Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Synthesized Gold Nanoparticles
3.2. Characterization of the Obtained SERS Substrates
3.3. SERS Analysis of Peptides: A Case of BSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scanlon, M.D.; Smirnov, E.; Stockmann, T.J.; Peljo, P. Gold Nanofilms at Liquid–Liquid Interfaces: An Emerging Platform for Redox Electrocatalysis, Nanoplasmonic Sensors, and Electrovariable Optics. Chem. Rev. 2018, 118, 3722–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdaniauskienė, A.; Charkova, T.; Ignatjev, I.; Melvydas, V.; Garjonytė, R.; Matulaitienė, I.; Talaikis, M.; Niaura, G. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy for Characterization of Living Yeast Cells. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 240, 118560. [Google Scholar] [CrossRef]
- Childs, A.; Vinogradova, E.; Ruiz-Zepeda, F.; Velazquez-Salazar, J.J.; Jose-Yacaman, M. Biocompatible Gold/Silver Nanostars for Surface-Enhanced Raman Scattering. J. Raman Spectrosc. 2016, 47, 651–655. [Google Scholar] [CrossRef]
- Ma, P.; Liang, F.; Diao, Q.; Wang, D.; Yang, Q.; Gao, D.; Song, D.; Wang, X. Selective and Sensitive SERS Sensor for Detection of Hg2+ in Environmental Water Base on Rhodamine-Bonded and Amino Group Functionalized SiO2-Coated Au-Ag Core-Shell Nanorods. RSC Adv. 2015, 5, 32168–32174. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Duan, J.; Hou, J.; Hou, Q.; Yang, Y.; Li, H.; Ai, S. Rapid and Ultrasensitive Detection of Mercury Ion (II) by Colorimetric and SERS Method Based on Silver Nanocrystals. Microchem. J. 2021, 161, 105790. [Google Scholar] [CrossRef]
- Balzerova, A.; Fargasova, A.; Markova, Z.; Ranc, V.; Zboril, R. Magnetically-Assisted Surface Enhanced Raman Spectroscopy (MA-SERS) for Label-Free Determination of Human Immunoglobulin G (IgG) in Blood Using Fe3O4@Ag Nanocomposite. Anal. Chem. 2014, 86, 11107–11114. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Identification of Synthetic Organic Pigments: The Role of a Comprehensive Digital Raman Spectral Library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman Spectroelectrochemistry. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Eremina, O.E.; Yarenkov, N.R.; Kapitanova, O.O.; Zelenetskaya, A.S.; Smirnov, E.A.; Shekhovtsova, T.N.; Goodilin, E.A.; Veselova, I.A. Molecular Immobilization and Resonant Raman Amplification by Complex-Loaded Enhancers (MIRRACLE) on Copper (II)–Chitosan–Modified SERS-Active Metallic Nanostructured Substrates for Multiplex Determination of Dopamine, Norepinephrine, and Epinephrine. Microchim. Acta 2022, 189, 211. [Google Scholar] [CrossRef]
- Holmberg, N.; Laasonen, K.; Peljo, P. Charge Distribution and Fermi Level in Bimetallic Nanoparticles. Phys. Chem. Chem. Phys. 2015, 18, 2924–2931. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Arul, R.; Nieuwoudt, M.K.; Dong, J.; Zhang, T.; Dai, L.; Greenham, N.C.; Rao, A.; Hoye, R.L.Z.; Gao, W.; et al. Understanding the Chemical Mechanism behind Photoinduced Enhanced Raman Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 4607–4616. [Google Scholar] [CrossRef]
- Lauterbur, P. © 1973 Nature Publishing Group. Nat. Phys. Sci. 1973, 22, 190–191. [Google Scholar] [CrossRef]
- Park, Y.-K.; Park, S. Directing Close-Packing of Midnanosized Gold Nanoparticles at a Water/Hexane Interface. Chem. Mater. 2008, 20, 2388–2393. [Google Scholar] [CrossRef]
- Smirnov, E. Assemblies of Gold Nanoparticles at Liquid-Liquid Interfaces; Springer Theses; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-77913-3. [Google Scholar]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Peljo, P.; Méndez, M.A.; Smirnov, E.; Girault, H.H. Charging and Discharging at the Nanoscale: Fermi Level Equilibration of Metallic Nanoparticles. Chem. Sci. 2015, 6, 2705–2720. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, E.; Peljo, P.; Scanlon, M.D.; Girault, H.H. Gold Nanofilm Redox Catalysis for Oxygen Reduction at Soft Interfaces. Electrochim. Acta 2016, 197, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Tian, X.D.; Liu, B.J.; Li, J.F.; Yang, Z.L.; Ren, B.; Tian, Z.Q. SHINERS and Plasmonic Properties of Au Core SiO2 Shell Nanoparticles with Optimal Core Size and Shell Thickness. J. Raman Spectrosc. 2013, 44, 994–998. [Google Scholar] [CrossRef]
- Li, J.F.; Tian, X.D.; Li, S.B.; Anema, J.R.; Yang, Z.L.; Ding, Y.; Wu, Y.F.; Zeng, Y.M.; Chen, Q.Z.; Ren, B.; et al. Surface Analysis Using Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nat. Protoc. 2013, 8, 52–65. [Google Scholar] [CrossRef]
- Chen, M.C.; Lord, R.C. Laser-Excited Raman Spectroscopy of Biomolecules. VIII. Conformational Study of Bovine Serum Albumin. J. Am. Chem. Soc. 1976, 98, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.; Foley, S.; Cardey, B.; Enescu, M. An Experimental and Theoretical Study of the Amino Acid Side Chain Raman Bands in Proteins. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 128, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Cui, J.; Chen, X. A Morpholinium Surfactant Crystallization Induced Formation of Au Nanoparticle Sheet-like Assemblies with Uniform SERS Activity. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 456, 100–107. [Google Scholar] [CrossRef]
- Xu, T.; Wang, X.; Huang, Y.; Lai, K.; Fan, Y. Rapid Detection of Trace Methylene Blue and Malachite Green in Four Fish Tissues by Ultra-Sensitive Surface-Enhanced Raman Spectroscopy Coated with Gold Nanorods. Food Control 2019, 106, 106720. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Hao, Y.; Duan, Q.; Wang, C.; Wang, T.; Li, D. Surface-Enhanced Raman Spectroscopy with Partial Least Squares Regression for Rapid and Accurate Detection of Malachite Green in Aquaculture Water Using Large-Size Gold Nanoparticles. Spectrosc. Lett. 2020, 53, 63–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Pei, L.; Lai, K.; Rasco, B.A.; Huang, Y. Rapid Analysis of Malachite Green and Leucomalachite Green in Fish Muscles with Surface-Enhanced Resonance Raman Scattering. Food Chem. 2015, 169, 80–84. [Google Scholar] [CrossRef]
- FRENS, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillie, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 75–82. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- X-ray Energy Reference. Available online: https://assets.thermofisher.com/TFS-Assets/CAD/posters/CAD-Niton-Periodictable-fxl.pdf (accessed on 11 January 2022).
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A Highly Sensitive, Flexible SERS Sensor for Malachite Green Detection Based on Ag Decorated Microstructured PDMS Substrate Fabricated from Taro Leaf as Template. Sens. Actuators B Chem. 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Huang, Q.; Wen, S.; Zhu, X. Synthesis and Characterization of an AgI/Ag Hybrid Nanocomposite with Surface-Enhanced Raman Scattering Performance and Photocatalytic Activity. RSC Adv. 2014, 4, 37187–37192. [Google Scholar] [CrossRef]
- Ogundare, S.A.; van Zyl, W.E. Amplification of SERS “Hot Spots” by Silica Clustering in a Silver-Nanoparticle/Nanocrystalline-Cellulose Sensor Applied in Malachite Green Detection. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 570, 156–164. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Le, N.T.; Hien, B.T.T.; Trung, D.Q.; Liem, N.Q. Preparation of SERS Substrates for the Detection of Organic Molecules at Low Concentration. Commun. Phys. 2017, 26, 261. [Google Scholar] [CrossRef] [Green Version]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Quantifying SERS Enhancements. MRS Bull. 2013, 38, 631–640. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Zhang, S.; Xu, P.; Song, B. Turn-on Fluorescence Probe for BSA Detection and Selective Cell Imaging. Dye. Pigment. 2022, 202, 110267. [Google Scholar] [CrossRef]
- Loughney, J.W.; Lancaster, C.; Ha, S.; Rustandi, R.R. Residual Bovine Serum Albumin (BSA) Quantitation in Vaccines Using Automated Capillary Western Technology. Anal. Biochem. 2014, 461, 49–56. [Google Scholar] [CrossRef]


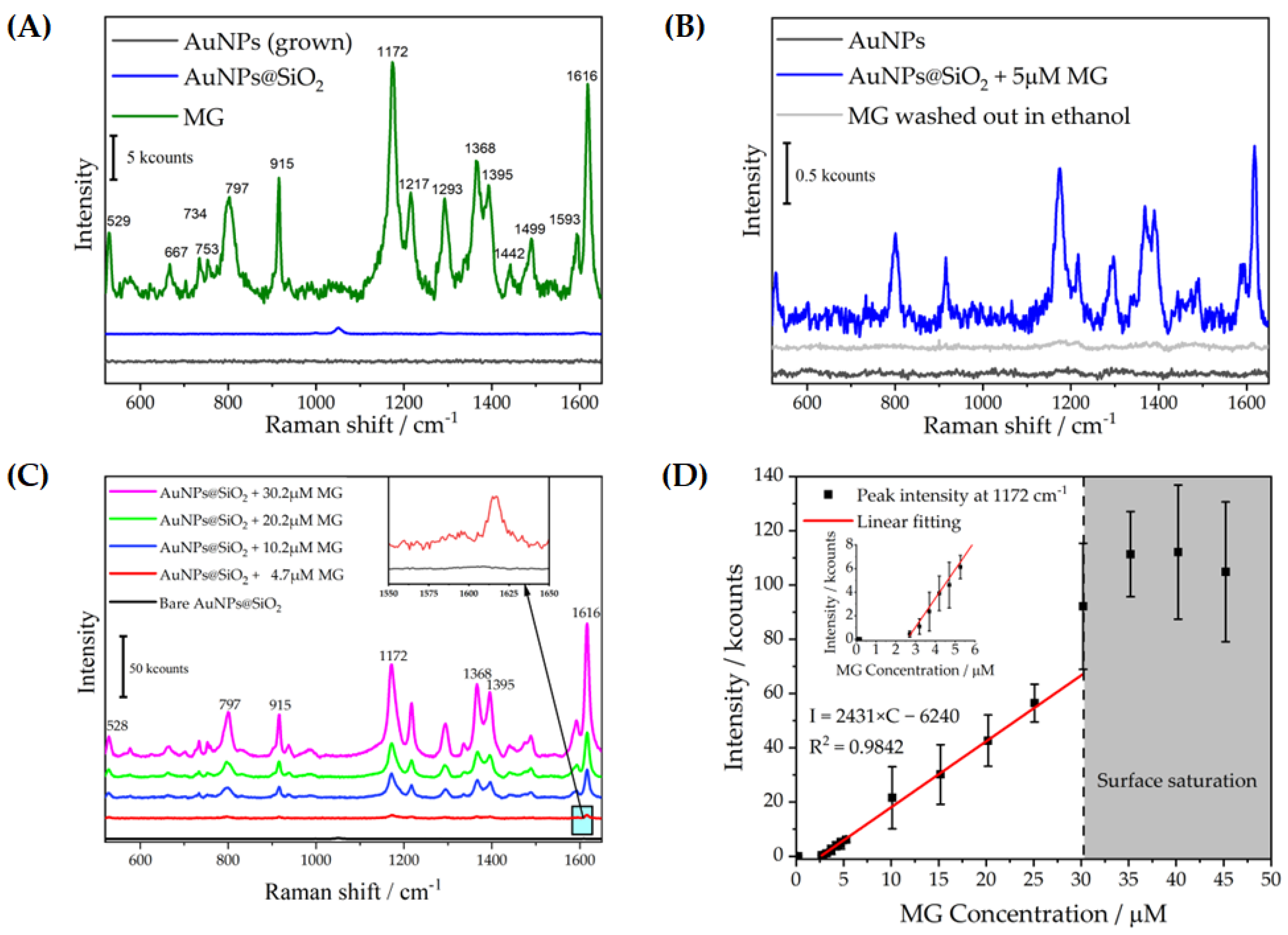
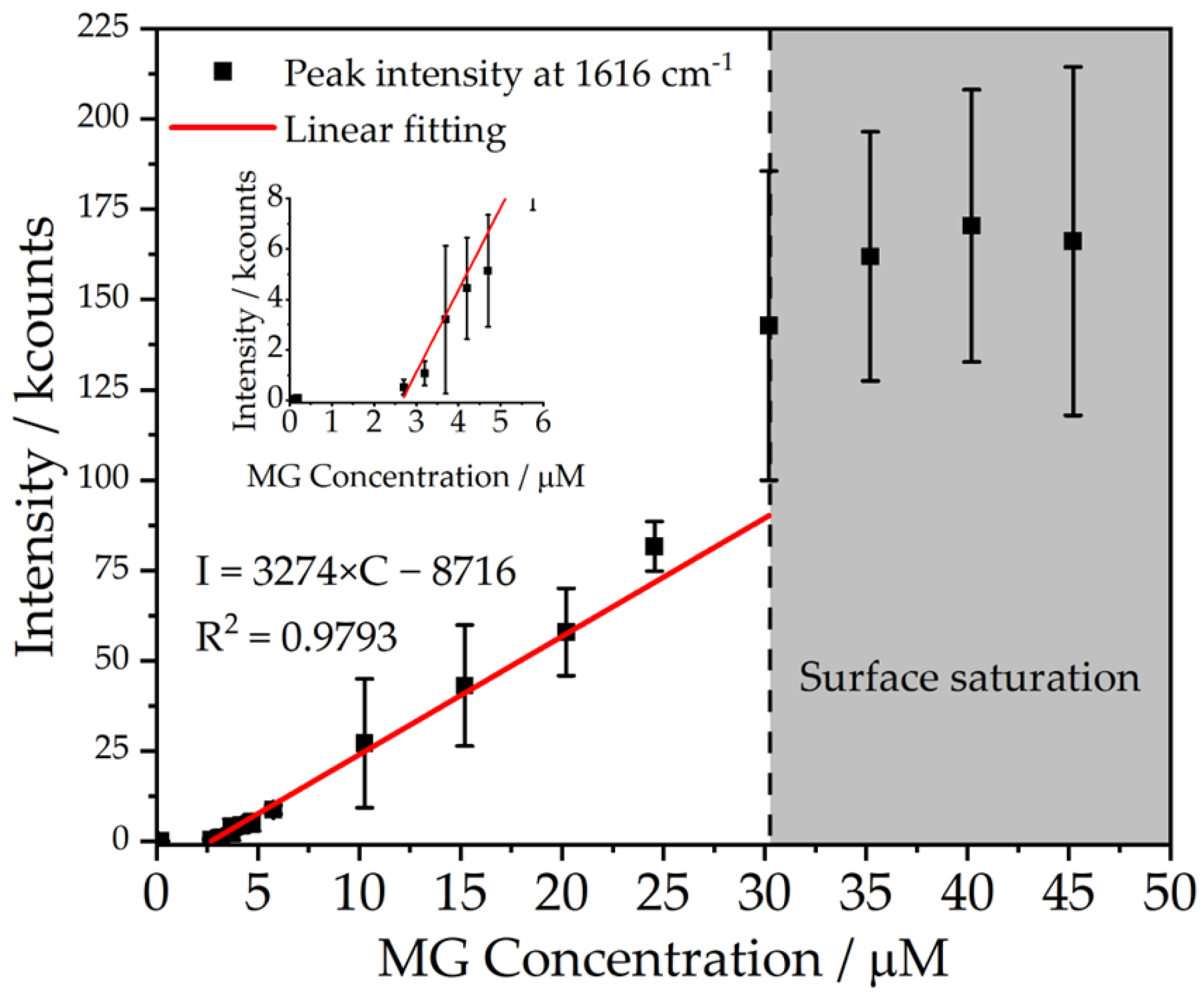

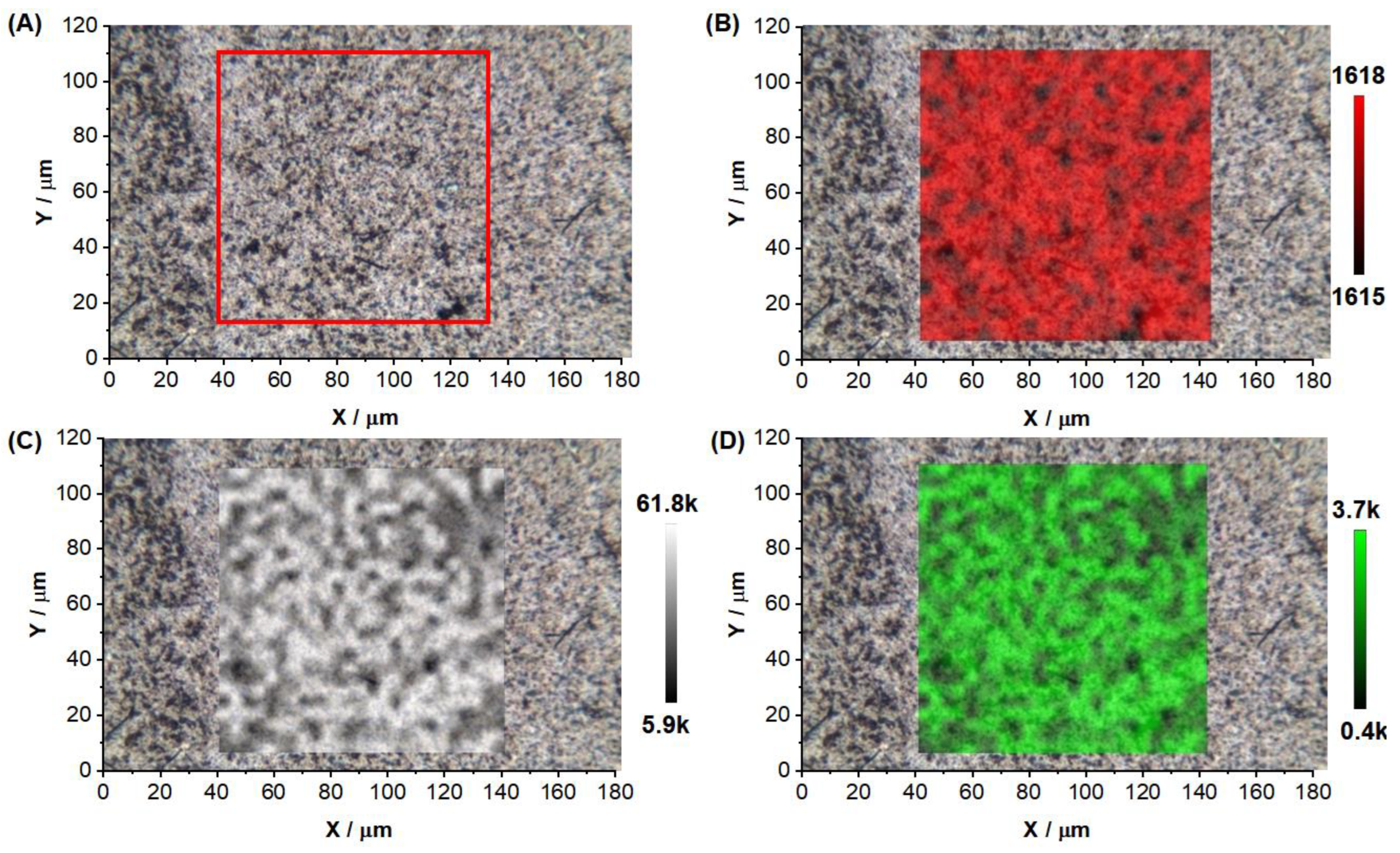

| Sample | <d>/nm | C/Particles μL−1 | <d>DLS/nm | <ζ>DLS/mV |
|---|---|---|---|---|
| Seed AuNPs | 13 | 4 × 109 | 17 ± 3 | −39 ± 3 |
| Seed-mediated grown AuNPs | 28 | 4 × 108 | 44 ± 10 | −39 ± 2 |
| Seed-mediated grown AuNPs with SiO2 shell | 35 | 3 × 108 | 52 ± 20 | −43 ± 2 |
| ν633экс. | ν633 | Vibration Type, Attribution | Refs. |
|---|---|---|---|
| 529 | – | Scissoring of phenyl-C-phenyl | 785 nm: [26,32] |
| 797 | 796/798 | Out-of-plane C-H (ring), wagging C-H of the benzene ring | 532 nm: [33], 633 nm: [25,27], 785 nm: [26,32,34] |
| 915 | 916 | Out-of-plane C-H (ring), stretching of the benzene ring | 532 nm: [33,35] 633 nm: [25,27] 785 nm: [26,32,34] |
| 1172 | 1173/1172 | In-plane C-H (ring), δ(C-H) | 532 nm: [33,35] 633 nm: [25,27] 785 nm: [26,32,34] |
| 1217 | — | Rocking of C-H bonds, δ(C–H) | 532 nm: [33] 633 nm: [2,26,34] |
| 1368 | 1366/1365 | Stretching of N-phenyl | 532 nm: [33,35] 633 nm: [25,27] 785 nm: [26,34] |
| 1395 | 1395 | Stretching of N-phenyl, δ(C–H), Stretching of the benzene ring | 532 nm: [33,35] 633 nm: [25] 785 nm: [26,32] |
| 1616 | 1615/1613 | Stretching of the benzene ring | 532 nm: [33,35] 633 nm: [25,27] 785 nm: [26,32,34] |
| ν633exp | ν633 | Vibration Type, Attribution | Refs. |
|---|---|---|---|
| 620 | 621 | Phenylalanine | [22] |
| 640 | 642 | Tyrosine | [22] |
| 712 | 713 | ν(C–S) | [3] |
| 762 | 764 | Tryptophan | [22] |
| 833 | 833/827 | Tyrosine/Tryptophan | [22,23] |
| 859 | 856 | Tyrosine | [23] |
| 880 | 883 | Tryptophan | [23] |
| 1005 | 1003/1004 | Phenylalanine | [3,22] |
| 1045 | 1035 | Phenylalanine | [22] |
| 1170 | 1178 | Tyrosine | [3] |
| 1241 | 1252/1248 | Various C-N (Amide III) | [3,22] |
| 1315 | 1319 | Histidine | [23] |
| 1355 | 1365 | Tryptophan | [23] |
| 1412 | 1409 | Lysine | [23] |
| 1451 | 1444/1449 | δ(CH3) in Methionine/Histidine/Lysine/Tryptophan | [3,22] |
| 1545 | 1556 | Tryptophan | [22] |
| —— | 1583/1587 | Phenylalanine | [3,22] |
| 1606 | 1609 | Phenylalanine | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Akhmetzhanov, T.; Pavlova, A.; Smirnov, E. Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection. Sensors 2023, 23, 6352. https://doi.org/10.3390/s23146352
Qi Z, Akhmetzhanov T, Pavlova A, Smirnov E. Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection. Sensors. 2023; 23(14):6352. https://doi.org/10.3390/s23146352
Chicago/Turabian StyleQi, Zhang, Timur Akhmetzhanov, Arina Pavlova, and Evgeny Smirnov. 2023. "Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection" Sensors 23, no. 14: 6352. https://doi.org/10.3390/s23146352
APA StyleQi, Z., Akhmetzhanov, T., Pavlova, A., & Smirnov, E. (2023). Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection. Sensors, 23(14), 6352. https://doi.org/10.3390/s23146352







