1. Introduction
Neural recording systems sense and communicate action potentials, local field potentials, post-synaptic potentials, or other relevant bioelectric signals. Sensing such signals can play a key role in the early detection of neurological disorders, identify motor intent, design therapeutic or functional neurostimulation systems, and contribute towards overall advances in neuroscience. The selectivity and sensitivity of the recorded signals are typically enhanced at the expense of the invasiveness of the sensory unit. EEGs (electroencephalograms) are least invasive as the signals are recorded from electrodes on the scalp [
1]. These electrodes typically capture the evoked or event-related potentials that are then appropriately decoded with signal processing to extract the features. In one class of applications, these features are translated to understand the motor intent in patients with spinal cord injuries and used to control assistive devices. However, EEGs do not provide a high signal-to-noise ratio and also do not have high-frequency sensitivity to identify the short-term activities with high spatial or temporal resolution.
Direct recording of action potentials with intracortical or depth electrodes provides the highest resolution for brain–computer interfaces (BCI) and therapeutic neurotechnologies [
2,
3]. These recordings, however, need proximal access to the neurons and are, therefore, the most invasive. They require craniotomy and can often cause neural damage. As a compromise, electrocorticograms (ECoG), which record signals from the surface of the cerebral cortex, provide adequate sensitivity without excessive risk of tissue scarring inside the brain [
4]. In spite of this, ECoG recordings are often performed under clinical settings during certain procedures, such as clinical monitoring to identify the epileptic foci [
5].
Neural recording systems offer a wide range of electrodes or channels, data rates, bandwidth, power budget, and size or volume depending on the intended applications. High-density microelectrode arrays are often needed to access brain neural circuits with enhanced spatial resolution. Examples of these include intracortical recording to access motor circuits that control the limb movement, mapping to reveal connectivity between neuronal functions or similar large-scale cortical processes. One clinical need where these functions become important is the diagnosis of chronic neurological disorders, such as epilepsy. In this case, the recording units obtain the interictal epileptiform discharges (IED) that occur between seizures. These aid in identifying the pathological area for surgical excision.
The sensing neural interfaces are fabricated at various scales depending on the channel count and bandwidth. Data rates of 50 Mbps or more are often needed with 32, 64, or 100 channels. In these applications, the power consumption often reaches 100 mW, resulting in a bit energy consumption of 0.1–2 nJ. The recording unit comprises a rigid package that is constructed using PCB (printed circuit boards) substrates with a communication module, an analog signal modulation front-end, and a power module with a battery, and is encapsulated in hermetic packages with electrode feedthroughs. As an example of such units, a system of ~74 cm
3 and a power consumption of 63.2 mW was designed, out of which 28 mW was consumed by the radio frequency transmitter [
6]. To reduce the burden of replacing batteries, rechargeable batteries as well as wireless telemetry were implemented. For example, a recording system for head-stage neural activity with a size of 5.6 × 4.2 × 0.9 cm
3 on a rigid package with a rechargeable battery was realized [
7]. The average power of the system will increase to 100 s of milliwatts when the battery is charging. Even though the system is powered by inductive coils, several problems are foreseen. It has been documented that 400 mW of overall power consumption may negatively affect tissue, as it has been shown that the tissue temperature is increased by 2 °C when more than 40 mW/cm
2 of dissipated power is applied [
8,
9].
Because of the extensive power and data telemetry electronics and the associated packaging, wireless neural recording systems are mounted on the top of the skull, whereas the microelectrode arrays (MEA) are typically implanted into the cortex surface. Systems mounted on the skull have a greater degree of design flexibility than the implanted version. They can, thus, accommodate large sizes in terms of the wireless system footprint and significant power consumption. Nevertheless, the intracranial wiring configuration is also accompanied by a wide variety of challenges and issues. It has been observed that the high failure rate is a major concern. Wireless head-stages with implanted microelectrode arrays are not yet safe and robust enough for use in long-term medical treatments [
10]. Wirelessly powered neural recording systems have been the primary focus to reduce the need for batteries and associated power conversion [
11] and reduce the overall package size. Such power telemetry is often achieved with an inductive link. With advanced coil designs, the inductive link can also be directly integrated onto the IC without compromising the performance [
12]. An integrated power rectifier on the IC also minimizes the need for other external components. A CMOS (complementary metal oxide semiconductor) integrated circuit (IC) amplifies, digitizes, and multiplexes the signal, and modulates the backscattered carrier with the serialized data.
Another type of neural recording system consists of distributed individual untethered units that are used to record from only a few sites or a single site. It is possible to miniaturize these down to the size of a few mm, as opposed to larger units of a few cm in size. These are commonly referred to as recording nodes. These provide many unique opportunities for neural recording through multiphysics-based transduction techniques that are derived from ultrasonic, RF, magnetoelectric, or multiferroic excitations [
13]. Unlike electromagnetic links that are current-based, these devices often power and communicate through acoustic actuation which scale to the sub-millimeter scale because of their smaller wavelengths compared to microwaves. These techniques have been proven to reduce the power consumption of communication modules as well as reduce the size of the communication modules [
14]. Such devices function both in the active mode where the incoming power is rectified to activate a CMOS signal processing circuit, or in a fully-passive mode which merely reflects or backscatters the modulated signal [
15].
Multiple coupling modes, such as acoustic, magnetoelectric, or RF backscattering techniques are utilized in fully-passive and implantable neural recording devices. In a typical RF backscattering implementation of this approach, a signal at
F GHz is transmitted from an interrogator and is received by the implant. The implant upconverts the signal to a
2F GHz signal, mixes it with a low-frequency low-amplitude neural signal (
fneuro), and sends the modulated signal (
2F GHz ±
fneuro) back to the interrogator. An antiparallel diode used as a subharmonic mixer, a matching network, and a bypass capacitor are incorporated together on a PCB substrate. Earlier reports demonstrated such passive backscattered sensors with a total size of 15 mm × 16 mm × 1.5 mm [
16] With further advances in antenna design, the implant’s size was further miniaturized to 9.5 mm × 8.7 mm × 0.7 mm on a flexible platform [
17]. However, in previous studies, implants were typically designed to match the 50 Ω telemetry system. This creates mismatch losses with the high electrode–tissue impedance at the physiological interfaces and limits the sensitivity of the signal. In order to address this limitation, Chen et al. proposed a buffer circuit to convert the high impedance from the electrode–tissue impedance to the matched RF impedance, thereby demonstrating a major advance in signal sensitivity [
18]. In this approach, the buffer circuit is integrated with the antenna but with no components to bypass high-frequency signals to improve signal sensitivity.
The applications of wireless passive telemetry systems also extend to wearable medical devices that can sense other biosignals, such as ECG (electrocardiogram), EMG (electromyogram), along with the aforementioned EEG. Passive telemetry can transform today’s obtrusive systems with thick electronic units on the skin to thin and disposable patches. This is because of their zero-power or battery-free operation with minimal signal processing components and simplified topologies. The key challenge with these systems, however, is the need for longer communication distance between the interrogator and the reader. This demands larger interrogation carrier power, which invariably puts a heavier power burden on the interrogator.
Impedance matched circuits and miniaturized antennas that are coupled with low-loss mixers are the key requirements towards enhancing neural recording and broadly all wireless sensor systems. Impedance-buffered electrodes are expected to improve the signal resolution with passive neural recording at low interrogating power. This forms the underlying hypothesis for this work. In order to advance the utility of this approach further, this manuscript describes a miniaturized integrated passive impedance-matching neural recording unit in a thin flexible package with improved sensitivity. A substrate with high permittivity was used to miniaturize the antennas. With these innovations, we capture signals as low as 170 µVpp when the impedance is 33 kΩ. The transmitted biosignals are detectable at a distance of 5 cm between the interrogator and the implanted antenna.
2. Neural Recording System Design
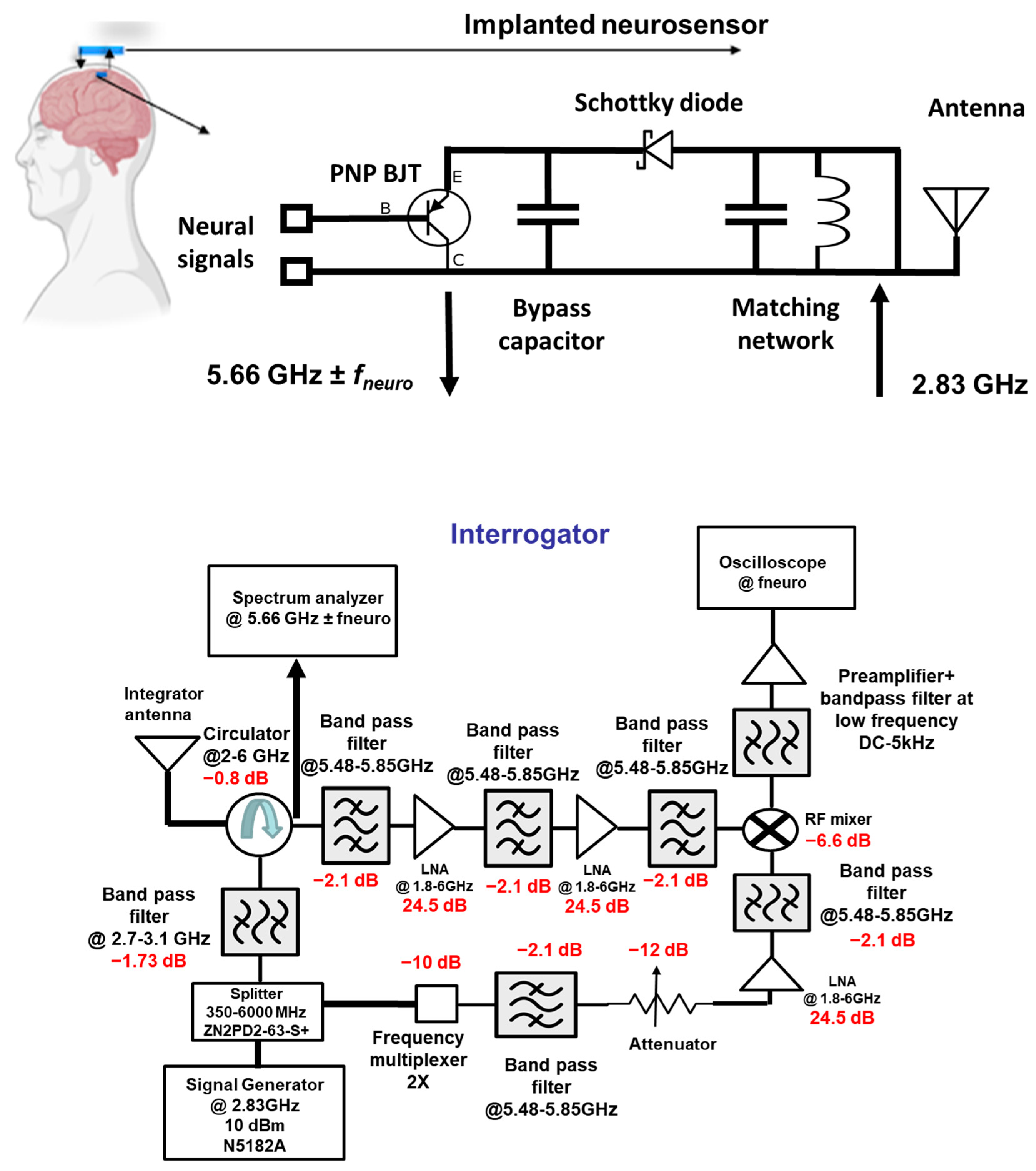
The neural recording system can be divided into two sections: (i) the implant and (ii) the interrogator, as shown in
Figure 1. The implanted neurosensor depends on the RF backscattering technique that is widely used in RFID applications. Electrode–tissue impedances are of the order of 10–100 kΩ for practical electrode designs. On the other hand, RF signal chains are designed at 50 Ohms. To deal with high impedance mismatch and DC offset, the backscattering circuit employs a BJT that is biased by an activated diode. The incident signal at
F1 GHz (typically at ~2.4 GHz) which comes from the interrogator is used to activate the diode in the forward region. The activated or turned-on diode acts as a rectifier circuit to bias the BJT transistor. In the second cycle of the sinewave, the activated transistor and the RF diode act as RF mixers. Thus, it backscatters the
fneuro signals ± 2
F1 (at ~4.8 GHz) GHz.
Figure 2 illustrates the concept of the RF backscattering of the implanted neurosensor. The designs in this paper were implemented at 2.83 and 5.66 GHz.
2.1. Passive Sensor
The circuit mechanism is divided into two modes: DC and RF. In DC mode, the interrogator sends a 2.83 GHz carrier signal to activate the implant. Upon receiving the signal, the Schottky diode acts as a rectifier to generate DC and bias the BJT. The BJT collector is connected to the ground while the emitter is connected to the diode in order to upconvert the baseband neuro-signal. The neuro-signal passes through the base when the BJT is biased or activated through the diode. The BJT can be operated either in the forward-active region or in the saturation region. In both scenarios, the signal can move from base to emitter, while the DC offset voltage at the base can be ignored. These unparallel characteristics suggest tolerance to DC offset. As the BJT has a high input impedance and is configured as an emitter follower, it matches the high electrode impedance and converts it into a low impedance. This will ensure that no circuit loading occurs.
In RF mode, the Schottky diode acts as a non-linear mixer, as shown in
Figure 3. The 2.83 GHz carrier signal is upconverted with the brain neuro-signal by the diode and generates the second-order component (5.66 GHz ±
fneuro). This second-order product is transmitted back to the interrogator and finally demodulated to retrieve the neural signal in the time–domain.
Thus, the implant features the following key innovations: (a) an implantable antenna for wireless communication, (b) a Schottky diode that operates in DC and RF modes, (c) an inductor for matching diode and antenna impedance, (d) a bypass capacitor to isolate low-frequency signals from high-frequency signals, and (e) a PNP bipolar transistor (BJT) that acts a buffer circuit. The followed components were utilized: a capacitor (Murata Mfg. Co., Ltd., Nagaokakyo, Kyoto, Japan, GRT155R61C105ME01D), diode (Skyworks Solutions, Inc., Irvine, CA, USA, SOT-23 Surface-Mount Mixer and Detector Schottky Diodes—sms7621-079LF), BJT (Onsemi, Scottsdale, Arizona, USA, MMBTH81), and inductor (Murata Mfg. Co., Nagaokakyo, Kyoto, Japan, JELF243C_0021J-01).
In order to achieve the operation in the two frequencies, a unique dual-band antenna that has resonance frequencies at 2.83 GHz and 5.66 GHz was designed. The dielectric substrate of the antenna is based on a ceramic-filled PTFE composite (Rogers RO3010TM) with a dielectric constant of 10.2 and a loss tangent of 0.001. We achieved the dual-band operation by cutting a U-shape slot on the patch. Dual-band operation is due to the U-slot’s increase in the current path.
2.2. Interrogator
The function of the interrogator is to generate and send a 2.83 GHz carrier signal and demodulate the 5.66 GHz ± fneuro. It is composed of an RF signal generator, splitter, circulator, multiplying chain, mixer, bandpass filter, low-noise amplifier block, spectrum analyzer, and oscilloscope for signal display and observation. The signal generator produces a 2.83 GHz carrier signal which a splitter routes into the two avenues. The first avenue routes the signal into Port 3 of the circulator and to the interrogator antenna. The second avenue of the splitter routes the signal through the multiplying chain block. With the first avenue, the signal reaches the implant. After signal up-conversion and mixing, the second-order backscattered and modulated signal reaches back to the interrogator. This 5.66 GHz ± fneuro signal is directed by the circulator to the filter and the amplifying block. This stage has three bandpass filters and two low-noise amplifiers (LNA). As their center frequency is at 5.66 GHz, the undesired second and third harmonic components are filtered out. The signal reaches the mixer with proper signal conditioning by the filter and amplifier stage. At the same time, in the second avenue, the 5.66 GHz signal from the multiplying chain block reaches the LO port of the mixer. Finally, the down-converted low-frequency neural signal is seen on the oscilloscope with proper filtering and amplification at the preamplifier.
5. Conclusions
Wireless neural recording systems with high-density circuitry and battery-powered components face constraints because of the need for battery replacement, surgical constraints during implantation that arise from size, and the generation of heat that damages tissues. Passive wireless neural recording devices using RF backscattering eliminate the need for high-density circuitry. However, passive recording units often use non-linear components with relatively low impedance and are, thus, not appropriate for neural recording electrodes as they generate high impedance at the electrode–tissue interface. This causes the system to attenuate the incoming biosignals. In order to address this challenge, the sensitivity of passive neural recording units was improved by implementing a BJT circuit as a buffer, a Schottky diode as a mixer and rectifier, and a bypass capacitor to isolate the signals at high and low frequencies. The sensing circuits were fabricated with high-permittivity Teflon-based dielectric substrates to aid miniaturization, flexibility, long-term stability, and reliability. When the prototypes were tested in a low-loss medium, such as air, neuro-signals with voltages as low as 80 µVpp were detected with an electrode–tissue impedance of 33 kΩ. In the representative phantom tissue, emulated biosignals of 170 µVpp were detected with the same electrode–tissue impedance. As we reach the lower amplitude range of the neural signal, the recovered time–domain signal becomes noisy with high-impedance electrodes.
Mixers with low conversion loss, antenna designs with smaller footprints but high efficiency, and matching networks and receiver circuits with higher signal-to-noise ratio are critical to further improve the signal sensitivity for neural recording applications. These improvements will broaden the applications to other product segments, such as multimodal wearable sensing with disposable on-skin patches that can seamlessly connect to smartphones.