An Instrumented Mouthguard for Real-Time Measurement of Head Kinematics under a Large Range of Sport Specific Accelerations
Abstract
1. Introduction
2. Materials and Methods
2.1. The Instrumented Mouthguard
2.2. Testing Protocol
2.3. The Finite Element Model of the Human Brain
2.4. Data and Statistical Analysis
3. Results
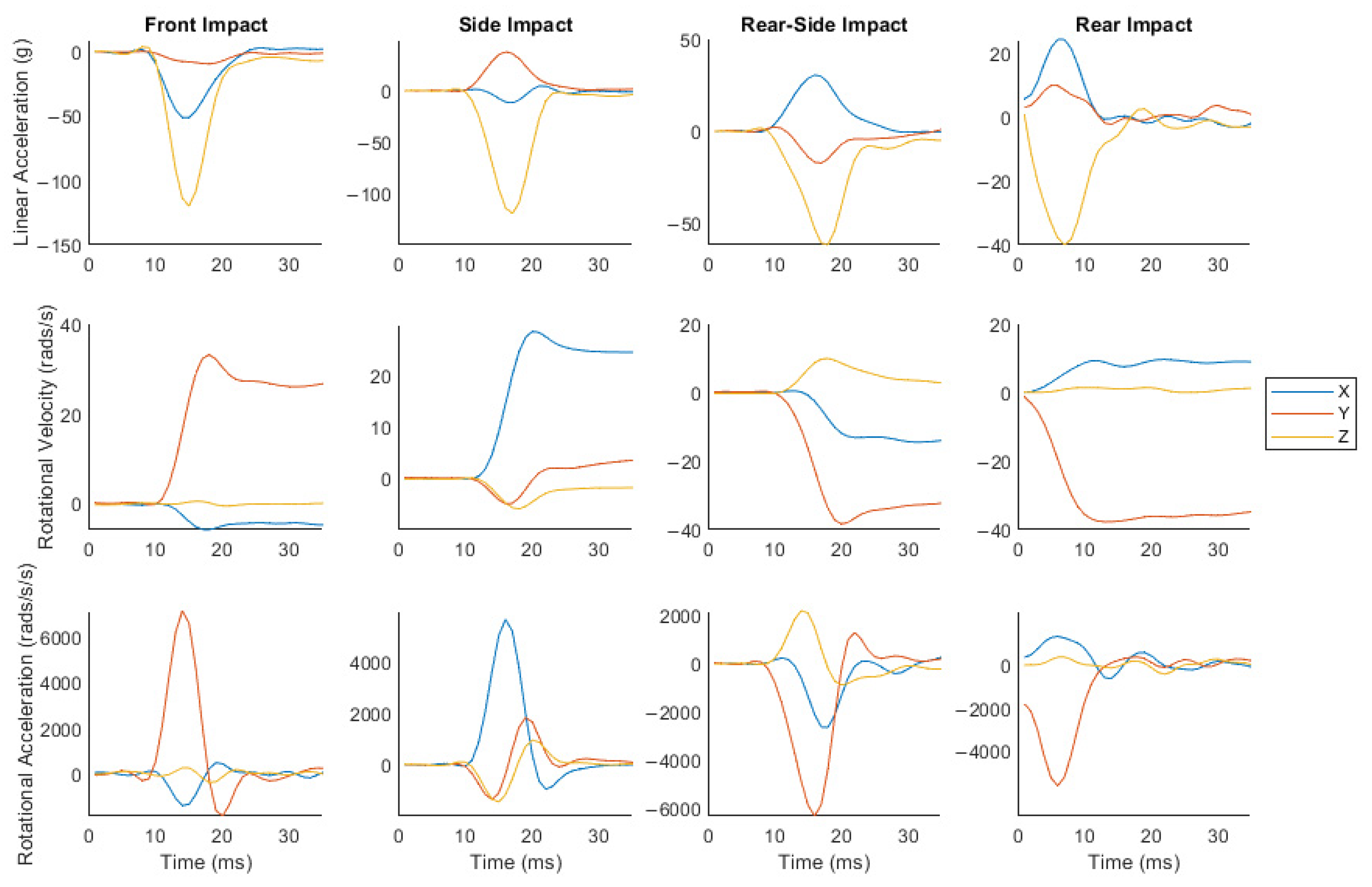
3.1. Impacts Produced a Large Range of Head Kinematics and Brain Strain
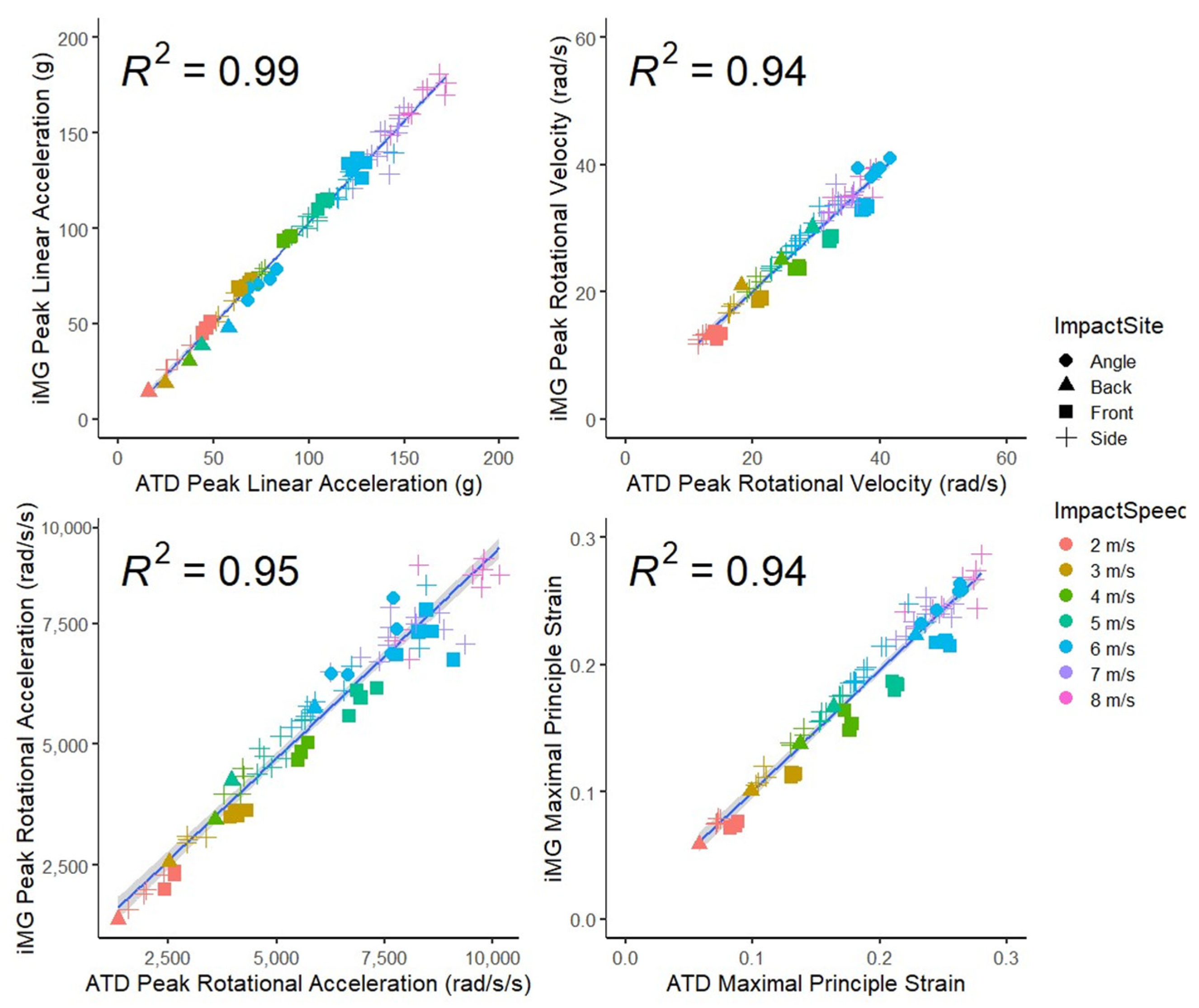
3.2. Good Agreement between the iMG and ATD Measurements of Peak Head Kinematics Values and Brain Strain
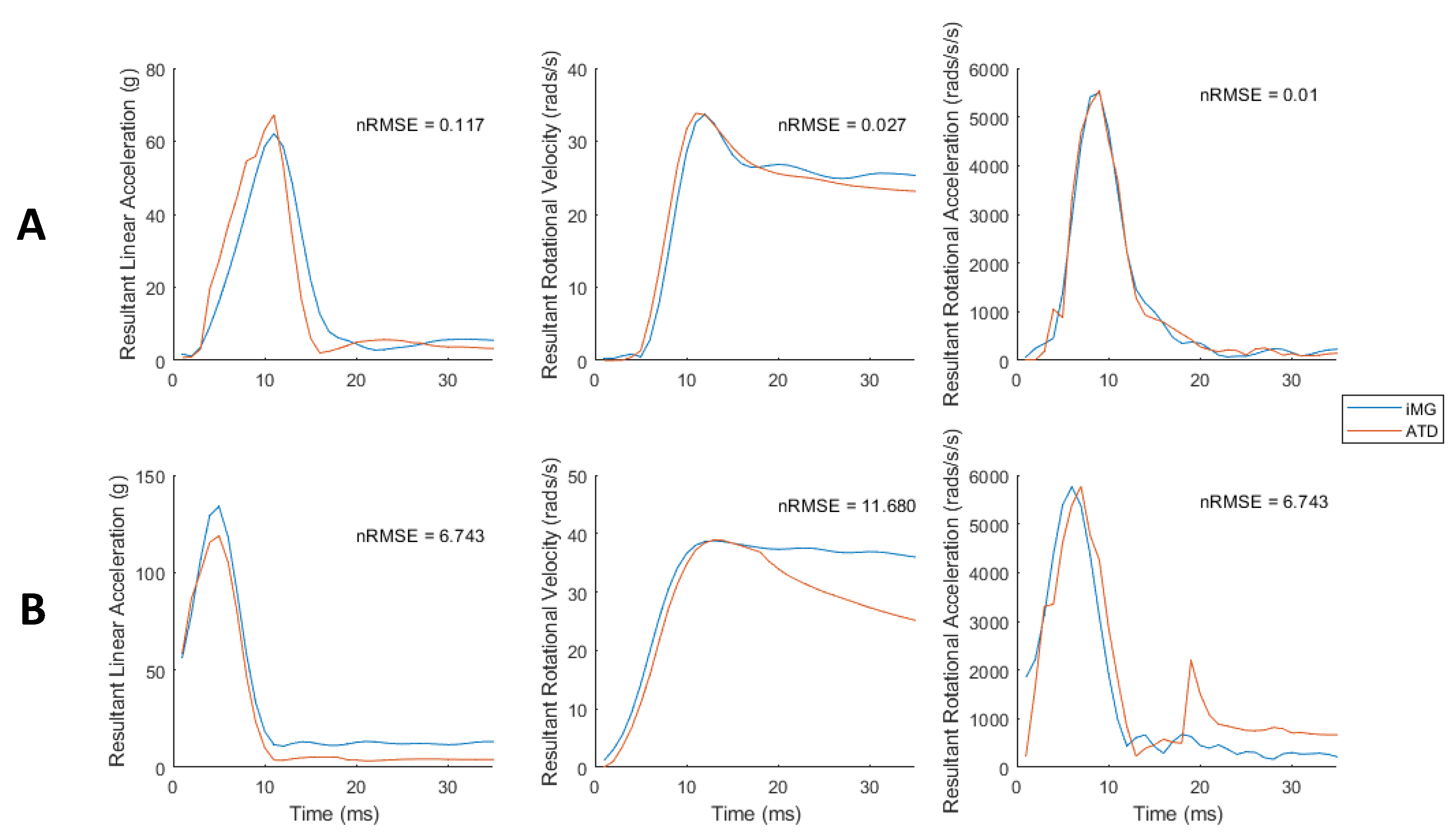
3.3. Low Error in iMG Measured Peak Values and Time-Series Data Compared with ATD
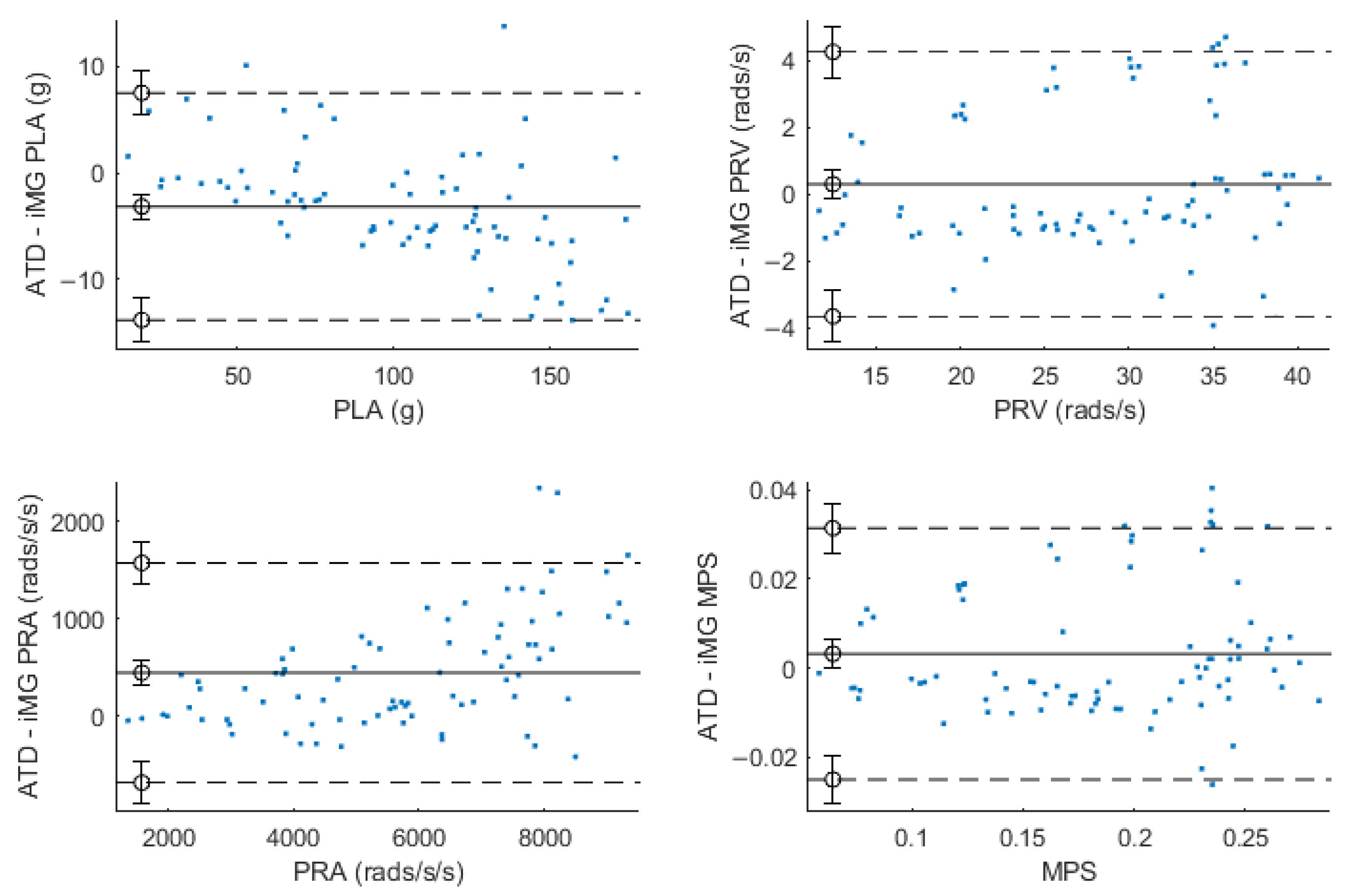
3.4. Evidence of Bias in Some of the iMG Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, V.; Motley, K.; Kemp, S.; Mian, S.; Patel, T.; James, L.; Pearce, N.; McElvenny, D. Concussion and long-term cognitive impairment among professional or elite sport-persons: A systematic review. J. Neurol. Neurosurg. Psychiatry 2020, 91, 455–468. [Google Scholar] [CrossRef]
- Patton, D.A.; Huber, C.M.; Jain, D.; Myers, R.K.; McDonald, C.C.; Margulies, S.S.; Master, C.L.; Arbogast, K.B. Head Impact Sensor Studies in Sports: A Systematic Review of Exposure Confirmation Methods. Ann. Biomed. Eng. 2020, 48, 2497–2507. [Google Scholar] [CrossRef]
- Mckee, A.C.; Alosco, M.; Huber, B.R. Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Neurosurg. Clin. N. Am. 2016, 27, 529–535. [Google Scholar] [CrossRef]
- Tyson, A.M.; Duma, S.M.; Rowson, S. Laboratory Evaluation of Low-Cost Wearable Sensors for Measuring Head Impacts in Sports. J. Appl. Biomech. 2018, 34, 320–326. [Google Scholar] [CrossRef]
- Owens, T.S.; Calverley, T.A.; Stacey, B.S.; Iannatelli, A.; Venables, L.; Rose, G.; Fall, L.; Tsukamoto, H.; Berg, R.M.G.; Jones, G.L.; et al. Contact events in rugby union and the link to reduced cognition: Evidence for impaired redox-regulation of cerebrovascular function. Exp. Physiol. 2021, 106, 1971–1980. [Google Scholar] [CrossRef]
- Ghajari, M.; Hellyer, P.J.; Sharp, D.J. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017, 140, 333–343. [Google Scholar] [CrossRef]
- Waldron, M.; Jones, C.M.; Melotti, L.; Brown, M.R.; Kilduff, L.P. Collision monitoring in elite male rugby union using a new instrumented mouth-guard. J. Sport Exerc. Sci. 2021, 5, 179–187. [Google Scholar]
- Jansen, A.E.; McGrath, M.; Samorezov, S.; Johnston, J.; Bartsch, A.; Alberts, J. Characterizing Head Impact Exposure in Men and Women during Boxing and Mixed Martial Arts. Orthop. J. Sports Med. 2021, 9. [Google Scholar] [CrossRef]
- Jones, B.; Tooby, J.; Weaving, D.; Till, K.; Owen, C.; Begonia, M.; Stokes, K.A.; Rowson, S.; Phillips, G.; Hendricks, S.; et al. Ready for impact? A validity and feasibility study of instrumented mouthguards (iMGs). Br. J. Sports Med. 2022, 56, 1171–1179. [Google Scholar] [CrossRef]
- Camarillo, D.B.; Shull, P.B.; Mattson, J.; Shultz, R.; Garza, D. An Instrumented Mouthguard for Measuring Linear and Angular Head Impact Kinematics in American Football. Ann. Biomed. Eng. 2013, 41, 1939–1949. [Google Scholar] [CrossRef]
- Liu, Y.; Domel, A.G.; Yousefsani, S.A.; Kondic, J.; Grant, G.; Zeineh, M.; Camarillo, D.B. Validation and Comparison of Instrumented Mouthguards for Measuring Head Kinematics and Assessing Brain Deformation in Football Impacts. Ann. Biomed. Eng. 2020, 48, 2580–2598, Erratum in Ann. Biomed. Eng. 2021, 49, 1119–1120. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Brown, R. Validation of an Instrumented Mouthguard. medRxiv 2022. [Google Scholar] [CrossRef]
- Stitt, D.; Draper, N.; Alexander, K.; Kabaliuk, N. Laboratory Validation of Instrumented Mouthguard for Use in Sport. Sensors 2021, 21, 6028. [Google Scholar] [CrossRef]
- Bartsch, A.J.; Hedin, D.S.; Gibson, P.L.; Miele, V.J.; Benzel, E.C.; Alberts, J.L.; Samorezov, S.; Shah, A.; Stemper, B.S.; McCrea, M.M. Laboratory and on-field data collected by a head impact monitoring mouthguard. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2068–2072. [Google Scholar]
- Siegmund, G.P.; Guskiewicz, K.M.; Marshall, S.W.; DeMarco, A.L.; Bonin, S.J. Laboratory Validation of Two Wearable Sensor Systems for Measuring Head Impact Severity in Football Players. Ann. Biomed. Eng. 2016, 44, 1257–1274. [Google Scholar] [CrossRef]
- Hoshizaki, T.B.; Post, A.; Kendall, M.; Cournoyer, J.; Rousseau, P.; Gilchrist, M.D.; Brien, S.; Cusimano, M.; Marshall, S. The development of a threshold curve for the understanding of concussion in sport. Trauma 2016, 19, 196–206. [Google Scholar] [CrossRef]
- Broglio, S.P.; Lapointe, A.; O’Connor, K.L.; McCrea, M. Head Impact Density: A Model to Explain the Elusive Concussion Threshold. J. Neurotrauma 2017, 34, 2675–2683. [Google Scholar] [CrossRef]
- O’Keeffe, E.; Kelly, E.; Liu, Y.; Giordano, C.; Wallace, E.; Hynes, M.; Tiernan, S.; Meagher, A.; Greene, C.; Hughes, S.; et al. Dynamic Blood–Brain Barrier Regulation in Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 347–356. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Mihalik, J.P. Biomechanics of Sport Concussion: Quest for the elusive injury threshold. Exerc. Sport Sci. Rev. 2011, 39, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Cournoyer, J.; Hoshizaki, T.B. Head dynamic response and brain tissue deformation for boxing punches with and without loss of consciousness. Clin. Biomech. 2019, 67, 96–101. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.S.; Patton, D.A.; Fréchède, B.; Pierré, P.-A.; Ferry, E.; Barthels, T. The biomechanics of concussion in unhelmeted football players in Australia: A case–control study. BMJ Open 2014, 4, e005078. [Google Scholar] [CrossRef]
- Donat, C.K.; Lopez, M.Y.; Sastre, M.; Baxan, N.; Goldfinger, M.; Seeamber, R.; Müller, F.; Davies, P.; Hellyer, P.; Siegkas, P.; et al. From biomechanics to pathology: Predicting axonal injury from patterns of strain after traumatic brain injury. Brain 2021, 144, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Elkin, B.S.; Morrison, B., III. Region-specific tolerance criteria for the living brain. Stapp Car Crash J. 2007, 51, 127. [Google Scholar] [PubMed]
- Morrison, B., III; Cater, H.L.; Wang, C.C.-B.; Thomas, F.C.; Hung, C.T.; Ateshian, G.A.; Sundstrom, L.E. A Tissue Level Tolerance Criterion for Living Brain Developed with an In Vitro Model of Traumatic Mechanical Loading. Stapp Car Crash J. 2003, 47, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bain, A.C.; Meaney, D.F. Tissue-Level Thresholds for Axonal Damage in an Experimental Model of Central Nervous System White Matter Injury. J. Biomech. Eng. 2000, 122, 615–622. [Google Scholar] [CrossRef]
- Kleiven, S. Predictors for Traumatic Brain Injuries Evaluated through Accident Reconstructions. Stapp Car Crash J. 2007, 51, 81–114. [Google Scholar] [CrossRef]
- Bian, K.; Mao, H. Mechanisms and variances of rotation-induced brain injury: A parametric investigation between head kinematics and brain strain. Biomech. Model. Mechanobiol. 2020, 19, 2323–2341. [Google Scholar] [CrossRef]
- Fahlstedt, M.; Abayazid, F.; Panzer, M.B.; Trotta, A.; Zhao, W.; Ghajari, M.; Gilchrist, M.D.; Ji, S.; Kleiven, S.; Li, X.; et al. Ranking and Rating Bicycle Helmet Safety Performance in Oblique Impacts Using Eight Different Brain Injury Models. Ann. Biomed. Eng. 2021, 49, 1097–1109. [Google Scholar] [CrossRef]
- Bartsch, A.; Samorezov, S.; Benzel, E.; Miele, V.; Brett, D. Validation of an “Intelligent Mouthguard” Single Event Head Impact Dosimeter. SAE Technical Papers. Stapp Car Crash J. 2014, 58, 1–27. [Google Scholar] [CrossRef]
- Kieffer, E.E.; Begonia, M.T.; Tyson, A.M.; Rowson, S. A Two-Phased Approach to Quantifying Head Impact Sensor Accuracy: In-Laboratory and On-Field Assessments. Ann. Biomed. Eng. 2020, 48, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Viano, D.C.; Casson, I.R.; Pellman, E.J.; Zhang, L.; King, A.I.; Yang, K.H. Concussion in Professional Football: Brain Responses by Finite Element Analysis: Part 9. Neurosurgery 2005, 57, 891–916. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.A.; Cournoyer, J.; Lai, H.; Snider, S.B.; Fischer, D.; Kemp, S.; Karton, C.; Hoshizaki, T.B.; Ghajari, M.; Sharp, D.J. The biomechanical signature of loss of consciousness: Computational modelling of elite athlete head injuries. Brain 2022, 146, 3063–3078. [Google Scholar] [CrossRef]
- Greybe, D.G.; Jones, C.M.; Brown, M.R.; Williams, E.M.P. Comparison of head impact measurements via an instrumented mouthguard and an anthropometric testing device. Sports Eng. 2020, 23, 12. [Google Scholar] [CrossRef]
- Gov.uk. Using the UKCA Marking. 2022. Available online: https://www.gov.uk/guidance/using-the-ukca-marking (accessed on 24 July 2023).
- Ng, T.P.; Bussone, W.R.; Duma, S.M. The effect of gender and body size on linear accelerations of the head observed during daily activities. Biomed. Sci. Instrum. 2006, 42, 25–30. [Google Scholar] [PubMed]
- Padgaonkar, A.J.; Krieger, K.W.; King, A.I. Measurement of angular acceleration of a rigid body using linear accelerometers. J. Appl. Mech. Sep. 1975, 42, 552–556. [Google Scholar] [CrossRef]
- Abayazid, F.; Ding, K.; Zimmerman, K.; Stigson, H.; Ghajari, M. A New Assessment of Bicycle Helmets: The Brain Injury Mitigation Effects of New Technologies in Oblique Impacts. Ann. Biomed. Eng. 2021, 49, 2716–2733. [Google Scholar] [CrossRef] [PubMed]
- Ghajari, M.; Galvanetto, U.; Iannucci, L.; Willinger, R. Influence of the body on the response of the helmeted head during impact. Int. J. Crashworthiness 2011, 16, 285–295. [Google Scholar] [CrossRef]
- Ghajari, M.; Peldschus, S.; Galvanetto, U.; Iannucci, L. Effects of the presence of the body in helmet oblique impacts. Accid. Anal. Prev. 2013, 50, 263–271. [Google Scholar] [CrossRef]
- Zimmerman, K.; Kim, J.; Karton, C.; Lochhead, L.; Sharp, D.; Hoshizaki, T.; Ghajari, M. Player position in American football influences the magnitude of mechanical strains produced in the location of chronic traumatic encephalopathy pathology: A computational modelling study. J. Biomech. 2021, 118, 110256. [Google Scholar] [CrossRef]
- Yu, X.; Logan, I.; Sarasola, I.d.P.; Dasaratha, A.; Ghajari, M. The Protective Performance of Modern Motorcycle Helmets Under Oblique Impacts. Ann. Biomed. Eng. 2022, 50, 1674–1688. [Google Scholar] [CrossRef]
- Yu, X.; Halldin, P.; Ghajari, M. Oblique impact responses of Hybrid III and a new headform with more biofidelic coefficient of friction and moments of inertia. Front. Bioeng. Biotechnol. 2022, 10, 860435. [Google Scholar] [CrossRef]
- Pintar, F.A.; Sances, A.; Yoganandan, N.; Reinartz, J.; Maiman, D.J.; Suh, J.K.; Unger, G.; Cusick, J.F.; Larson, S.J. Biodynamics of the Total Human Cadaveric Cervical Spine. SAE Technical Paper. In Proceedings of the Stapp Car Crash Conference, 34th, Orlando, FL, USA, 4–7 November 1990. [Google Scholar] [CrossRef]
- McIntosh, A.S.; Patton, D.A. Boxing headguard performance in punch machine tests. Br. J. Sports Med. 2015, 49, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Hume, P.A.; Brughelli, M.; Gissane, C. Instrumented Mouthguard Acceleration Analyses for Head Impacts in Amateur Rugby Union Players Over a Season of Matches. Am. J. Sports Med. 2014, 43, 614–624. [Google Scholar] [CrossRef]
- Viano, D.C.; Casson, I.R.; Pellman, E.J.; Bir, C.A.; Zhang, L.; Sherman, D.C.; Boitano, M.A. Concussion in Professional Football: Comparison with Boxing Head Impacts—Part 10. Neurosurgery 2005, 57, 1154–1172. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Hume, P.; Gissane, C.; Brughelli, M.; Clark, T. The Influence of Head Impact Threshold for Reporting Data in Contact and Collision Sports: Systematic Review and Original Data Analysis. Sports Med. 2016, 46, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Gabler, L.; Patton, D.; Begonia, M.; Daniel, R.; Rezaei, A.; Huber, C.; Siegmund, G.; Rooks, T.; Wu, L. Consensus Head Acceleration Measurement Practices (CHAMP): Laboratory Validation of Wearable Head Kinematic Devices. Ann. Biomed. Eng. 2022, 50, 1356–1371. [Google Scholar] [CrossRef]
- Arbogast, K.B.; Caccese, J.B.; Buckley, T.A.; McIntosh, A.S.; Henderson, K.; Stemper, B.D.; Solomon, G.; Broglio, S.P.; Funk, J.R.; Crandall, J.R. Consensus Head Acceleration Measurement Practices (CHAMP): Origins, Methods, Transparency and Disclosure. Ann. Biomed. Eng. 2022, 50, 1317–1345. [Google Scholar] [CrossRef]
- Ji, S.; Ghajari, M.; Mao, H.; Kraft, R.H.; Hajiaghamemar, M.; Panzer, M.B.; Willinger, R.; Gilchrist, M.D.; Kleiven, S.; Stitzel, J.D. Use of Brain Biomechanical Models for Monitoring Impact Exposure in Contact Sports. Ann. Biomed. Eng. 2022, 50, 1389–1408. [Google Scholar] [CrossRef]
- Nahum, A.M.; Smith, R.; Ward, C.C. Intracranial Pressure Dynamics During Head Impact. SAE Technical Paper. In Proceedings of the Stapp Car Crash Conference, Warrendale, PA, USA, 19–21 October 1977. [Google Scholar] [CrossRef]
- Alshareef, A.; Giudice, J.S.; Forman, J.; Salzar, R.S.; Panzer, M.B. A Novel Method for Quantifying Human In Situ Whole Brain Deformation under Rotational Loading Using Sonomicrometry. J. Neurotrauma 2018, 35, 780–789. [Google Scholar] [CrossRef]
- Alshareef, A.; Giudice, J.S.; Forman, J.; Shedd, D.F.; Reynier, K.A.; Wu, T.; Sochor, S.; Sochor, M.R.; Salzar, R.S.; Panzer, M.B. Biomechanics of the Human Brain during Dynamic Rotation of the Head. J. Neurotrauma 2020, 37, 1546–1555. [Google Scholar] [CrossRef]
- Yu, X.; Nguyen, T.-T.; Wu, T.; Ghajari, M. Non-Lethal Blasts can Generate Cavitation in Cerebrospinal Fluid While Severe Helmeted Impacts Cannot: A Novel Mechanism for Blast Brain Injury. Front. Bioeng. Biotechnol. 2022, 10, 808113. [Google Scholar] [CrossRef]
- Hallquist, J. LS-DYNA Keyword User’s Manual; Livermore Software Technology Corporation: Livermore, PA, USA, 2013; pp. 100–750. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Newcastle upon Tyne, UK, 2013; ISBN 1446274586. [Google Scholar]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- Baumgartner, T.A.; Chung, H. Confidence Limits for Intraclass Reliability Coefficients. Meas. Phys. Educ. Exerc. Sci. 2001, 5, 179–188. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Datta, D. Blandr: A Bland-Altman Method Comparison Package for R (0.5.1); R Studio: Boston, MA, USA, 2017. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Ludbrook, J. A primer for biomedical scientists on how to execute Model II linear regression analysis. Clin. Exp. Pharmacol. Physiol. 2012, 39, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Package “lmodel2” for R Studio (Version 1.7-3). 2018. Available online: https://cran.r-project.org/package=lmodel2 (accessed on 6 August 2023).
- Post, A.; Hoshizaki, T.B.; Gilchrist, M.D.; Cusimano, M.D. Peak linear and rotational acceleration magnitude and duration effects on maximum principal strain in the corpus callosum for sport impacts. J. Biomech. 2017, 61, 183–192. [Google Scholar] [CrossRef] [PubMed]

| iMG (PROTECHTTM System) | ATD (Reference) | |
|---|---|---|
| Sampling rate (Accelerometer) | 1000 Hz | 50,000 Hz |
| Sampling rate (Gyroscope) | 1666 Hz and downsampled to 1000 Hz | NA |
| Measurement range and resolution (Accelerometer) | ±400 g per axis | ±1000 g |
| Measurement range and resolution (Gyroscope) | ±35 rad/s per axis | N/A |
| Output time windows | [−10, 94] ms | [−2, 48] ms (after data processing) |
| Output coordinate axes direction | Not standard but transformed to ISO reference frame. | ISO reference frame |
| Output coordinate origin | Sensor which is then transformed to Centre of Mass | Centre of Mass |
| Filter | Post analysis identified a 160 Hz low pass 4th order Butterworth filter | 4th order Butterworth low-pass filter with a 1 kHz cut-off frequency |
| Derivation of rotational acceleration | 5-point stencil derivative | Padgaonkar’s method [36] |
| CCC | Mean Relative Error (±SD) % | RMSE | nRMSE | Bland-Altman (% Difference) | |||
|---|---|---|---|---|---|---|---|
| (95% CI) | (±SD) | (±SD) % | Bias (95% CI) | Lower Limit | Upper Limit | ||
| PLA (g) | 0.989 | 5.2 | 2.76 | 2.88 | −2.16 | −14.43 | 10.10 |
| 0.984–0.992 | (±4.4) | (±1.61) | (±1.27) | (−4.51–0.19) | |||
| PRV (rad/s) | 0.970 | 0.8 | 1.52 | 5.47 | 0.69 | −13.74 | 15.12 |
| 0.953–0.980 | (±7.4) | (±0.87) | (±2.61) | (−2.07–3.46) | |||
| PRA (rad/s2) | 0.945 | 6.7 | 78.27 | 1.24 | 6.16 | −8.72 | 21.04 |
| 0.921–0.962 | (±8.2) | (±71.97) | (±0.86) | (3.31–9.02) | |||
| MPS | 0.970 | 1.67 | N/A | N/A | 1.35 | −13.85 | 16.59 |
| 0.955–0.981 | (±8.1) | (−1.59–4.29) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, C.M.; Austin, K.; Augustus, S.N.; Nicholas, K.J.; Yu, X.; Baker, C.; Chan, E.Y.K.; Loosemore, M.; Ghajari, M. An Instrumented Mouthguard for Real-Time Measurement of Head Kinematics under a Large Range of Sport Specific Accelerations. Sensors 2023, 23, 7068. https://doi.org/10.3390/s23167068
Jones CM, Austin K, Augustus SN, Nicholas KJ, Yu X, Baker C, Chan EYK, Loosemore M, Ghajari M. An Instrumented Mouthguard for Real-Time Measurement of Head Kinematics under a Large Range of Sport Specific Accelerations. Sensors. 2023; 23(16):7068. https://doi.org/10.3390/s23167068
Chicago/Turabian StyleJones, Chris M., Kieran Austin, Simon N. Augustus, Kieran Jai Nicholas, Xiancheng Yu, Claire Baker, Emily Yik Kwan Chan, Mike Loosemore, and Mazdak Ghajari. 2023. "An Instrumented Mouthguard for Real-Time Measurement of Head Kinematics under a Large Range of Sport Specific Accelerations" Sensors 23, no. 16: 7068. https://doi.org/10.3390/s23167068
APA StyleJones, C. M., Austin, K., Augustus, S. N., Nicholas, K. J., Yu, X., Baker, C., Chan, E. Y. K., Loosemore, M., & Ghajari, M. (2023). An Instrumented Mouthguard for Real-Time Measurement of Head Kinematics under a Large Range of Sport Specific Accelerations. Sensors, 23(16), 7068. https://doi.org/10.3390/s23167068





