Human Olfactory Receptor Sensor for Odor Reconstitution
Abstract
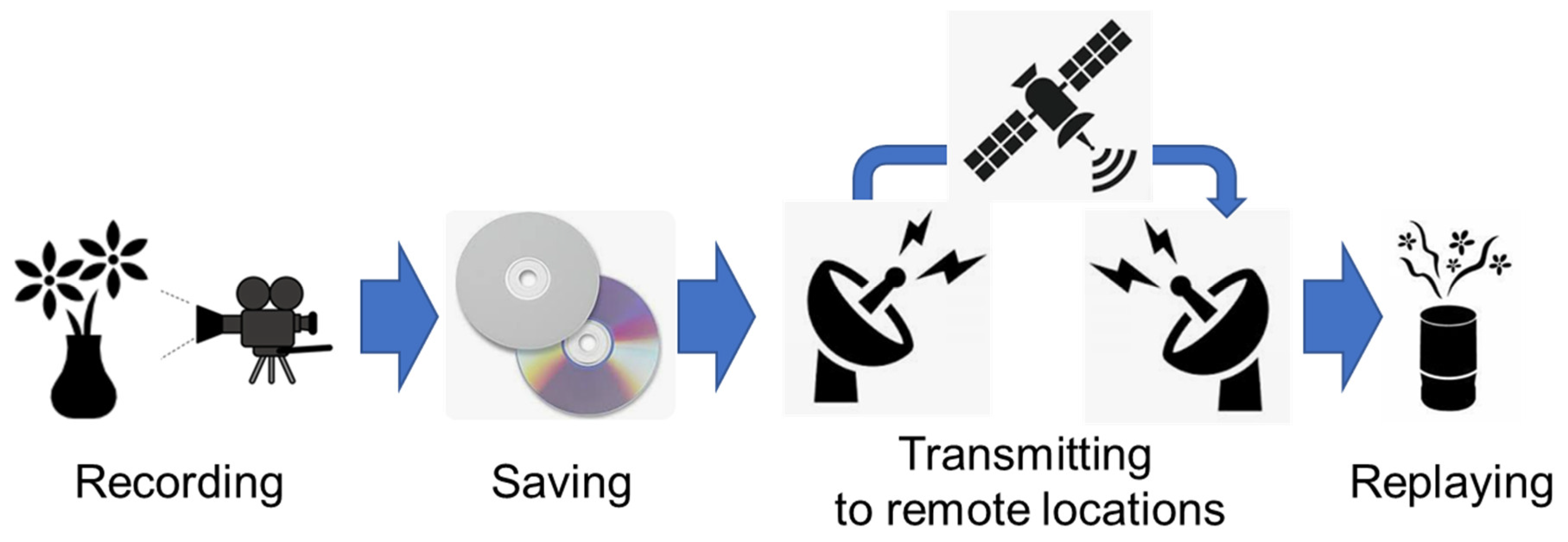
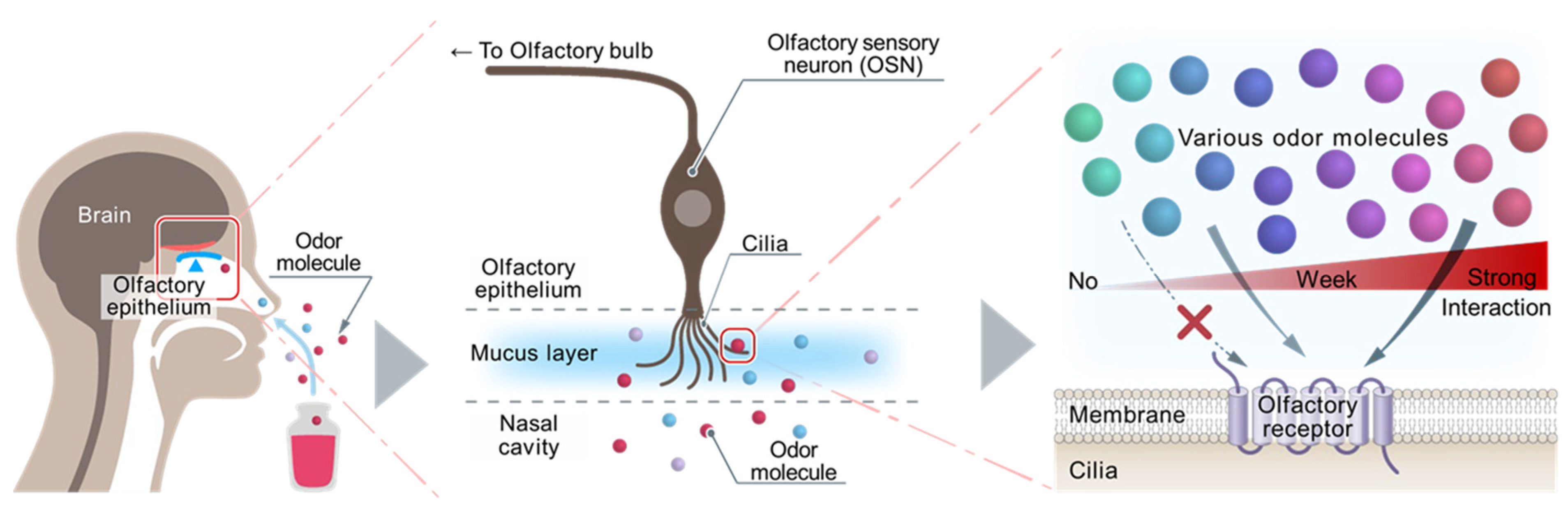
1. Introduction
2. Establishment of Heterologous Cells Expressing ORs
3. Importance of Measuring OR Response in Real Time
4. Human Olfactory Receptor-Expressing Cell Array Sensor (Human OR Sensor)
5. Towards Human Olfactory DX Realization
6. Preliminary Odor Reconstitution
7. Future Improvements of Human OR Sensor
8. Possible Alternatives for Human OR Sensor
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurot, C.; Brenet, S.; Buhot, A.; Barou, E.; Belloir, C.; Briand, L.; Hou, Y. Highly sensitive olfactory biosensors for the detection of volatile organic compounds by surface plasmon resonance imaging. Biosens. Bioelectron. 2019, 123, 230–236. [Google Scholar] [CrossRef]
- Pelosi, P.; Zhu, J.; Knoll, W. Odorant-binding proteins as sensing elements for odour monitoring. Sensors 2018, 18, 3248. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. A highly selective biosensor based on peptide directly derived from the HarmOBP7 aldehyde binding site. Sensors 2019, 19, 4284. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Colbert, D.; Cheema, J.; Malmström, J.; Kralicek, A.; Travas-Sejdic, J. An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants. Biosens. Bioelectron. 2019, 126, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshimoto, N.; Shimono, K.; Kuroda, S. Deciphering the receptor repertoire encoding specific odorants by time-lapse single-cell array cytometry. Sci. Rep. 2016, 6, 19934. [Google Scholar] [CrossRef]
- Peterlin, Z.; Firestein, S.; Rogers, M.E. The state of the art of odorant receptor deorphanization: A report from the orphanage. J. Gen. Physiol. 2014, 143, 527–542. [Google Scholar] [CrossRef]
- Lu, M.; Echeverri, F.; Moyer, B.D. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic 2003, 4, 416–433. [Google Scholar] [CrossRef]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 2004, 119, 679–691. [Google Scholar] [CrossRef]
- Wu, L.; Pan, Y.; Chen, G.Q.; Matsunami, H.; Zhuang, H. Receptor-transporting protein 1 short (RTP1S) mediates translocation and activation of odorant receptors by acting through multiple steps. J. Biol. Chem. 2012, 287, 22287–22294. [Google Scholar] [CrossRef]
- Shepard, B.D.; Natarajan, N.; Protzko, R.J.; Acres, O.W.; Pluznick, J.L. A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS ONE 2013, 8, e68758. [Google Scholar] [CrossRef]
- Noe, F.; Frey, T.; Fiedler, J.; Geithe, C.; Nowak, B.; Krautwurst, D. IL-6-HaloTag® enables live-cell plasma membrane staining, flow cytometry, functional expression, and de-orphaning of recombinant odorant receptors. J. Biol. Methods 2017, 4, e81. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Uberti, M.A.; Chen, Z.; Bush, C.F.; Jones, S.V.; Ressler, K.J.; Hall, R.A.; Minneman, K.P. Olfactory receptor surface expression is driven by association with the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 13672–13676. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Matsunami, H. Activation state of the M3 muscarinic acetylcholine receptor modulates mammalian odorant receptor signaling. Sci. Signal 2011, 4, ra1. [Google Scholar] [CrossRef] [PubMed]
- Mashukova, A.; Spehr, M.; Hatt, H.; Neuhaus, E.M. β-arrestin 2-mediated internalization of mammalian odorant receptors. J. Neurosci. 2006, 26, 9902–9912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.R.; Tian, H.; Ma, M.; Matsunami, H. Muscarinic acetylcholine receptor M3 modulates odorant receptor activity via inhibition of β-arrestin-2 recruitment. Nat. Commun. 2015, 6, 6448. [Google Scholar] [CrossRef]
- Jones, D.T.; Reed, R.R. Golf: An olfactory neuron specific-G protein involved in odorant signal transduction. Science 1989, 244, 790–795. [Google Scholar] [CrossRef]
- Papasergi, M.M.; Patel, B.R.; Tall, G.G. The G protein α chaperone Ric-8 as a potential therapeutic target. Mol. Pharmacol. 2015, 87, 52–63. [Google Scholar] [CrossRef]
- Kida, H.; Fukutani, Y.; Mainland, J.D.; de March, C.A.; Vihani, A.; Li, Y.R.; Chi, Q.; Toyama, A.; Liu, L.; Kameda, M.; et al. Vapor detection and discrimination with a panel of odorant receptors. Nat. Commun. 2018, 9, 4556. [Google Scholar] [CrossRef]
- Saito, H.; Chi, Q.; Zhuang, H.; Matsunami, H.; Mainland, J.D. Odor coding by a Mammalian receptor repertoire. Sci. Signal. 2009, 2, ra9. [Google Scholar] [CrossRef]
- Ikegami, K.; de March, C.A.; Nagai, M.H.; Ghosh, S.; Do, M.; Sharma, R.; Bruguera, E.S.; Lu, Y.E.; Fukutani, Y.; Vaidehi, N.; et al. Structural instability and divergence from conserved residues underlie intracellular retention of mammalian odorant receptors. Proc. Natl. Acad. Sci. USA 2020, 117, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Fukutani, Y.; Nakamura, Y.; Muto, N.; Miyanaga, S.; Kanemaki, R.; Ikegami, K.; Noguchi, K.; Ohsawa, I.; Matsunami, H.; Yohda, M. Hot spot mutagenesis improves the functional expression of unique mammalian odorant receptors. Int. J. Mol. Sci. 2021, 23, 277. [Google Scholar] [CrossRef]
- Menini, A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999, 9, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Takai, E.; Hattori, S.; Tatematsu, K.; Kuroda, S. Effects of 3-octen-2-one on human olfactory receptor responses to vanilla flavor. Biosci. Biotechnol. Biochem. 2022, 86, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Emkey, R.; Rankl, N.B. Screening G protein-coupled receptors: Measurement of intracellular calcium using the fluometric imaging plate reader. Methods Mol. Biol. 2009, 565, 145–158. [Google Scholar] [CrossRef]
- Shirokova, E.; Schmiedeberg, K.; Bedner, P.; Niessen, H.; Willecke, K.; Raguse, J.D.; Meyerhof, W.; Krautwurst, D. Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J. Biol. Chem. 2005, 280, 11807–11815. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Moberly, A.H.; Adipietro, K.A.; Liu, W.L.; Zhuang, H.; et al. The missense of smell: Functional variability in the human odorant receptor repertoire. Nat. Neurosci. 2014, 17, 114–120. [Google Scholar] [CrossRef]
- Jaeger, S.R.; McRae, J.F.; Bava, C.M.; Beresford, M.K.; Hunter, D.; Jia, Y.; Chheang, S.L.; Jin, D.; Peng, M.; Gamble, J.C.; et al. A mendelian trait for olfactory sensitivity affects odor experience and food selection. Curr. Biol. 2013, 23, 1601–1605. [Google Scholar] [CrossRef]
- Trimmer, C.; Keller, A.; Murphy, N.R.; Snyder, L.L.; Willer, J.R.; Nagai, M.H.; Katsanis, N.; Vosshall, L.B.; Matsunami, H.; Mainland, J.D. Genetic variation across the human olfactory receptor repertoire alters odor perception. Proc. Natl. Acad. Sci. USA 2019, 116, 9475–9480. [Google Scholar] [CrossRef]
- Le Magnen, J. Odeurs et Parfums, 2e ed.; Presses Universitaires de France: Paris, France, 1961; pp. 128–137. [Google Scholar]
- Debnath, T.; Nakamoto, T. Extraction of sensing data for desired scent impressions using mass spectra of odorant molecules. Sci. Rep. 2022, 12, 16297. [Google Scholar] [CrossRef]
- Home Page of Komi Hakko Corp. Available online: https://komi-hakko.co.jp/archives/393 (accessed on 24 May 2023).
- Gupta, P.; Albeanu, D.F.; Bhalla, U.S. Olfactory bulb coding of odors, mixtures and sniffs is a linear sum of odor time profiles. Nat. Neurosci. 2015, 18, 272–281. [Google Scholar] [CrossRef]
- Reddy, G.; Zak, J.D.; Vergassola, M.; Murthy, V.N. Antagonism in olfactory receptor neurons and its implications for the perception of odor mixtures. Elife 2018, 7, e34958. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Murphy, N.R.; Balasubramanian, V.; Mainland, J.D. Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc. Natl. Acad. Sci. USA 2019, 116, 9598–9603. [Google Scholar] [CrossRef]
- Inagaki, S.; Iwata, R.; Iwamoto, M.; Imai, T. Widespread inhibition, antagonism, and synergy in mouse olfactory sensory neurons in vivo. Cell Rep. 2020, 31, 107814. [Google Scholar] [CrossRef] [PubMed]
- Cygnar, K.D.; Zhao, H. Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat. Neurosci. 2009, 12, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, A.Z.; Chan, G.C.; Baker, L.P.; Impey, S.; Beavo, J.A.; Storm, D.R. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in Neurons: A mechanism for attenuation of olfactory signals. Neuron 1998, 21, 495–504. [Google Scholar] [CrossRef]
- Castillo, K.; Delgado, R.; Bacigalupo, J. Plasma membrane Ca(2+)-ATPase in the cilia of olfactory receptor neurons: Possible role in Ca(2+) clearance. Eur. J. Neurosci. 2007, 26, 2524–2531. [Google Scholar] [CrossRef]
- Matarazzo, V.; Zsürger, N.; Guillemot, J.C.; Clot-Faybesse, O.; Botto, J.M.; Dal Farra, C.; Crowe, M.; Demaille, J.; Vincent, J.P.; Mazella, J.; et al. Porcine odorant-binding protein selectively binds to a human olfactory receptor. Chem. Senses 2002, 27, 691–701. [Google Scholar] [CrossRef]
- Nakashima, N.; Nakashima, K.; Taura, A.; Takaku-Nakashima, A.; Ohmori, H.; Takano, M. Olfactory marker protein directly buffers cAMP to avoid depolarization-induced silencing of olfactory receptor neurons. Nat. Commun. 2020, 11, 2188. [Google Scholar] [CrossRef]
- Nakashima, N.; Nakashima, K.; Nakashima, A.; Takano, M. Olfactory marker protein elevates basal cAMP concentration. Biochem. Biophys. Res. Commun. 2020, 531, 203–208. [Google Scholar] [CrossRef]
- Mayberry, C.L.; Wilczek, M.P.; Fong, T.M.; Nichols, S.L.; Maginnis, M.S. GRK2 mediates β-arrestin interactions with 5-HT2 receptors for JC polyomavirus endocytosis. J. Virol. 2021, 95, e02139-20. [Google Scholar] [CrossRef] [PubMed]
- Wallrabenstein, I.; Kuklan, J.; Weber, L.; Zborala, S.; Werner, M.; Altmüller, J.; Becker, C.; Schmidt, A.; Hatt, H.; Hummel, T.; et al. Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS ONE 2013, 8, e54950. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Q. TAAR agonists. Cell. Mol. Neurobiol. 2020, 40, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Gisladottir, R.S.; Ivarsdottir, E.V.; Helgason, A.; Jonsson, L.; Hannesdottir, N.K.; Rutsdottir, G.; Arnadottir, G.A.; Skuladottir, A.; Jonsson, B.A.; Norddahl, G.L.; et al. Sequence variants in TAAR5 and other loci affect human odor perception and naming. Curr. Biol. 2020, 30, 4643–4653.e3. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Verbeurgt, C.; Wilkin, F.; Tarabichi, M.; Gregoire, F.; Dumont, J.E.; Chatelain, P. Profiling of olfactory receptor gene expression in whole human olfactory mucosa. PLoS ONE 2014, 9, e96333. [Google Scholar] [CrossRef]
- Olender, T.; Keydar, I.; Pinto, J.M.; Tatarskyy, P.; Alkelai, A.; Chien, M.S.; Fishilevich, S.; Restrepo, D.; Matsunami, H.; Gilad, Y.; et al. The human olfactory transcriptome. BMC Genom. 2016, 17, 619. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Riveros-McKay, F.; Mezzavilla, M.; Abou-Moussa, E.H.; Arayata, C.J.; Makhlouf, M.; Trimmer, C.; Ibarra-Soria, X.; Khan, M.; Van Gerven, L.; et al. A transcriptomic atlas of mammalian olfactory mucosae reveals an evolutionary influence on food odor detection in humans. Sci. Adv. 2019, 5, eaax0396. [Google Scholar] [CrossRef]
- Marenco, L.; Wang, R.; McDougal, R.; Olender, T.; Twik, M.; Bruford, E.; Liu, X.; Zhang, J.; Lancet, D.; Shepherd, G.; et al. ORDB, HORDE, ODORactor and other on-line knowledge resources of olfactory receptor-odorant interactions. Database 2016, 2016, baw132. [Google Scholar] [CrossRef]
- Liu, X.; Su, X.; Wang, F.; Huang, Z.; Wang, Q.; Li, Z.; Zhang, R.; Wu, L.; Pan, Y.; Chen, Y.; et al. ODORactor: A web server for deciphering olfactory coding. Bioinformatics 2011, 27, 2302–2303. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Saha, B.K.; Kumar, R.; Varadwaj, P.K. OlfactionBase: A repository to explore odors, odorants, olfactory receptors and odorant-receptor interactions. Nucleic Acids Res. 2022, 50, D678–D686. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Ren, W.; Pacalon, J.; Xu, R.; Xu, L.; Li, X.; de March, C.A.; Matsunami, H.; Yu, H.; Yu, Y.; et al. Large-scale G protein-coupled olfactory receptor-ligand pairing. ACS Cent. Sci. 2022, 8, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Semwal, R.; Aier, I.; Tyagi, P.; Varadwaj, P.K. DeepOlf: Deep neural network based architecture for predicting odorants and their interacting olfactory receptors. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 418–428. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Hu, J.; Wang, T.; Liu, D.; Dong, J.; Lu, Y. Artificial olfactory biohybrid system: An evolving sense of smell. Adv. Sci. 2023, 10, e2204726. [Google Scholar] [CrossRef]
- Son, M.; Cho, D.G.; Lim, J.H.; Park, J.; Hong, S.; Ko, H.J.; Park, T.H. Real-time monitoring of geosmin and 2-methylisoborneol, representative odor compounds in water pollution using bioelectronic nose with human-like performance. Biosens. Bioelectron. 2015, 74, 199–206. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Ko, H.J.; Hong, S.; Park, T.H. A portable and multiplexed bioelectronic sensor using human olfactory and taste receptors. Biosens. Bioelectron. 2017, 87, 901–907. [Google Scholar] [CrossRef]
- Lee, M.; Yang, H.; Kim, D.; Yang, M.; Park, T.H.; Hong, S. Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose. Sci. Rep. 2018, 8, 13945. [Google Scholar] [CrossRef]
- Castro, J.B.; Ramanathan, A.; Chennubhotla, C.S. Categorical dimensions of human odor descriptor space revealed by non-negative matrix factorization. PLoS ONE 2013, 8, e73289. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, S.; Nakaya-Kishi, Y.; Tatematsu, K.; Hinuma, S. Human Olfactory Receptor Sensor for Odor Reconstitution. Sensors 2023, 23, 6164. https://doi.org/10.3390/s23136164
Kuroda S, Nakaya-Kishi Y, Tatematsu K, Hinuma S. Human Olfactory Receptor Sensor for Odor Reconstitution. Sensors. 2023; 23(13):6164. https://doi.org/10.3390/s23136164
Chicago/Turabian StyleKuroda, Shun’ichi, Yukiko Nakaya-Kishi, Kenji Tatematsu, and Shuji Hinuma. 2023. "Human Olfactory Receptor Sensor for Odor Reconstitution" Sensors 23, no. 13: 6164. https://doi.org/10.3390/s23136164
APA StyleKuroda, S., Nakaya-Kishi, Y., Tatematsu, K., & Hinuma, S. (2023). Human Olfactory Receptor Sensor for Odor Reconstitution. Sensors, 23(13), 6164. https://doi.org/10.3390/s23136164







