. 2000, 1, 338–340. [Google Scholar] [CrossRef]
- Jengelley, D.H.; Zimmers, T.A. The Role of Interleukin-6/GP130 Cytokines in Cancer Cachexia. In The Systemic Effects of Advanced Cancer: A Textbook on Cancer-Associated Cachexia; Springer: Berlin/Heidelberg, Germany, 2022; pp. 97–117. [Google Scholar]
- Anik, Ü. Electrochemical medical biosensors for POC applications. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–292. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. A review on electrochemical sensors and biosensors used in assessing antioxidant activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Casanovas, J.; Redondo, E.; Armelin, E.; Alemán, C. A rational design for the selective detection of dopamine using conducting polymers. Phys. Chem. Chem. Phys. 2014, 16, 7850–7861. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.; Li, H.; Luo, X.; Cui, X. A graphene oxide/conducting polymer nanocomposite for electrochemical dopamine detection: Origin of improved sensitivity and specificity. J. Mater. Chem. B 2014, 2, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.; Bossi, A.; Whitcombe, M.J.; Chianella, I.; Fowler, S.A.; Subrahmanyam, S.; Piletska, E.V.; Piletsky, S.A. Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal. Chem. 2009, 81, 3576–3584. [Google Scholar] [CrossRef]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric detection of dopamine in human serumby electrochemical sensor based on gold nanoparticles doped molecularly imprinted polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef]
- Du, Y.; Dai, L.; Yang, F.; Zhang, Y.; An, C. In-situ polymerization confining synthesis of ultrasmall MoTe2 nanoparticles for electrochemical detection of dopamine. Inorg. Chem. Front. 2022, 9, 4121–4126. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Electrochemical sensor for detecting dopamine using graphene quantum dots incorporated with multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Bala, K.; Sharma, D.; Gupta, N. Carbon—Nanotube—Based Materials for Electrochemical Sensing of the Neurotransmitter Dopamine. ChemElectroChem 2019, 6, 274–288. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Bukhari, N.; Wabaidur, S.M.; Haider, S. Simultaneous electrochemical determination of dopamine and acetaminophen using multiwall carbon nanotubes modified glassy carbon electrode. Sens. Actuators B: Chem. 2010, 146, 314–320. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Said, D.M. Novel design of a layered electrochemical dopamine sensor in real samples based on gold nanoparticles/β-cyclodextrin/nafion-modified gold electrode. ACS Omega 2019, 4, 17947–17955. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Brink, R.; Nandi, D.; Siwal, S.; Mallick, K. Gold nanoparticle within the polymer chain, a multi-functional composite material, for the electrochemical detection of dopamine and the hydrogen atom-mediated reduction of Rhodamine-B, a mechanistic approach. J. Mater. Sci. 2017, 52, 770–781. [Google Scholar] [CrossRef]
- Pandikumar, A.; How, G.T.S.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, K.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, S.A. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. Rsc Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Bhat, S.A.; Pandit, S.A.; Rather, G.M.; Khan, K.Z.; Bhat, M.A. Imidazolium based surface active ionic liquids as novel micellar media for simultaneous and sensitive electrochemical detection of dopamine and ascorbic acid. Electroanalysis 2017, 29, 1772–1782. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, L.; Guo, Y.; Zeng, Y.; Sun, W.; Huang, X. Electrochemical detection of dopamine on a Ni/Al layered double hydroxide modified carbon ionic liquid electrode. Sens. Actuators B Chem. 2010, 151, 146–152. [Google Scholar] [CrossRef]
- Liang, T.; Pal, R.K.; Zou, X.; Root, A.; Mazzeo, A.D.; Hussain, M.; El-Atab, N. Flexible and Stretchable Paper-based Structures for Electronic Applications. In Handbook of Flexible and Stretchable Electronics; CRC Press: Boca Raton, FL, USA, 2019; pp. 337–375. [Google Scholar]
- Mazzeo, A.D.; Liang, T.; Zou, X.; Xie, J.; Ashraf, A.; Salvi, D.; Berthiaume, F.; Pal, R.K. Paper as a substrate and smart material for electronics, packaging, and robotics. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 5 July 2021; pp. 1–3. [Google Scholar]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef]
- Liang, T.; Zou, X.; Pal, R.K.; Xie, J.; Assasie-Gyimah, M.K.; Liu, J.; Guo, W.; Chen, C.; Tenorio, M.; Sullivan, D. Tunable electrical properties of embossed, cellulose-based paper for skin-like sensing. ACS Appl. Mater. Interfaces 2020, 12, 51960–51968. [Google Scholar] [CrossRef]
- Zou, X.; Chen, C.; Liang, T.; Xie, J.; Gillette-Henao, E.N.; Oh, J.; Tumalle, J.; Mazzeo, A.D. Paper–Based Resistive Networks for Scalable Skin-Like Sensing. Adv. Electron. Mater. 2018, 4, 1800131. [Google Scholar] [CrossRef]
- Adkins, J.; Boehle, K.; Henry, C. Electrochemical paper—based microfluidic devices. Electrophoresis 2015, 36, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.W.; Pui, T.-S. Cytokine and cancer biomarkers detection: The dawn of electrochemical paper-based biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef] [PubMed]
- Mantione, D.; Del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly (3, 4-ethylenedioxythiophene)(PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.M.; dos Santos Klem, M.; Nogueira, G.L.; Gomes, T.C.; Alves, N. Low cost humidity sensor based on PANI/PEDOT: PSS printed on paper. IEEE Sens. J. 2018, 18, 2647–2651. [Google Scholar] [CrossRef]
- Cho, K.H.; Yu, H.; Lee, J.S.; Jang, J. Facile synthesis of palladium-decorated three-dimensional conducting polymer nanofilm for highly sensitive H2 gas sensor. J. Mater. Sci. 2020, 55, 5156–5165. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An overview on the synthesis and recent applications of conducting poly (3, 4-ethylenedioxythiophene)(PEDOT) in industry and biomedicine. J. Mater. Sci. 2020, 55, 7575–7611. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.A. Fabrication and characterization of an ammonia gas sensor based on PEDOT-PSS with N-doped graphene quantum dots dopant. IEEE Sens. J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Karuwan, C.; Sriprachuabwong, C.; Wisitsoraat, A.; Phokharatkul, D.; Sritongkham, P.; Tuantranont, A. Inkjet-printed graphene-poly (3, 4-ethylenedioxythiophene): Poly (styrene-sulfonate) modified on screen printed carbon electrode for electrochemical sensing of salbutamol. Sens. Actuators B Chem. 2012, 161, 549–555. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A highly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ruecha, N.; Shin, K.; Chailapakul, O.; Rodthongkum, N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens. Actuators B Chem. 2019, 279, 298–304. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Pandey, C.M.; Malhotra, B.D. Conducting paper based sensor for cancer biomarker detection. J. Phys. Conf. Ser. 2016, 704, 012010. [Google Scholar] [CrossRef]
- Russell, C.; Ward, A.C.; Vezza, V.; Hoskisson, P.; Alcorn, D.; Steenson, D.P.; Corrigan, D.K. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin-6 (IL-6) in real time. Biosens. Bioelectron. 2019, 126, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; He, T.; Jiang, F.; Shi, J.-J.; Zhu, J.-J. A competitive electrochemical immunosensor for the detection of human interleukin-6 based on the electrically heated carbon electrode and silver nanoparticles functionalized labels. Talanta 2014, 122, 135–139. [Google Scholar] [CrossRef]
- Peng, J.; Feng, L.N.; Ren, Z.J.; Jiang, L.P.; Zhu, J.J. Synthesis of Silver Nanoparticle—Hollow Titanium Phosphate Sphere Hybrid as a Label for Ultrasensitive Electrochemical Detection of Human Interleukin-6. Small 2011, 7, 2921–2928. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Zhang, G.; Zhang, X.; Fang, B.; Wang, L. Dual amplification strategy for the fabrication of highly sensitive interleukin-6 amperometric immunosensor based on poly-dopamine. Langmuir 2011, 27, 1224–1231. [Google Scholar] [CrossRef]
- Longo, A.; Baraket, A.; Vatteroni, M.; Zine, N.; Baussells, J.; Di Francesco, F.; Karanasiou, G.S.; Fotiadis, D.I.; Menciassi, A.; Errachid, A. Highly sensitive electrochemical BioMEMS for TNF-α detection in humansaliva: Heart failure. Procedia Eng. 2016, 168, 97–100. [Google Scholar] [CrossRef]
- Wang, H.; Ohnuki, H.; Endo, H.; Izumi, M. Impedimetric and amperometric bifunctional glucose biosensor based on hybrid organic–inorganic thin films. Bioelectrochemistry 2015, 101, 1–7. [Google Scholar] [CrossRef]
- Ryu, G.H.; Lee, J.; Kang, D.; Jo, H.J.; Shin, H.S.; Lee, Z. Effects of dry oxidation treatments on monolayer graphene. 2D Mater. 2017, 4, 024011. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Sheng, G.; Chen, C.; Wang, X.; Nagatsu, M. Removal of polychlorinated biphenyls from aqueous solutions using β-cyclodextrin grafted multiwalled carbon nanotubes. Chemosphere 2010, 79, 679–685. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Uygun, H.D.E. A short footnote: Circuit design for faradaic impedimetric sensors and biosensors. Sens. Actuators B: Chem. 2014, 202, 448–453. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; McNeil, C.J.; Rawson, K. Electrochemical impedance spectroscopy studies of polymer degradation: Application to biosensor development. TrAC Trends Anal. Chem. 2005, 24, 37–48. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance biosensors: Applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Alves, J.F.; Frasco, M.F.; Piloto, A.M.; Serrano, V.; Mateus, D.; Sebastião, A.I.; Matos, A.M.; Carmo, A.; Cruz, T. An ultra-sensitive electrochemical biosensor using the Spike protein for capturing antibodies against SARS-CoV-2 in point-of-care. Mater. Today Bio 2022, 16, 100354. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Ito, K.; Nishimura, W.; Imai, K.; Maeda, M. Detection limit estimated from slope of calibration curve: An application to competitive ELISA. Anal. Sci. 2005, 21, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Dong, M. Handbook of Pharmaceutical Analysis by HPLC; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Yang, L.; Liu, S.; Zhang, Q.; Li, F. Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@ polyaniline core–shell nanocomposites modified electrode. Talanta 2012, 89, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Sridevi, V. In situ electrodeposited gold nanoparticles on polyaniline-modified electrode surface for the detection of dopamine in presence of ascorbic acid and uric acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT: PSS organic electrochemical transistor. Sci. Rep. 2016, 6, 35419. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-based electrochemical potentiostat detection system using pedot: Pss/chitosan/graphene modified screen-printed electrodes for dopamine detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Tsai, T.-H.; Chen, S.-M. Performing enzyme-free H2O2 biosensor and simultaneous determination for AA, DA, and UA by MWCNT–PEDOT film. Biosens. Bioelectron. 2010, 26, 608–614. [Google Scholar] [CrossRef]
- Amara, U.; Sarfraz, B.; Mahmood, K.; Mehran, M.T.; Muhammad, N.; Hayat, A.; Nawaz, M.H. Fabrication of ionic liquid stabilized MXene interface for electrochemical dopamine detection. Microchim. Acta 2022, 189, 64. [Google Scholar] [CrossRef]
- Moallem, Q.A.; Beitollahi, H. Electrochemical sensor for simultaneous detection of dopamine and uric acid based on a carbon paste electrode modified with nanostructured Cu-based metal-organic frameworks. Microchem. J. 2022, 177, 107261. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Fayemi, O.E.; Adekunle, A.S.; Mamba, B.B.; Nkambule, T.T.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine using carbon quantum dots/copper oxide nanocomposite modified electrode. FlatChem 2022, 33, 100372. [Google Scholar] [CrossRef]
- Zhou, R.; Tu, B.; Xia, D.; He, H.; Cai, Z.; Gao, N.; Chang, G.; He, Y. High-performance Pt/Ti3C2Tx MXene based graphene electrochemical transistor for selective detection of dopamine. Anal. Chim. Acta 2022, 1201, 339653. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Engelhard, M.H.; Lin, Y. Sensitive immunoassay of a biomarker tumor necrosis factor-α based on poly (guanine)-functionalized silica nanoparticle label. Anal. Chem. 2006, 78, 6974–6979. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, Y.; Jiang, L.-P.; Zhu, J.-J. Electrochemical immunosensor of tumor necrosis factor α based on alkaline phosphatase functionalized nanospheres. Biosens. Bioelectron. 2011, 26, 1890–1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef]
- Kongsuphol, P.; Ng, H.H.; Pursey, J.P.; Arya, S.K.; Wong, C.C.; Stulz, E.; Park, M.K. EIS-based biosensor for ultra-sensitive detection of TNF-α from non-diluted human serum. Biosens. Bioelectron. 2014, 61, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Sri, S.; Chauhan, D.; Lakshmi, G.; Thakar, A.; Solanki, P.R. MoS2 nanoflower based electrochemical biosensor for TNF alpha detection in cancer patients. Electrochim. Acta 2022, 405, 139736. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Baraket, A.; Zine, N.; Streklas, A.; Bausells, J.; Errachid, A.; López, M. A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-α by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2018, 100, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Baraket, A.; Zine, N.; Ali, M.B.; Bausells, J.; Errachid, A. Capacitance electrochemical biosensor based on silicon nitride transducer for TNF-α cytokine detection in artificial human saliva: Heart failure (HF). Talanta 2020, 209, 120501. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Niu, W.; Fam, D.W.; Palaniappan, A.; Larisika, M.; Faulkner, S.H.; Nowak, C.; Nimmo, M.A.; Liedberg, B. Highly manufacturable graphene oxide biosensor for sensitive Interleukin-6 detection. Rsc Adv. 2015, 5, 39245–39251. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]


| Electrode Structure | Technique | Linear Range | Limit of Detection (LOD) | References |
|---|---|---|---|---|
| Polyaniline (PANI)–Au nanoparticles (NPs) | Differential pulse voltammetry (DPV) | 10–1700 µM | 5 µM | [77] |
| Polyaniline (PANI)–Au NPs | Linear sweep voltammetry (LSV) | 20–100 μM | 16 μM | [78] |
| PEDOT:PSS (FET) | CV, DPV | 5–100 µM | 6 µM | [79] |
| PEDOT: PSS/Chitosan/Graphene | CV, DPV | 0.05–70 µM | 0.29 µM | [80] |
| MWCNT-PEDOT | CV, DPV | 10–330 µM | 10 µM | [81] |
| Ti3C2Cl2/graphitic pencil electrode | CV, DPV, EIS | 10−2000 μM | 702 nM | [82] |
| Cu-benzene-1,3,5-tricarboxylic acid/carbon paste electrode | CV, DPV | 0.05-500 μM | 0.03 μM | [83] |
| Glassy carbon electrode (GCE)/Carbon quantum dots/CuO | Square Wave voltammetry (SWV) | 1–800 μM | 25.4 μM | [84] |
| GCE/Pt/Ti3C2Tx | CV, Constant Voltage Deposition | 50 nM–9 mM | 50 nM | [85] |
| G-PEDOT:PSS | EIS | 12.5–400 µM | 3.4 µM | This work |
| Biosensor Structure | Sensing Matrix | Technique | Detection Range | LOD | References |
|---|---|---|---|---|---|
| Poly(guanine)-functionalized silica NPs | Antibody | Square wave voltammograms | 0.1–100 ng/mL | 50 pg/mL | [86] |
| Alkaline phosphatase functionalized nanospheres | Antibody | EIS | 0.02–200 ng/mL | 0.01 ng/mL | [87] |
| Au working electrode | Aptamer | CV | 10–100 ng/mL | 10 ng/mL | [88] |
| Comb-structured Au microelectrode arrays | Antibody | EIS | 0.001-1 ng/mL | 1 pg/mL | [89] |
| MoS2 nanoflower | Antibody | CV & EIS | 1-200 pg/mL | 0.202 pg/mL | [90] |
| Au W.E. | Antibody | EIS | 266–666,000 pg/mL | 266 pg/mL | [91] |
| Si3N4/SiO2/Si[P]/Al | Antibody | Capacitive | 1–30 pg/mL | 1 pg/mL | [92] |
| G-PEDOT:PSS | Antibody | EIS | 0.005–50 ng/mL | 5.97 pg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Pal, R.K.; Islam, N.; Freeman, R.; Berthiaume, F.; Mazzeo, A.; Ashraf, A. A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection. Sensors 2023, 23, 8115. https://doi.org/10.3390/s23198115
Rahman MA, Pal RK, Islam N, Freeman R, Berthiaume F, Mazzeo A, Ashraf A. A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection. Sensors. 2023; 23(19):8115. https://doi.org/10.3390/s23198115
Chicago/Turabian StyleRahman, Md Ashiqur, Ramendra Kishor Pal, Nazmul Islam, Robert Freeman, Francois Berthiaume, Aaron Mazzeo, and Ali Ashraf. 2023. "A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection" Sensors 23, no. 19: 8115. https://doi.org/10.3390/s23198115
APA StyleRahman, M. A., Pal, R. K., Islam, N., Freeman, R., Berthiaume, F., Mazzeo, A., & Ashraf, A. (2023). A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection. Sensors, 23(19), 8115. https://doi.org/10.3390/s23198115







