Evaluation of a Scintillating Plastic Optical Fiber Device for Measuring kV-Cone Beam Computed Tomography Dose
Abstract
1. Introduction
2. Materials and Methods
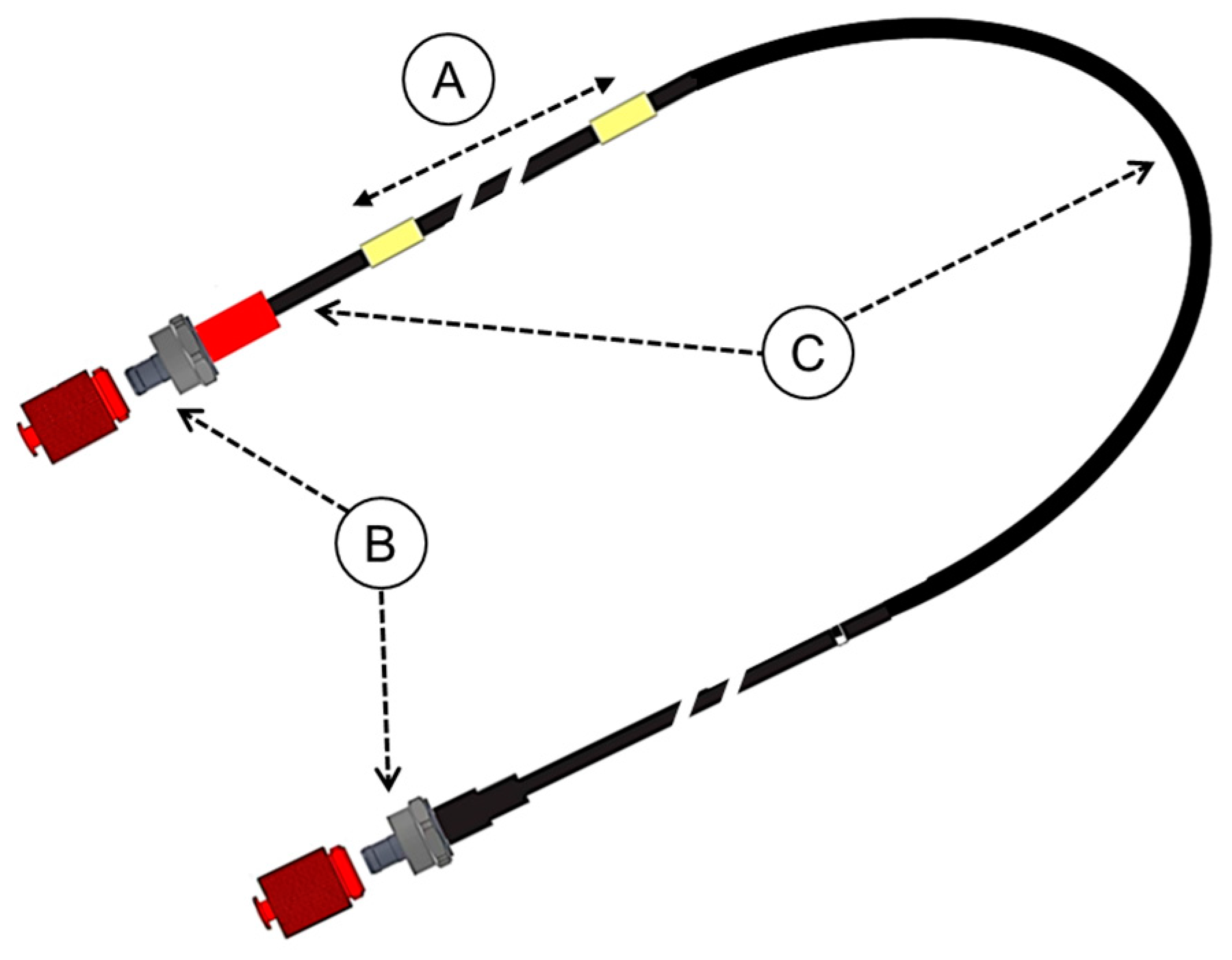
2.1. Properties of the Dosimeter
2.2. Installation of the Device on the Irradiation Device
2.3. Calibration of the Device for Specific CBCT Protocols
2.4. Determining the Impact of the Fiber on the Delivery of the Treatment Dose and Imaging
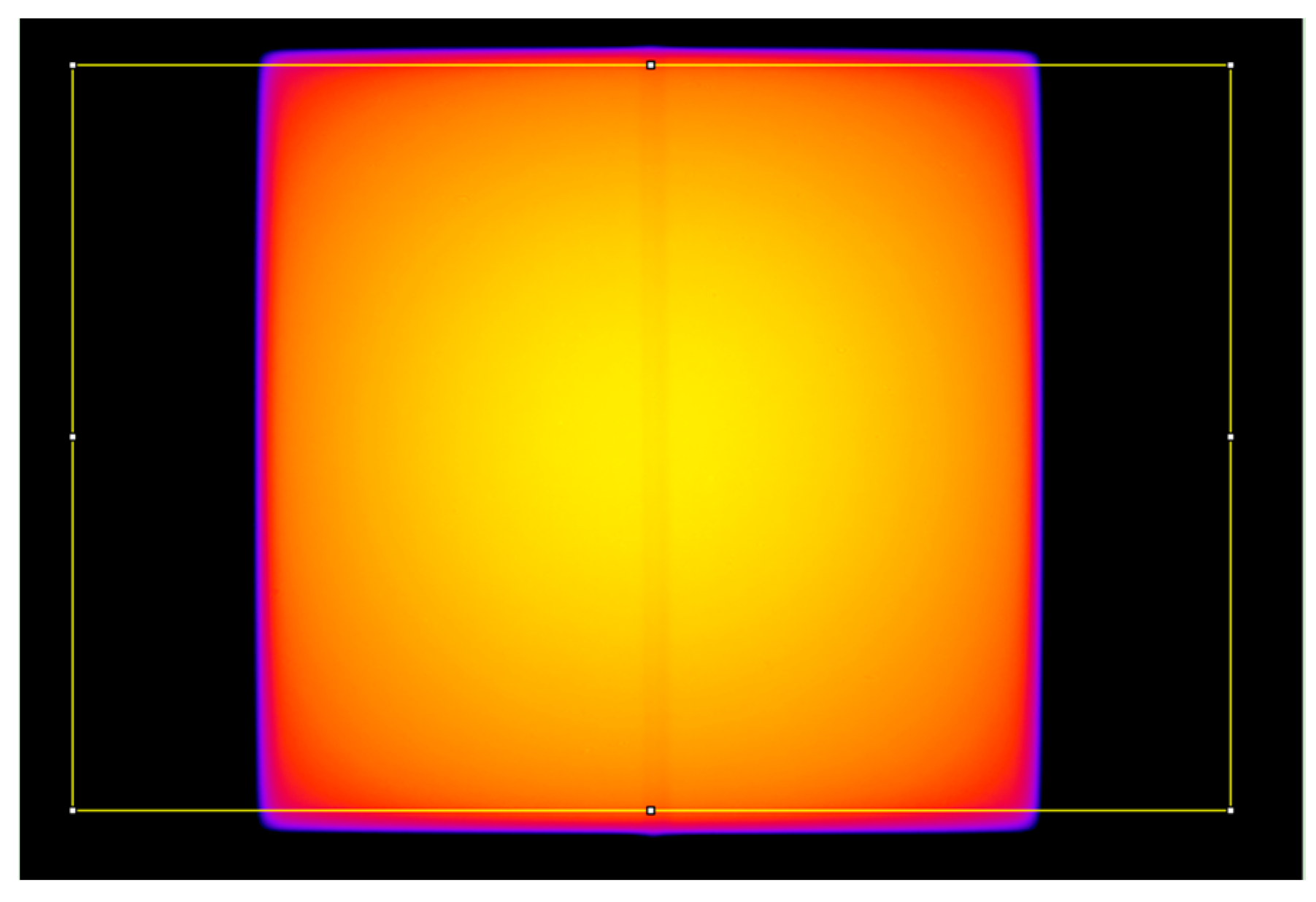
2.5. Comparison of the Device, Pencil Ionization Chamber, and Accelerator CTDIs
3. Results
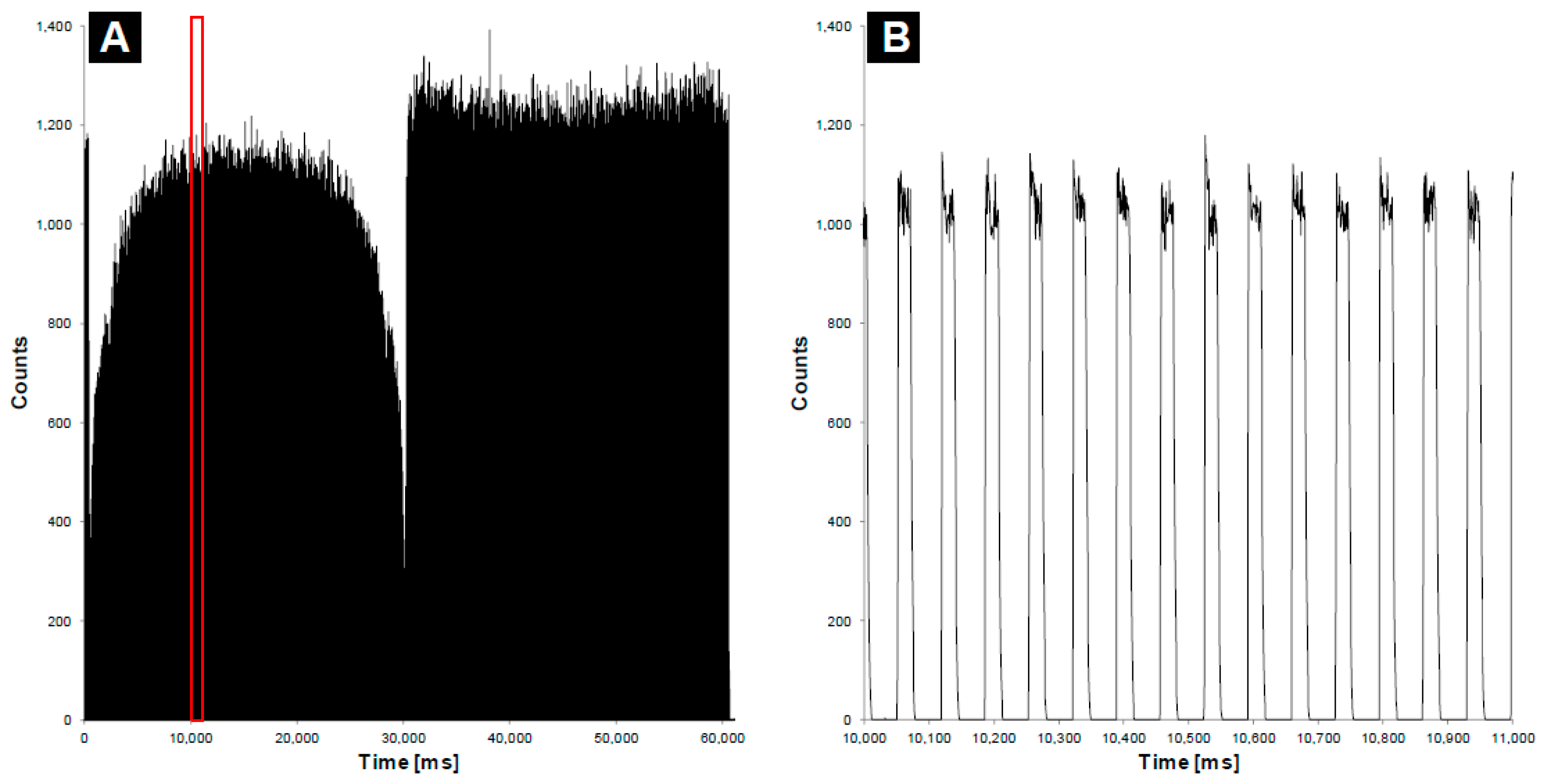
3.1. Determining the Impact of the Fiber on the Delivery of the Treatment Dose
3.2. Determining the Impact of the Fiber on the Delivery of the Imaging
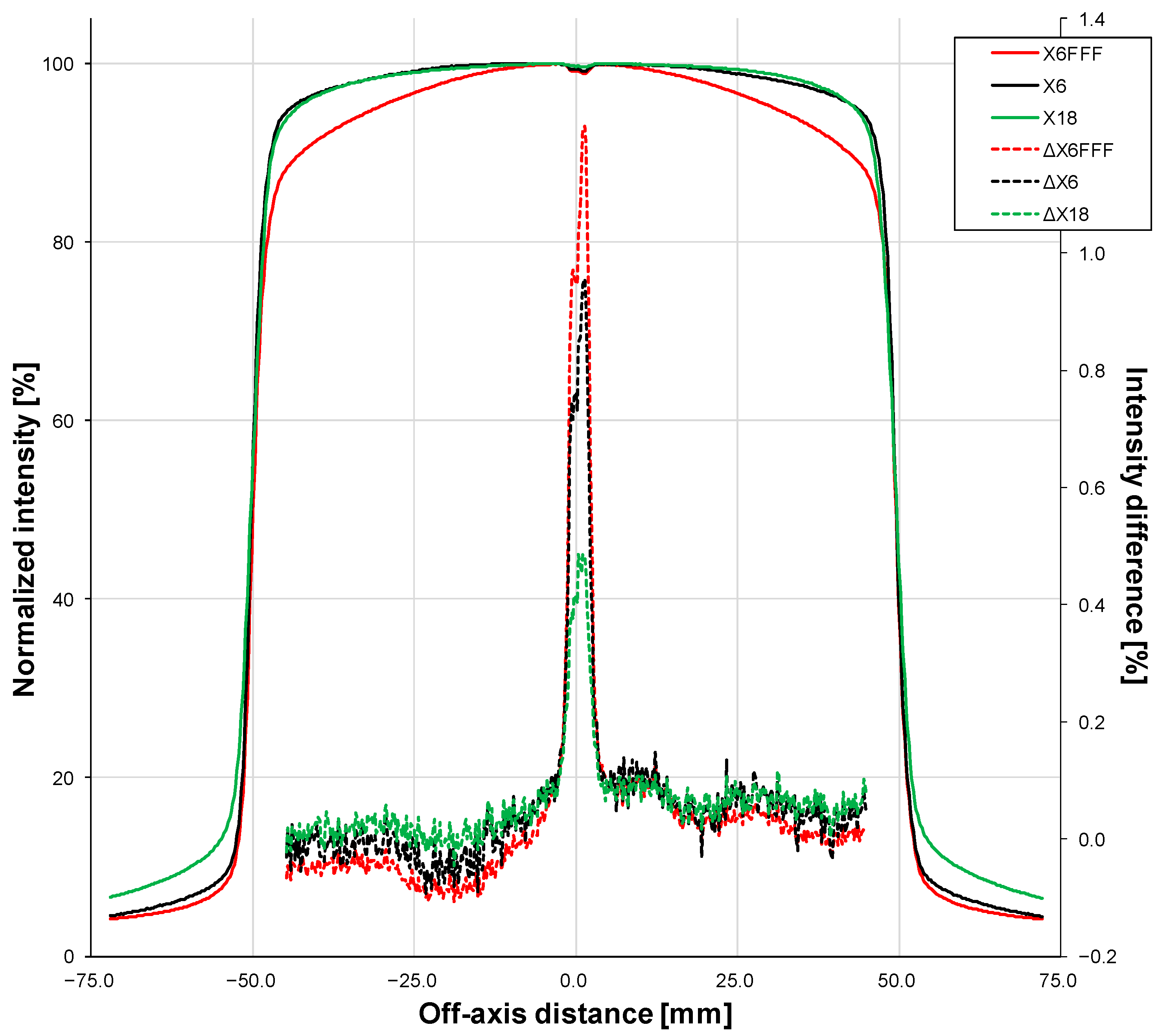
3.3. Comparison of the Device, Pencil Ionization Chamber, and Accelerator CTDIs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Mourik, A.; van Kranen, S.; den Hollander, S.; Sonke, J.-J.; van Herk, M.; van Vliet-Vroegindeweij, C. Effects of Setup Errors and Shape Changes on Breast Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Siebers, J.V.; Keall, P.J.; Wu, Q.; Williamson, J.F.; Schmidt-Ullrich, R.K. Effect of Patient Setup Errors on Simultaneously Integrated Boost Head and Neck IMRT Treatment Plans. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Frøseth, T.C.; Strickert, T.; Solli, K.S.; Salvesen, Ø.; Frykholm, G.; Reidunsdatter, R.J. A Randomized Study of the Effect of Patient Positioning on Setup Reproducibility and Dose Distribution to Organs at Risk in Radiotherapy of Rectal Cancer Patients. Radiat. Oncol. 2015, 10, 217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.; Yoshizumi, T.T.; Toncheva, G.; Yoo, S.; Yin, F.F. Comparison of Radiation Doses between Cone Beam CT and Multi Detector CT: TLD Measurements. Radiat. Prot. Dosim. 2008, 132, 339–345. [Google Scholar] [CrossRef]
- Islam, M.K.; Purdie, T.G.; Norrlinger, B.D.; Alasti, H.; Moseley, D.J.; Sharpe, M.B.; Siewerdsen, J.H.; Jaffray, D.A. Patient Dose from Kilovoltage Cone Beam Computed Tomography Imaging in Radiation Therapy. Med. Phys. 2006, 33, 1573–1582. [Google Scholar] [CrossRef]
- Ding, G.X.; Coffey, C.W. Radiation Dose From Kilovoltage Cone Beam Computed Tomography in an Image-Guided Radiotherapy Procedure. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 610–617. [Google Scholar] [CrossRef]
- Kim, S.; Yoshizumi, T.; Toncheva, G.; Yoo, S.; Yin, F.-F.; Frush, D. Estimation of Computed Tomography Dose Index in Cone Beam Computed Tomography: Mosfet Measurements and Monte Carlo Simulations. Health Phys. 2010, 98, 683. [Google Scholar] [CrossRef]
- Spezi, E.; Downes, P.; Jarvis, R.; Radu, E.; Staffurth, J. Patient-Specific Three-Dimensional Concomitant Dose From Cone Beam Computed Tomography Exposure in Image-Guided Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 419–426. [Google Scholar] [CrossRef]
- Hu, N.; McLean, D. Measurement of Radiotherapy CBCT Dose in a Phantom Using Different Methods. Australas. Phys. Eng. Sci. Med. 2014, 37, 779–789. [Google Scholar] [CrossRef]
- Giaddui, T.; Cui, Y.; Galvin, J.; Yu, Y.; Xiao, Y. Comparative Dose Evaluations between XVI and OBI Cone Beam CT Systems Using Gafchromic XRQA2 Film and NanoDot Optical Stimulated Luminescence Dosimeters. Med. Phys. 2013, 40, 062102. [Google Scholar] [CrossRef]
- Scandurra, D.; Lawford, C.E. A Dosimetry Technique for Measuring Kilovoltage Cone-Beam CT Dose on a Linear Accelerator Using Radiotherapy Equipment. J. Appl. Clin. Med. Phys. 2014, 15, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Marchant, T.; Sykes, J.; Czajka, J.; Moore, C. Imaging Doses from the Elekta Synergy X-Ray Cone Beam CT System. Br. J. Radiol. 2007, 80, 476–482. [Google Scholar] [CrossRef]
- Kim, D.W.; Chung, W.K.; Yoon, M. Imaging Doses and Secondary Cancer Risk From Kilovoltage Cone-Beam CT in Radiation Therapy. Health Phys. 2013, 104, 499. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Eidemüller, M.; Lumniczky, K.; Mancuso, M.; Ramadan, R.; Stolarczyk, L.; Moertl, S. Out-of-Field Effects: Lessons Learned from Partial Body Exposure. Radiat. Environ. Biophys. 2022, 61, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Kry, S.F.; Bednarz, B.; Howell, R.M.; Dauer, L.; Followill, D.; Klein, E.; Paganetti, H.; Wang, B.; Wuu, C.-S.; Xu, X.G. AAPM TG 158: Measurement and Calculation of Doses Outside the Treated Volume from External-Beam Radiation Therapy. Med. Phys. 2017, 44, e391–e429. [Google Scholar] [CrossRef]
- Conrad, M.; Bolard, G.; Nowak, M.; De Bari, B.; Jeanneret-Sozzi, W.; Bourhis, J.; Germond, J.-F.; Bochud, F.; Moeckli, R. Determination of the Effective Dose Delivered by Image Guided Radiotherapy in Head & Neck and Breast Treatments. Z. Für Med. Phys. 2018, 28, 276–285. [Google Scholar] [CrossRef]
- Hempel, D.; Chrenowicz, R.; Filipowski, T.; Wojtukiewicz, M.Z.; Sierko, E. Testicular Dose Contributed by X-ray Volume Image-(XVI)-Guided Intensity-Modulated Radiotherapy (IMRT) in Prostate Cancer Patients. Biul. Pol. Tow. Onkol. Nowotw. 2020, 5, 64–70. [Google Scholar]
- Martin, C.J.; Kron, T.; Vassileva, J.; Wood, T.J.; Joyce, C.; Ung, N.M.; Small, W.; Gros, S.; Roussakis, Y.; Plazas, M.C.; et al. An International Survey of Imaging Practices in Radiotherapy. Phys. Medica 2021, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Anderson, J.; Bakalyar, D.; Boedeker, K.; Boone, J.; Cody, D.; Fahrig, R.; Jaffray, D.; Kyprianou, I.; McCollough, C.; et al. Comprehensive Methodology for the Evaluation of Radiation Dose in X-ray Computed Tomography; AAPM: Alexandria, VA, USA, 2010. [Google Scholar]
- INTERNATIONAL ATOMIC ENERGY AGENCY. Status of Computed Tomography Dosimetry for Wide Cone Beam Scanners; IAEA Human Health Reports No. 5; IAEA: Wien, Austria, 2011. [Google Scholar]
- De las Hugo, H.G.; Alberto, T.; Alexandru, D.; Osvaldo, R.; Harry, D.; Irene, H.G.; Chrysoula, T.; Jonas, A.; John, H.; Mats, N.; et al. Quality Control in Cone-Beam Computed Tomography (Cbct) Efomp-Estro-Iaea Protocol, 1st ed.; European Federation of Organisations for Medical Physics: Utrech, The Netherlands, 2017. [Google Scholar]
- Munier, M.; Sohier, T.; Jung, J.-M.; Barillon, R.; Torres, M. Method for Determining the Irradiation Dose Deposited in a Scintillator by Ionising Radiation and Associated Device. U.S. Patent 9,244,178, 26 January 2016. [Google Scholar]
- Devic, C.; Plagnard, J.; Munier, M. Characterization of an Innovative Detector Based on Scintillating Fiber for Personalized Computed Tomography Dosimetry. Sensors 2021, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Guillochon, N.; Balde, M.; Popotte, C.; Pondard, S.; Kien, N.; Desport, C.; Carbillet, F.; Moreno, R.; Munier, M. Validation of a New Scintillating Fiber Dosimeter for Radiation Dose Quality Control in Computed Tomography. Sensors 2023, 23, 2614. [Google Scholar] [CrossRef]
- IEC 61267; Medical Diagnostic X-ray Equipment. Radiation Conditions for Use in the Determination of Characteristics. BSI British Standards: London, UK, 2005.
- Pardo, E.; Novais, J.C.; Molina López, M.Y.; Maqueda, S.R. On Flattening Filter-Free Portal Dosimetry. J. Appl. Clin. Med. Phys. 2016, 17, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 617–675. [Google Scholar] [CrossRef] [PubMed]
- Mijnheer, B. EPID-Based Dosimetry and Its Relation to Other 2D and 3D Dose Measurement Techniques in Radiation Therapy. J. Phys. Conf. Ser. 2017, 847, 012024. [Google Scholar] [CrossRef]
- Stevens, S.; Dvorak, P.; Spevacek, V.; Pilarova, K.; Bray-Parry, M.; Gesner, J.; Richmond, A. An Assessment of a 3D EPID-Based Dosimetry System Using Conventional Two- and Three-Dimensional Detectors for VMAT. Phys. Medica Eur. J. Med. Phys. 2018, 45, 25–34. [Google Scholar] [CrossRef]
- Van Elmpt, W.; McDermott, L.; Nijsten, S.; Wendling, M.; Lambin, P.; Mijnheer, B. A Literature Review of Electronic Portal Imaging for Radiotherapy Dosimetry. Radiother. Oncol. 2008, 88, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Marinello, G.; Pan, J. Systèmes d’ Imagerie Intégrés Ou Associés Aux Appareils de Radiothérapie Coordonné Par Ginette Marinello et Jianji Pan; EDP Sciences: Les Ulis, France, 2019; ISBN 978-2-7598-2298-0. [Google Scholar]


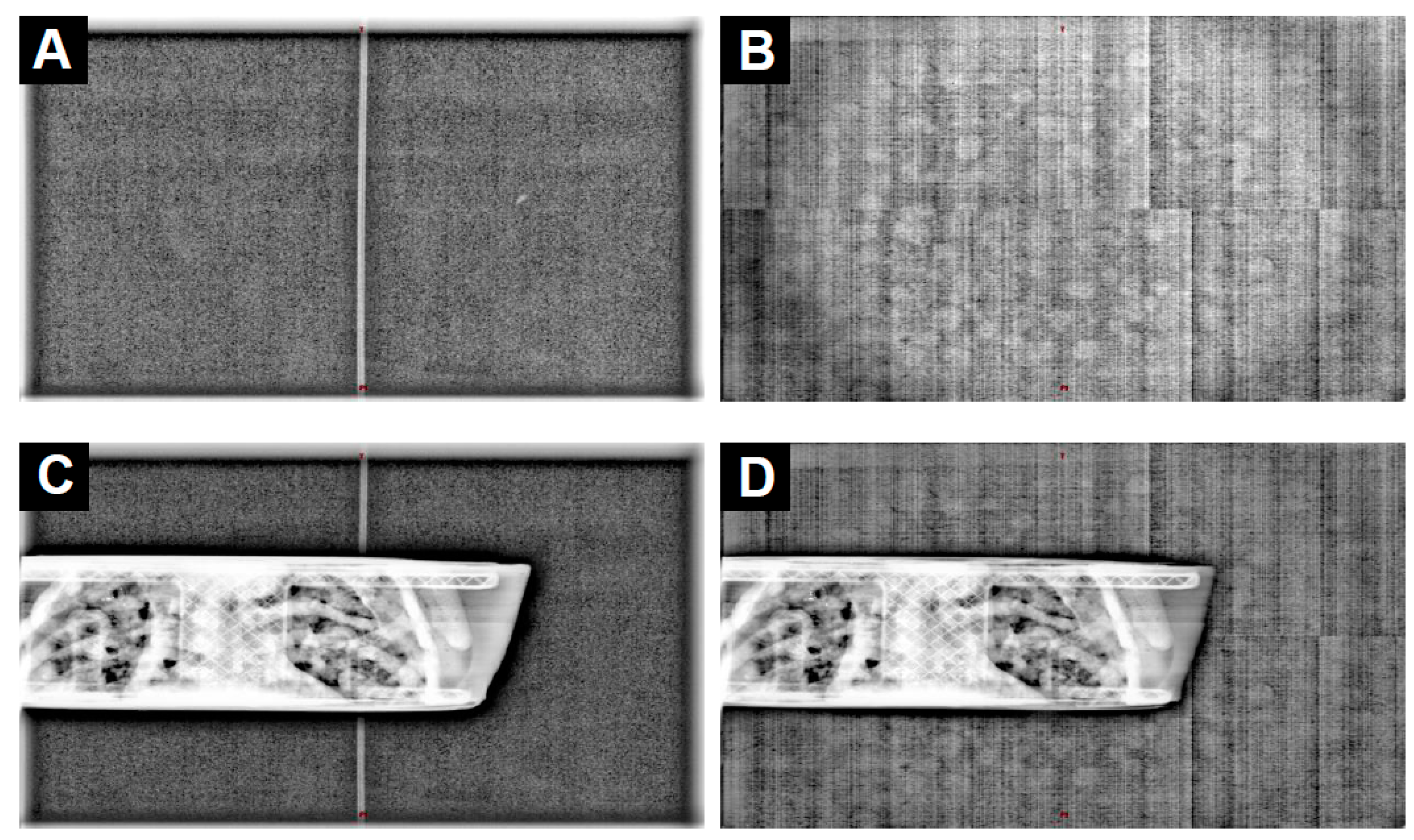
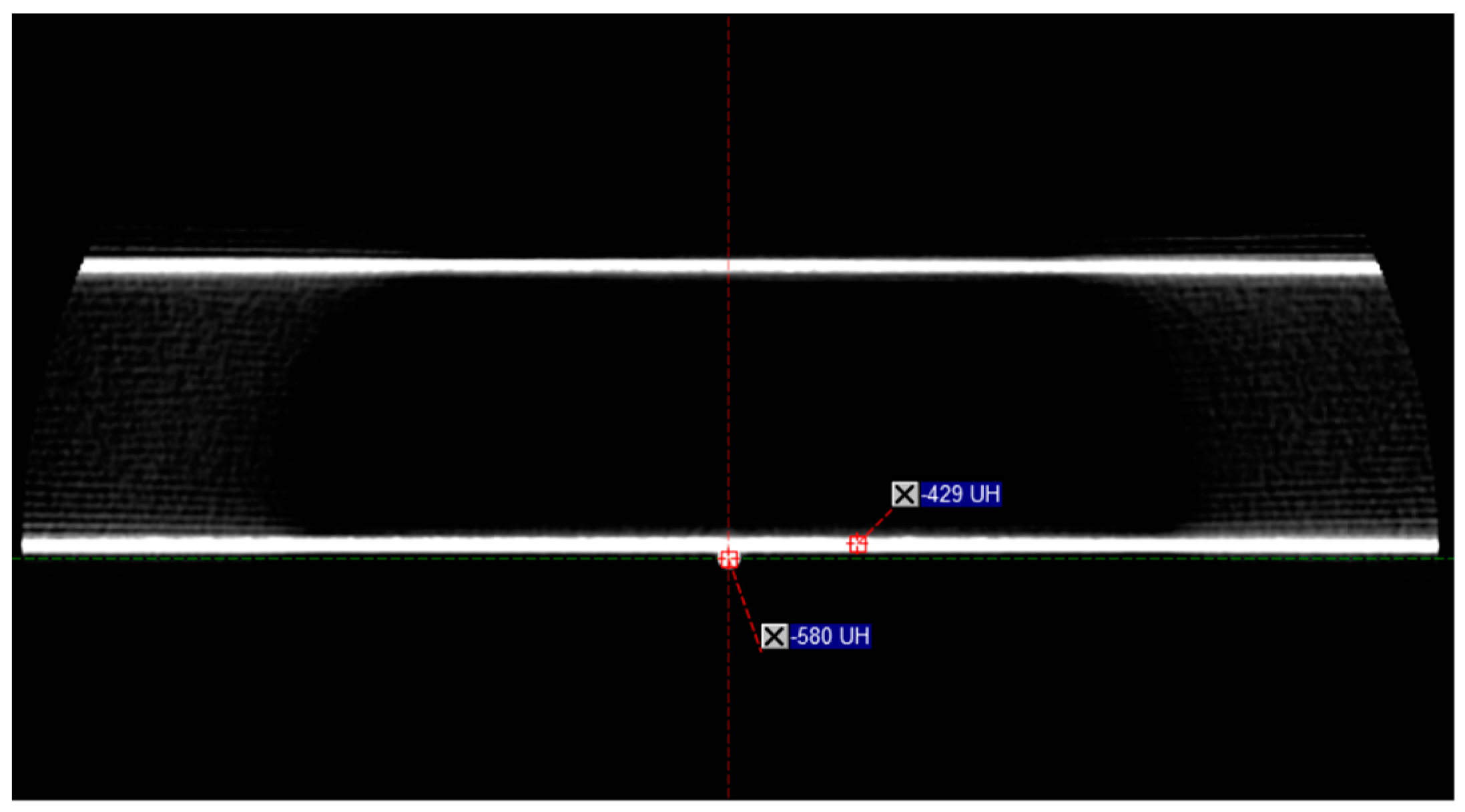
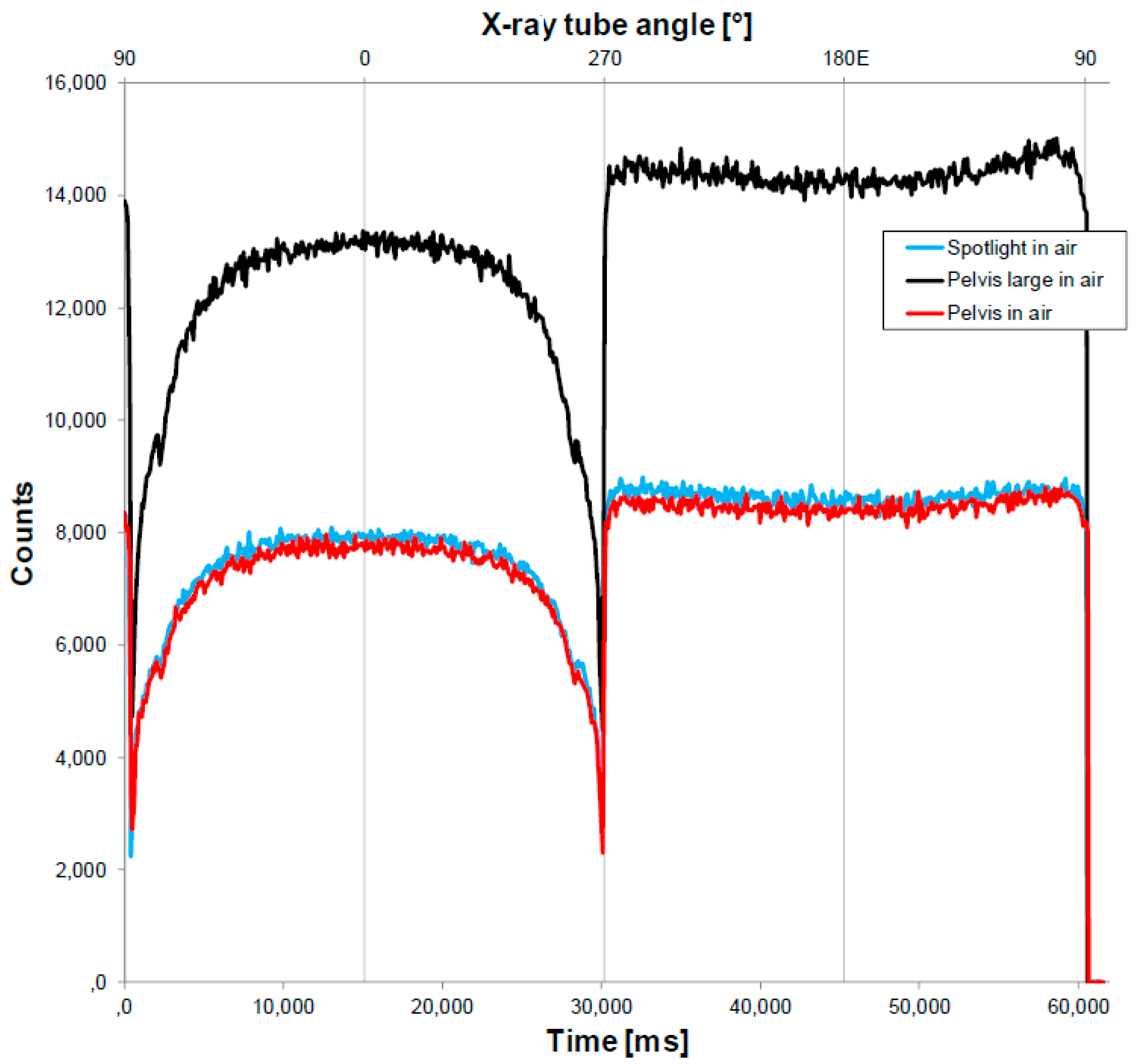

| Protocol Details | Pelvis | Pelvis Large | Spotlight |
|---|---|---|---|
| Fan type | Half fan | Half fan | Full fan |
| Trajectory | Full | Full | Full |
| Start angle (°) | 180 | 180 | 180 |
| End angle (°) | 180E 1 | 180E 1 | 180E 1 |
| Tube voltage (kV) | 125 | 140 | 125 |
| mAs | 1080 | 1687.5 | 1350 |
| Field of view (cm) | 46.5 | 46.5 | 26.2 |
| Collimation (cm) | 17.5 | 17.5 | 18.5 |
| Source of CTDI Value | CBCT Protocols | ||
|---|---|---|---|
| Pelvis | Pelvis Large | Spotlight | |
| IVI-CBCT | 19.14 ± 0.02 mGy [0.1%] | 40.45 ± 0.03 mGy [0.1%] | 25.34 ± 0.11 mGy [0.5%] |
| Pencil chamber | 19.14 ± 0.33 mGy [1.7%] | 40.45 ± 0.15 mGy [0.5%] | 25.34 ± 0.12 mGy [0.5%] |
| Reported by accelerator | 15.89 mGy | 36.54 mGy | 22.02 mGy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popotte, C.; Letellier, R.; Paul, D.; Waltener, A.; Guillochon, N.; Munier, M.; Retif, P. Evaluation of a Scintillating Plastic Optical Fiber Device for Measuring kV-Cone Beam Computed Tomography Dose. Sensors 2023, 23, 7778. https://doi.org/10.3390/s23187778
Popotte C, Letellier R, Paul D, Waltener A, Guillochon N, Munier M, Retif P. Evaluation of a Scintillating Plastic Optical Fiber Device for Measuring kV-Cone Beam Computed Tomography Dose. Sensors. 2023; 23(18):7778. https://doi.org/10.3390/s23187778
Chicago/Turabian StylePopotte, Christian, Romain Letellier, Didier Paul, Alexandre Waltener, Nicolas Guillochon, Mélodie Munier, and Paul Retif. 2023. "Evaluation of a Scintillating Plastic Optical Fiber Device for Measuring kV-Cone Beam Computed Tomography Dose" Sensors 23, no. 18: 7778. https://doi.org/10.3390/s23187778
APA StylePopotte, C., Letellier, R., Paul, D., Waltener, A., Guillochon, N., Munier, M., & Retif, P. (2023). Evaluation of a Scintillating Plastic Optical Fiber Device for Measuring kV-Cone Beam Computed Tomography Dose. Sensors, 23(18), 7778. https://doi.org/10.3390/s23187778







