Reliability and Validity of a Smartphone Device and Clinical Tools for Thoracic Spine Mobility Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Participants
2.4. Clinical Tools (Equipment)
2.5. Observers (Raters)
2.6. Procedures
2.6.1. Lumbar-Locked Trunk Rotation
2.6.2. Standing Full Extension
2.6.3. Standing Full Flexion
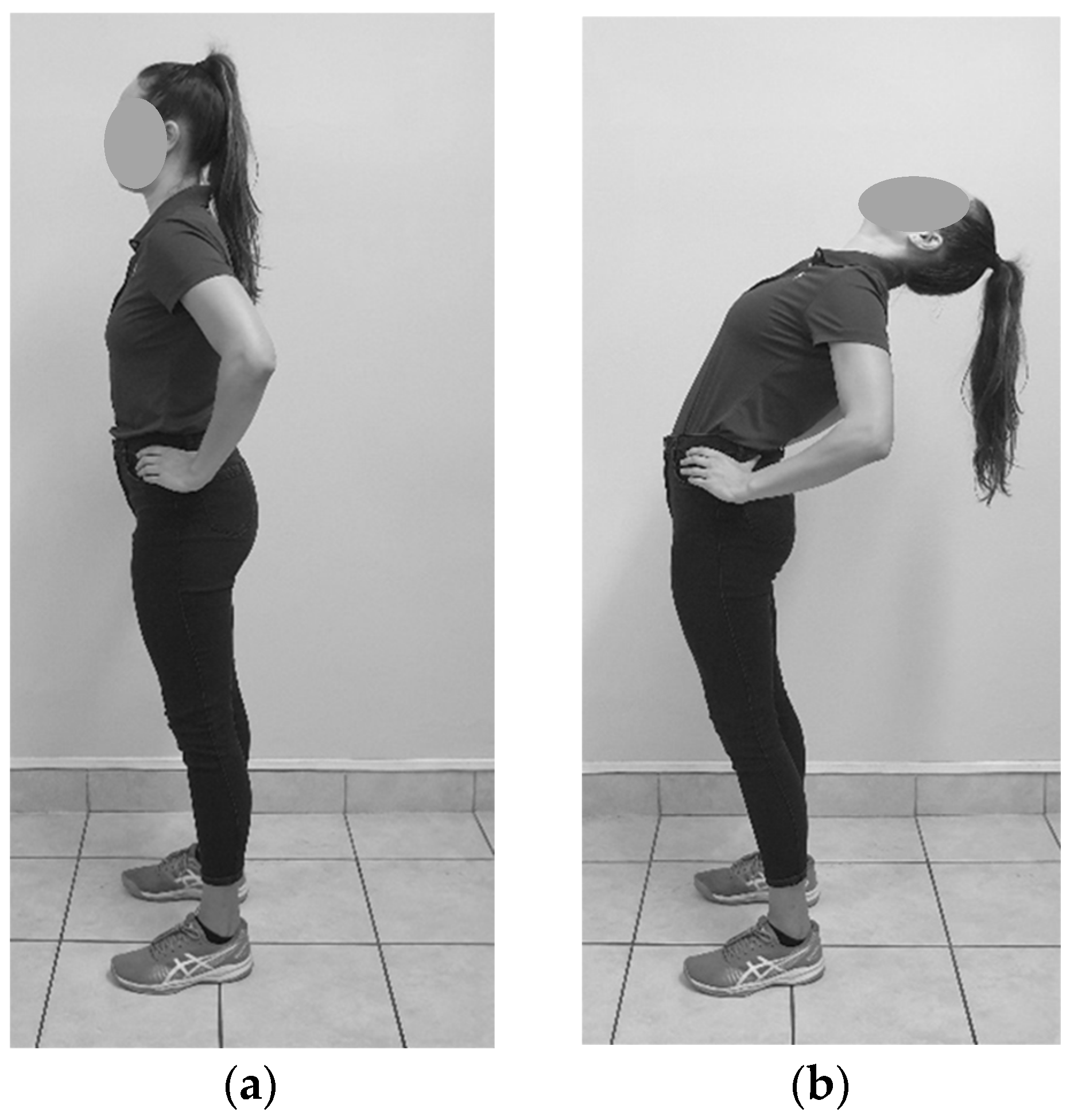
2.6.4. Standing Relaxed Curve
2.6.5. Seated Trunk Rotation
2.7. Statistical Analysis
2.7.1. Reliability
2.7.2. Validity
3. Results
3.1. Reliability
3.1.1. Intra-Rater Reliability
Lumbar Locked Trunk Rotation
Standing Full Extension
Standing Full Flexion
Standing Relaxed Curve
Seated Trunk Rotation
3.1.2. Inter-Rater Reliability
Lumbar-Locked Trunk Rotation
Standing Full Extension and Standing Full Flexion
Standing Relaxed Curve
Seated Trunk Rotation
3.2. Validity
3.2.1. Lumbar-Locked Trunk Rotation
3.2.2. Standing Relaxed Curve
3.2.3. Seated Trunk Rotation
4. Discussion
4.1. Lumbar-Locked Trunk Rotation
4.1.1. Intra-Rater Reliability
4.1.2. Inter-Rater Reliability
4.1.3. Validity
4.2. Standing Full Flexion and Extension
4.2.1. Intra-Rater Reliability
4.2.2. Inter-Rater Reliability
4.3. Standing Relaxed Curve
4.3.1. Intra-Rater Reliability
4.3.2. Inter-Rater Reliability
4.3.3. Validity
4.4. Seated Trunk Rotation
4.4.1. Intra-Rater Reliability
4.4.2. Inter-Rater Reliability
4.4.3. Validity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K.D.; Kim, K.-M.; Yu, B.-K.; Saliba, S.A.; Grindstaff, T.L. Reliability of Thoracic Spine Rotation Range-of-Motion Measurements in Healthy Adults. J. Athl. Train. 2012, 47, 52–60. [Google Scholar] [CrossRef]
- Furness, J.; Schram, B.; Cox, A.J.; Anderson, S.L.; Keogh, J. Reliability and Concurrent Validity of the IPhone® Compass Application to Measure Thoracic Rotation Range of Motion (ROM) in Healthy Participants. PeerJ 2018, 6, e4431. [Google Scholar] [CrossRef] [PubMed]
- Bucke, J.; Spencer, S.; Fawcett, L.; Sonvico, L.; Rushton, A.; Heneghan, N.R. Validity of the Digital Inclinometer and Iphone When Measuring Thoracic Spine Rotation. J. Athl. Train. 2017, 52, 820–825. [Google Scholar] [CrossRef]
- Grobler, B.K.; Ellapen, T.J.; Paul, Y.; Strydom, G.L. The Strategic Development and Strengthening of the Profession of Biokinetics. Afr. J. Health Prof. Educ. 2021, 13, 8–9. [Google Scholar] [CrossRef]
- Ravi, B.; Kapoor, M.; Player, D. Feasibility and Reliability of a Web-Based Smartphone Application for Joint Position Measurement. J. Rehabil. Med. 2021, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Devaney, L.; Bohannon, R.; Rizzo, J.; Capetta, M.; Vigneault, J.; Van Deveire, K. Inclinometric Measurement of Kyphotic Curvature: Description and Clinimetric Properties. Physiother. Theory Pract. 2017, 33, 797–804. [Google Scholar] [CrossRef]
- Hunter, D.J.; Rivett, D.A.; McKiernan, S.; Weerasekara, I.; Snodgrass, S.J. Is the Inclinometer a Valid Measure of Thoracic Kyphosis? A Cross-Sectional Study. Braz. J. Phys. Ther. 2018, 22, 310–317. [Google Scholar] [CrossRef]
- Lewis, J.S.; Valentine, R.E. Clinical Measurement of the Thoracic Kyphosis. A Study of the Intra-Rater Reliability in Subjects with and without Shoulder Pain. BMC Musculoskelet. Disord. 2010, 11, 39. [Google Scholar] [CrossRef]
- Faramarzi Kohneh Shahri, Y.; Ghani Zadeh Hesar, N. Validity and Reliability of Smartphone-Based Goniometer-Pro App for Measuring the Thoracic Kyphosis. Musculoskelet. Sci. Pract. 2020, 49, 102216. [Google Scholar] [CrossRef]
- Feijen, S.; Kuppens, K.; Tate, A.; Baert, I.; Struyf, T.; Struyf, F. Intra- and Interrater Reliability of the ‘Lumbar-Locked Thoracic Rotation Test’ in Competitive Swimmers Ages 10 through 18 Years. Phys. Ther. Sport 2018, 32, 140–144. [Google Scholar] [CrossRef]
- Charlton, P.C.; Mentiplay, B.F.; Pua, Y.H.; Clark, R.A. Reliability and Concurrent Validity of a Smartphone, Bubble Inclinometer and Motion Analysis System for Measurement of Hip Joint Range of Motion. J. Sci. Med. Sport 2015, 18, 262–267. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Bissolotti, L.; Zaina, F.; Arienti, C.; Donzelli, S.; Negrini, S. Thoracic Hyperkyphosis Non Invasively Measured by General Practitioners Is Associated with Chronic Low Back Pain: A Cross-Sectional Study of 1364 Subjects. J. Bodyw. Mov. Ther. 2018, 22, 752–756. [Google Scholar] [CrossRef]
- Johnson, K.D.; Grindstaff, T.L. Thoracic Rotation Measurement Techniques: Clinical Commentary. N. Am. J. Sports Phys. Ther. 2010, 5, 252–256. [Google Scholar] [PubMed]
- Nguyen, B.T.; Baicoianu, N.A.; Howell, D.B.; Peters, K.M.; Steele, K.M. Accuracy and Repeatability of Smartphone Sensors for Measuring Shank-to-Vertical Angle. Prosthet. Orthot. Int. 2020, 44, 172–179. [Google Scholar] [CrossRef]
- Koong, D.P.; Lee, J.; Cheng, T.L.; Little, D.G. Validity and Reliability of Smartphone Inclinometer Applications for Measurement of Elbow Range of Motion in Paediatric Patients. J. Child. Orthop. 2020, 14, 488–494. [Google Scholar] [CrossRef]
- Franko, O.I.; Tirrell, T.F. Smartphone App Use among Medical Providers in ACGME Training Programs. J. Med. Syst. 2012, 36, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Meigal, A.Y.; Gerasimova-Meigal, L.I.; Reginya, S.A.; Soloviev, A.V.; Moschevikin, A.P. Gait Characteristics Analyzed with Smartphone IMU Sensors in Subjects with Parkinsonism under the Conditions of “Dry” Immersion. Sensors 2022, 22, 7915. [Google Scholar] [CrossRef]
- Thomas What Size Smartphone Do I Need? Available online: https://www.coolblue.be/en/advice/smartphone-screens.html#:~:text=Average%20phones%3A%205.8%20to%206.2,the%20size%20of%20your%20hands (accessed on 24 May 2023).
- Balsalobre-Fernández, C.; Romero-Franco, N.; Jiménez-Reyes, P. Concurrent Validity and Reliability of an IPhone App for the Measurement of Ankle Dorsiflexion and Inter-Limb Asymmetries. J. Sports Sci. 2019, 37, 249–253. [Google Scholar] [CrossRef]
- Humphrey, R.A.; Lakomy, J. An Evaluation of Pre-Exercise Screening Questionnaires Used within the Health and Fitness Industry in the United Kingdom. Phys. Ther. Sport 2003, 4, 187–191. [Google Scholar] [CrossRef]
- Edmondston, S.J.; Singer, K.P. Thoracic Spine: Anatomical and Biomechanical Considerations for Manual Therapy. Man. Ther. 1997, 2, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Roghani, T.; Zavieh, M.K.; Manshadi, F.D.; King, N.; Katzman, W. Age-Related Hyperkyphosis: Update of Its Potential Causes and Clinical Impacts—Narrative Review. Aging Clin. Exp. Res. 2017, 29, 567–577. [Google Scholar] [CrossRef]
- A Healthy Lifestyle—WHI Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 3 June 2023).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Int. J. Nurs. Stud. 2010, 47, 937–938. [Google Scholar] [CrossRef]
- Salamh, P.A.; Kolber, M. The Reliability, Minimal Detectable Change and Concurrent Validity of a Gravity-Based Bubble Inclinometer and Iphone Application for Measuring Standing Lumbar Lordosis. Physiother. Theory Pract. 2014, 30, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pourahmadi, M.R.; Taghipour, M.; Jannati, E.; Mohseni-Bandpei, M.A.; Ebrahimi Takamjani, I.; Rajabzadeh, F. Reliability and Validity of an IPhone ® Application for the Measurement of Lumbar Spine Flexion and Extension Range of Motion. PeerJ 2016, 4, e2355. [Google Scholar] [CrossRef] [PubMed]
- Kolber, M.J.; Mdt, C.; Pizzini, M.; Robinson, A.; Yanez, D.; Hanney, W.J. The Reliability and Concurrent Validity of Measurements Used to Quantify Lumbar Spine Mobility: An Analysis of an Iphone ® Application and Gravity Based Inclinometry. Int. J. Sports Phys. Ther. 2013, 8, 129–137. [Google Scholar] [PubMed]





| Clinical Assessments | Clinical Tools | |
|---|---|---|
| Preferred Tool | New Tool | |
| Lumbar-locked trunk rotation | Bubble inclinometer | Smartphone |
| Standing full extension | Bubble inclinometer | |
| Standing full flexion | Bubble inclinometer | |
| Standing relaxed curve | Bubble inclinometer | Smartphone |
| Seated trunk rotation | Universal goniometer | Smartphone |
| Clinical Assessment | Rater (R) | Tool | ICC (95% CI) | SEM (°) | MDC (°) |
|---|---|---|---|---|---|
| LL Left | R1 | Preferred tool | 0.855 (0.679 to 0.943) | 3.4 | 9.4 |
| LL Left | R2 | Preferred tool | 0.866 (0.682 to 0.952) | 4.1 | 11.3 |
| LL Left | R3 | Preferred tool | 0.903 (0.774 to 0.964) | 3.3 | 9.2 |
| LL Left | R1 | Smartphone | 0.878 (0.727 to 0.952) | 2.9 | 8.1 |
| LL Left | R2 | Smartphone | 0.871 (0.698 to 0.953) | 3.8 | 10.5 |
| LL Left | R3 | Smartphone | 0.914 (0.798 to 0.969) | 3.1 | 8.6 |
| LL Right | R1 | Preferred tool | 0.776 (0.495 to 0.912) | 4.3 | 11.9 |
| LL Right | R2 | Preferred tool | 0.816 (0.566 to 0.933) | 3.8 | 10.4 |
| LL Right | R3 | Preferred tool | 0.761 (0.427 to 0.913) | 4.4 | 12.1 |
| LL Right | R1 | Smartphone | 0.739 (0.409 to 0.898) | 4.2 | 11.6 |
| LL Right | R2 | Smartphone | 0.889 (0.737 to 0.960) | 2.9 | 8.1 |
| LL Right | R3 | Smartphone | 0.890 (0.738 to 0.960) | 2.8 | 7.7 |
| SFE P1 | R1 | Preferred tool | 0.955 (0.897 to 0.983) | 4.0 | 11.0 |
| SFE P1 | R2 | Preferred tool | 0.955 (0.870 to 0.985) | 3.1 | 8.5 |
| SFE P1 | R3 | Preferred tool | 0.889 (0.732 to 0.961) | 5.2 | 14.3 |
| SFE P2 | R1 | Preferred tool | 0.973 (0.938 to 0.990) | 3.0 | 8.2 |
| SFE P2 | R2 | Preferred tool | 0.950 (0.866 to 0.966) | 3.7 | 10.4 |
| SFE P2 | R3 | Preferred tool | 0.888 (0.730 to 0.961) | 4.9 | 13.7 |
| SFF P1 | R1 | Preferred tool | 0.969 (0.931 to 0.988) | 2.8 | 7.8 |
| SFF P1 | R2 | Preferred tool | 0.880 (0.689 to 0.958) | 4.8 | 13.3 |
| SFF P1 | R3 | Preferred tool | 0.854 (0.617 to 0.949) | 5.7 | 15.8 |
| SFF P2 | R1 | Preferred tool | 0.981 (0.981 to 0.958) | 1.9 | 5.2 |
| SFF P2 | R2 | Preferred tool | 0.934 (0.847 to 0.976) | 3.5 | 9.8 |
| SFF P2 | R3 | Preferred tool | 0.917 (0.805 to 0.970) | 3.6 | 10.1 |
| SRC | R1 | Preferred tool | 0.929 (0.841 to 0.972) | 1.7 | 4.7 |
| SRC | R2 | Preferred tool | 0.691 (0.294 to 0.886) | 4.3 | 11.9 |
| SRC | R3 | Preferred tool | 0.822 (0.589 to 0.935) | 2.8 | 7.6 |
| SRC | R1 | Smartphone | 0.942 (0.872 to 0.977) | 1.5 | 4.3 |
| SRC | R2 | Smartphone | 0.624 (0.145 to 0.861) | 4.0 | 11.1 |
| SRC | R3 | Smartphone | 0.824 (0.545 to 0.938) | 2.7 | 7.5 |
| TR Left | R1 | Smartphone | 0.918 (0.816 to 0.968 | 2.6 | 7.2 |
| TR Left | R2 | Smartphone | 0.908 (0.781 to 0.966) | 2.6 | 7.3 |
| TR Left | R3 | Smartphone | 0.933 (0.844 to 0.976) | 2.1 | 6.0 |
| TR Left | R1 | Preferred tool | 0.958 (0.906 to 0.983) | 2.2 | 6.1 |
| TR Left | R2 | Preferred tool | 0.940 (0.860 to 0.978) | 3.0 | 8.4 |
| TR Left | R3 | Preferred tool | 0.850 (0.652 to 0.945) | 4.0 | 11.2 |
| TR Right | R1 | Smartphone | 0.949 (0.886 to 0.980) | 2.0 | 5.5 |
| TR Right | R2 | Smartphone | 0.868 (0.560 to 0.957) | 3.4 | 9.5 |
| TR Right | R3 | Smartphone | 0.918 (0.802 to 0.970) | 2.2 | 6.0 |
| TR Right | R1 | Preferred tool | 0.920 (0.823 to 0.969) | 3.2 | 9.0 |
| TR Right | R2 | Preferred tool | 0.938 (0.852 to 0.978) | 3.3 | 9.1 |
| TR Right | R3 | Preferred tool | 0.701 (0.290 to 0.890) | 4.5 | 12.6 |
| Clinical Assessment | Tool | ICC (95% CI) | SEM (°) | MDC (°) |
|---|---|---|---|---|
| LL Left | Preferred tool | 0.936 (0.837 to 0.977) | 2.3 | 6.4 |
| LL Left | Smartphone | 0.928 (0.793 to 0.976) | 2.4 | 6.7 |
| LL Right | Preferred tool | 0.882 (0.600 to 0.962) | 2.6 | 7.2 |
| LL Right | Smartphone | 0.860 (0.461 to 0.957) | 2.8 | 7.7 |
| SFE P1 | Preferred tool | 0.960 (0.904 to 0.986) | 3.1 | 8.6 |
| SFE P2 | Preferred tool | 0.968 (0.922 to 0.989) | 2.8 | 7.8 |
| SFF P1 | Preferred tool | 0.953 (0.899 to 0.983) | 3.0 | 8.4 |
| SFF P2 | Preferred tool | 0.957 (0.895 to 0.984) | 2.6 | 7.3 |
| SRC | Preferred tool | 0.819 (0.555 to 0.935) | 2.6 | 7.1 |
| SRC | Smartphone | 0.671 (0.080 to 0.890) | 3.2 | 9.0 |
| TR Left | Smartphone | 0.942 (0.751 to 0.983) | 1.9 | 5.4 |
| TR Left | Preferred tool | 0.958 (0.895 to 0.985) | 2.1 | 5.9 |
| TR Right | Smartphone | 0.955 (0.896 to 0.984) | 1.7 | 4.8 |
| TR Right | Preferred tool | 0.873 (0.519 to 0.961) | 3.6 | 9.9 |
| Clinical Assessment | Tool | Mean° (SE°) | (95% CI) | Residuals | |
|---|---|---|---|---|---|
| Mean° (SE°) | (95% CI) | ||||
| LL Left | Preferred tool | 49.6 (1.3) | (46.9 to 52.3) | −2.4 (0.5) | (−3.5 to −1.3) |
| Smartphone | 47.2 (1.3) | (44.6 to 49.9) | |||
| LL Right | Preferred tool | 50.4 (1.1) | (48.2 to 52.7) | −1.1 (0.5) | (−2.1 to −0.1) |
| Smartphone | 49.4 (1.2) | (47.1 to 51.7) | |||
| SRC | Preferred tool | 33.3 (0.9) | (31.5 to 35.2) | −2.1 (0.5) | (−3.1 to −1.1) |
| Smartphone | 31.3 (1.0) | (29.3 to 33.3) | |||
| TR Left | Preferred tool | 57.2 (1.5) | (54.1 to 60.2) | −2.6 (1.0) | (−4.5 to −0.6) |
| Smartphone | 54.6 (1.2) | (52.2 to 57.0) | |||
| TR Right | Preferred tool | 57.2 (1.6) | (54.0 to 60.4) | −1.9 (0.9) | (−3.8 to 0.0) |
| Smartphone | 55.3 (1.2) | (53.0 to 57.7) | |||
| Clinical Assessment | Tool | ICC (95% CI) |
|---|---|---|
| LL Left | Preferred tool vs. smartphone | 0.942 (0.823 to 0.975) |
| LL Right | Preferred tool vs. smartphone | 0.947 (0.902 to 0.971) |
| SRC | Preferred tool vs. smartphone | 0.903 (0.744 to 0.956) |
| TR Left | Preferred tool vs. smartphone | 0.836 (0.691 to 0.911) |
| TR Right | Preferred tool vs. smartphone | 0.867 (0.759 to 0.927) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Baalen, G.B.; Vanwanseele, B.; Venter, R.R. Reliability and Validity of a Smartphone Device and Clinical Tools for Thoracic Spine Mobility Assessments. Sensors 2023, 23, 7622. https://doi.org/10.3390/s23177622
van Baalen GB, Vanwanseele B, Venter RR. Reliability and Validity of a Smartphone Device and Clinical Tools for Thoracic Spine Mobility Assessments. Sensors. 2023; 23(17):7622. https://doi.org/10.3390/s23177622
Chicago/Turabian Stylevan Baalen, Gabriela Bella, Benedicte Vanwanseele, and Ranel Rachel Venter. 2023. "Reliability and Validity of a Smartphone Device and Clinical Tools for Thoracic Spine Mobility Assessments" Sensors 23, no. 17: 7622. https://doi.org/10.3390/s23177622
APA Stylevan Baalen, G. B., Vanwanseele, B., & Venter, R. R. (2023). Reliability and Validity of a Smartphone Device and Clinical Tools for Thoracic Spine Mobility Assessments. Sensors, 23(17), 7622. https://doi.org/10.3390/s23177622







