Spatial Multiplexing of Whispering Gallery Mode Sensors
Abstract
1. Introduction
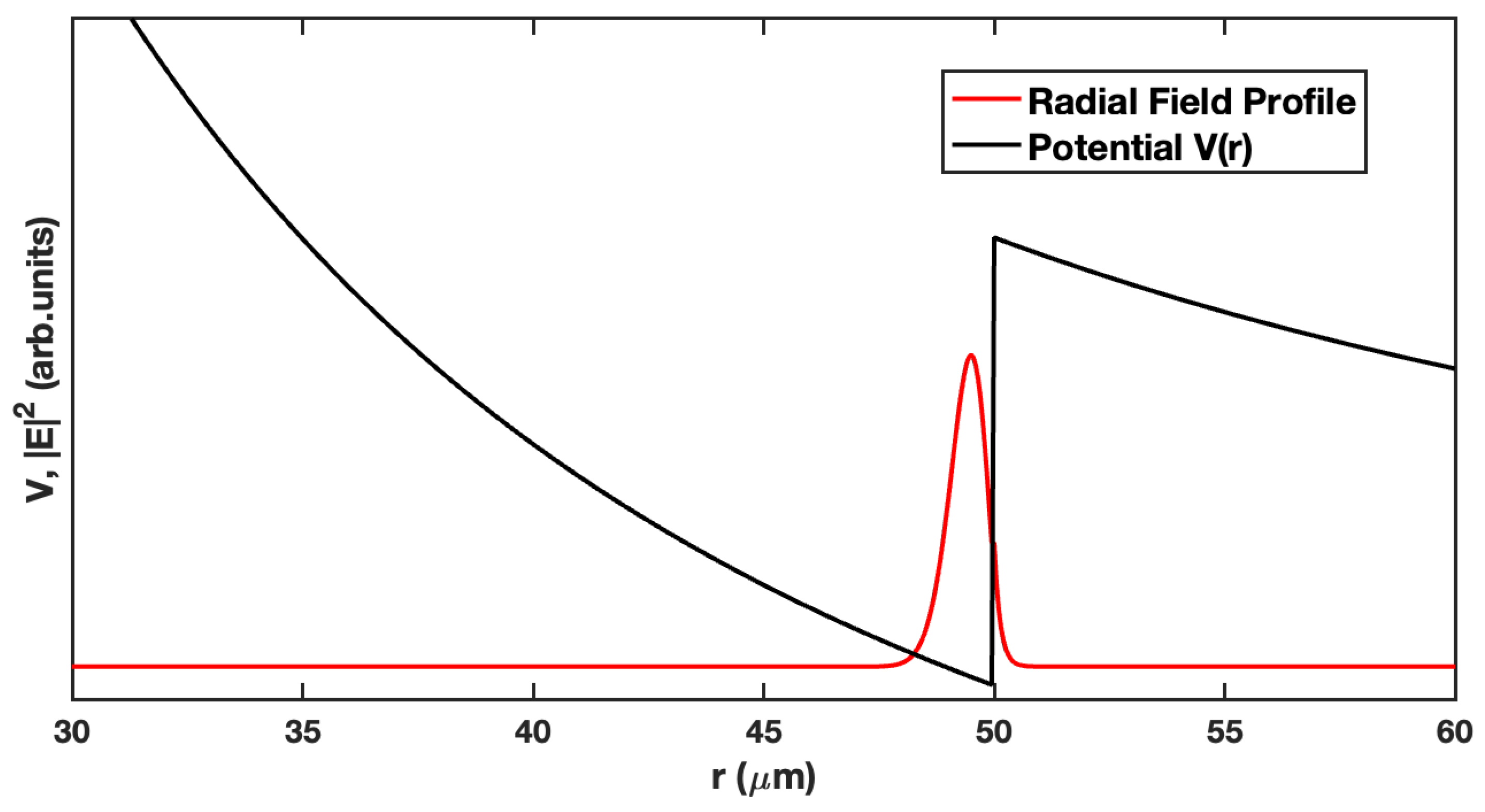
2. The Whispering Gallery Mode Biosensor
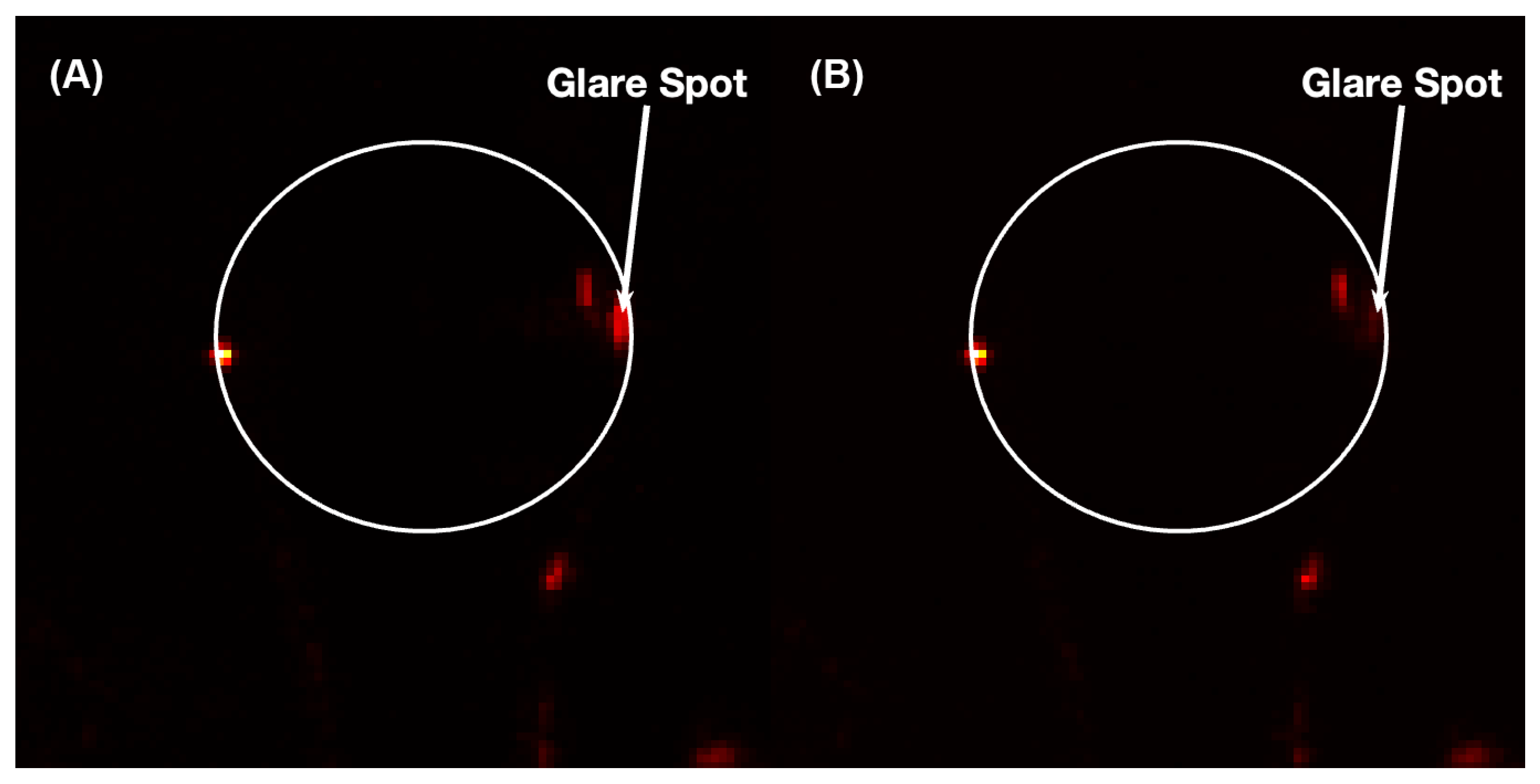
3. Experimental Apparatus
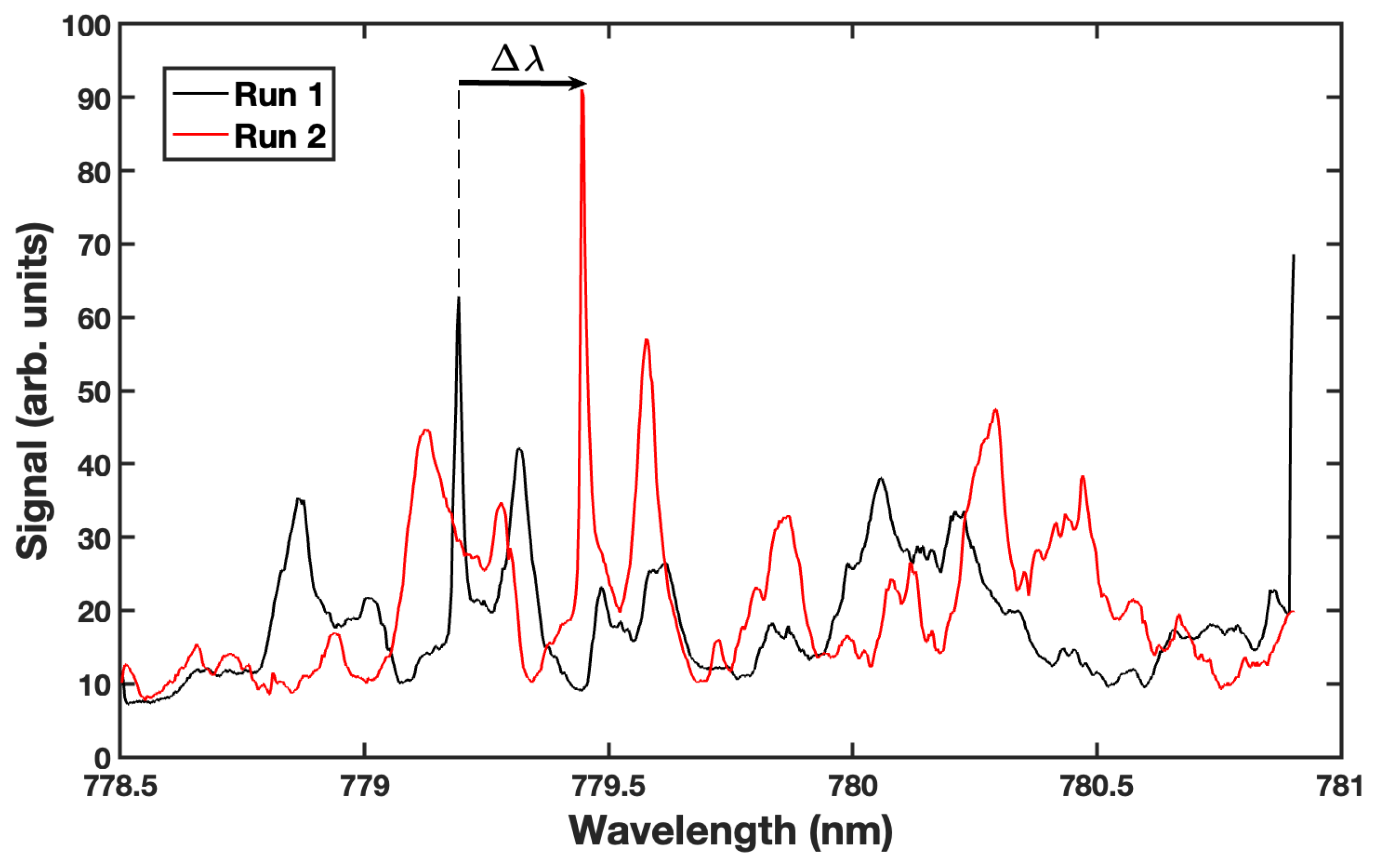
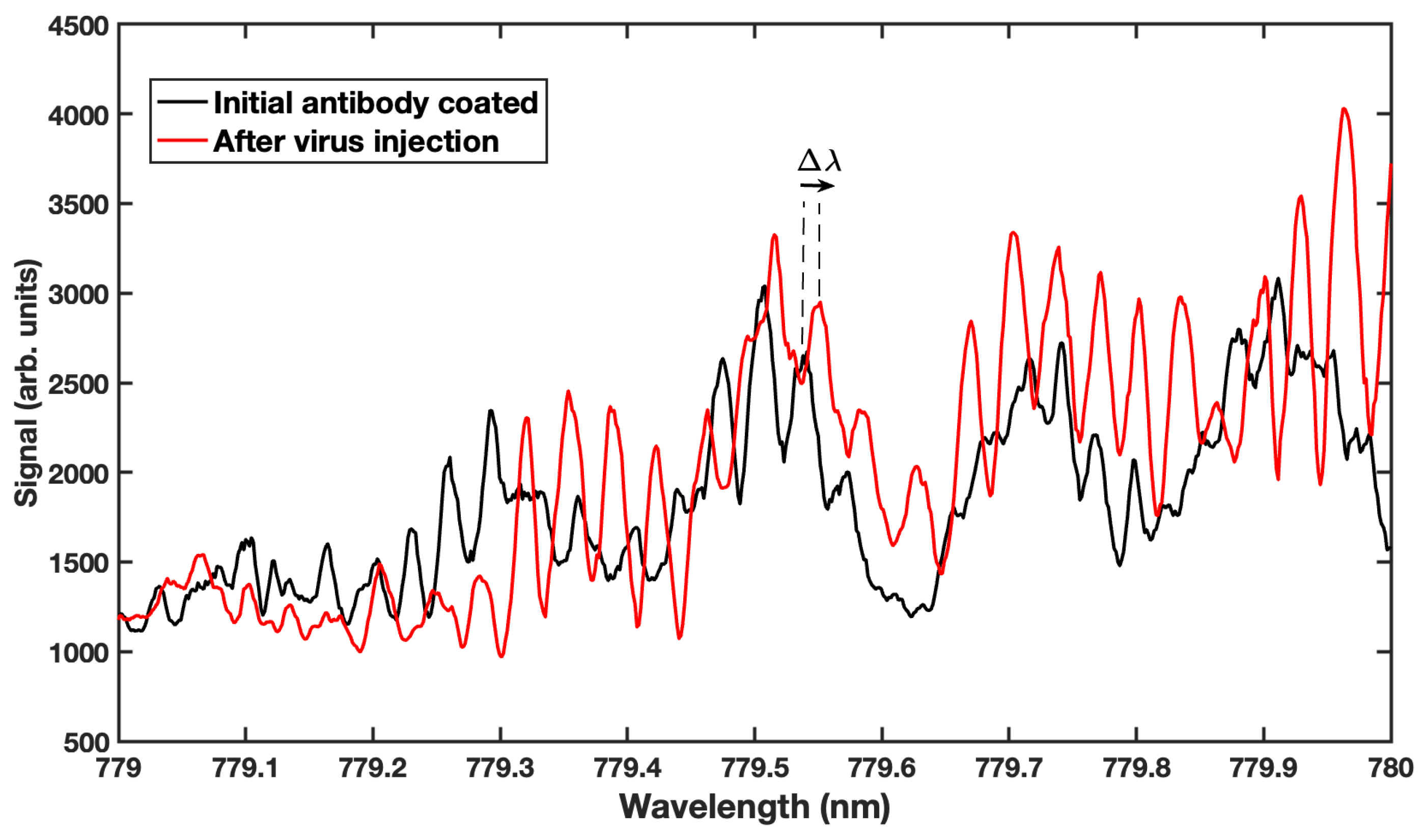
4. Results and Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFB | Distributed Feedback |
| HPV | Human Paplillomavirus |
| MDR | Morphology-Dependent Resonance |
| RSP | Reactive Sensing Principle |
| TIR | Total Internal Reflection |
| WGM | Whispering Gallery Mode |
References
- Fauci, A.S.; Lane, H.C.; Redfield, R.R. COVID-19—Navigating the Uncharted. N. Engl. J. Med. 2020, 382, 1268–1269. [Google Scholar] [CrossRef]
- Ferreira, M.F.S.; Castro-Camus, E.; Ottaway, D.J.; López-Higuera, J.M.; Feng, X.; Jin, W.; Jeong, Y.; Picqué, N.; Tong, L.; Reinhard, B.M.; et al. Roadmap on optical sensors. J. Opt. 2017, 19, 083001. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, Y.; Li, Y.; Miao, Y.; Gao, S.; Lin, F.; Deng, Y.; Geng, L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens. Bioelectron. 2019, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Holler, S.; Speck, M. Spatial multiplexing of whispering gallery mode sensors for trace species detection. In Proceedings of the Advanced Environmental, Chemical, and Biological Sensing Technologies XIII; Vo-Dinh, T., Lieberman, R.A., Gauglitz, G.G., Eds.; International Society for Optics and Photonics; SPIE: Bellingham, WA, USA, 2016; Volume 9862, p. 986203. [Google Scholar] [CrossRef]
- Hill, S.C.; Benner, R.E. Morphology-Dependent Resonances. In Optical Effects Associated with Small Particles; Barber, P.W., Chang, R.K., Eds.; World Scientific: Singapore, 1988; Chapter 1; pp. 1–61. [Google Scholar]
- Zhang, J.X.; Aiello, D.; Aker, P.M. A spectroscopic tour through the liquid aerosol interface: Implications for atmospheric chemistry. J. Geophys. Res. Atmos. 1994, 99, 25667–25672. [Google Scholar] [CrossRef]
- Arnold, S.; Holler, S.; Druger, S.D. Imaging enhanced energy transfer in a levitated aerosol particle. J. Chem. Phys. 1996, 104, 7741–7748. [Google Scholar] [CrossRef]
- Mitchem, L.; Buajarern, J.; Ward, A.D.; Reid, J.P. A Strategy for Characterizing the Mixing State of Immiscible Aerosol Components and the Formation of Multiphase Aerosol Particles through Coagulation. J. Phys. Chem. B 2006, 110, 13700–13703. [Google Scholar] [CrossRef]
- Moridnejad, A.; Preston, T.C.; Krieger, U.K. Tracking Water Sorption in Glassy Aerosol Particles using Morphology-Dependent Resonances. J. Phys. Chem. A 2017, 121, 8176–8184. [Google Scholar] [CrossRef]
- Qian, S.X.; Snow, J.B.; Tzeng, H.M.; Chang, R.K. Lasing Droplets: Highlighting the Liquid-Air Interface by Laser Emission. Science 1986, 231, 486–488. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tomisawa, H.; Kido, J. Ultra-low-threshold europium chelate laser in morphology-dependent resonances. Appl. Phys. Lett. 1995, 66, 1578–1580. [Google Scholar] [CrossRef]
- Wei, G.Q.; Wang, X.D.; Liao, L.S. Recent Advances in Organic Whispering-Gallery Mode Lasers. Laser Photonics Rev. 2020, 14, 2000257. [Google Scholar] [CrossRef]
- Arnold, S.; Khoshsima, M.; Teraoka, I.; Holler, S.; Vollmer, F. Shift of whispering-gallery modes in microspheres by protein adsorption. Opt. Lett. 2003, 28, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Ioppolo, T.; Ayaz, U.K.; Ötügen, M.V. High-resolution force sensor based on morphology dependent optical resonances of polymeric spheres. J. Appl. Phys. 2009, 105, 013535. [Google Scholar] [CrossRef]
- Rahman, A. Temperature sensor based on dielectric optical microresonator. Opt. Fiber Technol. 2011, 17, 536–540. [Google Scholar] [CrossRef]
- Ali, A.R.; Ioppolo, T. Effect of Angular Velocity on Sensors Based on Morphology Dependent Resonances. Sensors 2014, 14, 7041–7048. [Google Scholar] [CrossRef]
- Xia, R.L.; Liu, B.; Hu, Y.; Liu, J.; Fu, Y.; He, X.D.; Lu, P.; Farrell, G.; Yuan, J.; Wu, Q. Rapid Detection of SARS-CoV-2 Nucleocapsid Protein by a Label-free biosensor based on Optical Fiber Cylindrical Micro-resonator. IEEE Sens. J. 2023, 23, 12511–12518. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Johnson, B.R. Theory of morphology-dependent resonances: Shape resonances and width formulas. J. Opt. Soc. Am. A 1993, 10, 343–352. [Google Scholar] [CrossRef]
- Chowdhury, D.Q.; Hill, S.C.; Mazumder, M.M. Quality factors and effective-average modal gain or loss in inhomogeneous spherical resonators: Application to two-photon absorption. IEEE J. Quantum Electron. 1993, 29, 2553–2561. [Google Scholar] [CrossRef]
- Zhu, J.; Özdemir, S.K.; He, L.; Chen, D.R.; Yang, L. Single virus and nanoparticle size spectrometry by whispering-gallery-mode microcavities. Opt. Exp. 2011, 19, 16195. [Google Scholar] [CrossRef]
- Stoian, R.I.; Bui, K.; Rosenberger, A. Silica hollow bottle resonators for use as whispering gallery mode based chemical sensors. J. Opt. 2015, 17, 125001. [Google Scholar] [CrossRef]
- Malmir, K.; Habibiyan, H.; Ghafoorifard, H. An ultrasensitive optical label-free polymeric biosensor based on concentric triple microring resonators with a central microdisk resonator. Opt. Commun. 2016, 365, 150–156. [Google Scholar] [CrossRef]
- Ghali, H.; Chibli, H.; Nadeau, J.L.; Peter, Y.A. Real-Time Detection of Staphylococcus Aureus Using Whispering Gallery Mode Optical Microdisks. Biosensors 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Ajad, A.K.; Islam, J.; Kaysir, R.; Atai, J. Highly sensitive bio sensor based on WGM ring resonator for hemoglobin detection in blood samples. Optik 2021, 226, 166009. [Google Scholar] [CrossRef]
- Suebka, S.; Nguyen, P.D.; Gin, A.; Su, J. How Fast It Can Stick: Visualizing Flow Delivery to Microtoroid Biosensors. ACS Sens. 2021, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Meth. 2008, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Dantham, V.R.; Holler, S.; Kolchenko, V.; Wan, Z.; Arnold, S. Taking whispering gallery-mode single virus detection and sizing to the limit. Appl. Phys. Lett. 2012, 101, 043704. [Google Scholar] [CrossRef]
- Keng, D.; Tan, X.; Arnold, S. Whispering gallery micro-global positioning system for nanoparticle sizing in real time. Appl. Phys. Lett. 2014, 105, 071105. [Google Scholar] [CrossRef]
- Foreman, M.R.; Keng, D.; Treasurer, E.; Lopez, J.R.; Arnold, S. Whispering gallery mode single nanoparticle detection and sizing: The validity of the dipole approximation. Opt. Lett. 2017, 42, 963–966. [Google Scholar] [CrossRef]
- Graybill, R.M.; Para, C.S.; Bailey, R.C. PCR-free, Multiplexed Expression Profiling of microRNAs using Silicon Photonic Microring Resonators. Anal. Chem. 2016, 88, 10347–10351. [Google Scholar] [CrossRef]
- Serpengüzel, A.; Griffel, G.; Arnold, S. Excitation of resonances of microspheres on an optical fiber. Opt. Lett. 1995, 20, 654–656. [Google Scholar] [CrossRef]
- Gorodetsky, M.; Ilchenko, V. High-Q optical whispering-gallery microresonators: Precession approach for spherical mode analysis and emission patterns with prism couplers. Opt. Comm. 1994, 113, 133–143. [Google Scholar] [CrossRef]
- Knight, J.C.; Cheung, G.; Jacques, F.; Birks, T.A. Phase-matched excitation of whispering-gallery-mode resonances by a fiber taper. Opt. Lett. 1997, 22, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.L.; Chang, R.K. Highly efficient prism coupling to whispering gallery modes of a square μ-cavity. Appl. Phys. Lett. 2003, 82, 487–489. [Google Scholar] [CrossRef]
- Huckabay, H.A.; Dunn, R.C. Whispering gallery mode imaging for the multiplexed detection of biomarkers. Sens. Actuators B Chem. 2011, 160, 1262–1267. [Google Scholar] [CrossRef]
- Huckabay, H.A.; Wildgen, S.M.; Dunn, R.C. Label-free detection of ovarian cancer biomarkers using whispering gallery mode imaging. Biosens. Bioelectron. 2013, 45, 223–229. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef]
- Garrett, C.G.B.; Kaiser, W.; Bond, W.L. Stimulated. Emission into Optical Whispering Modes of Spheres. Phys. Rev. 1961, 124, 1807–1809. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Observation of optical resonances of dielectric spheres by light scattering. Appl. Opt. 1981, 20, 1803–1814. [Google Scholar] [CrossRef]
- Arnold, S.; Hill, S.C.; Holler, S.; Li, J.H.; Serpengüzel, A.; Auffermann, W.F. Aerosol particle microphotography and glare-spot absorption spectroscopy. Opt. Lett. 1995, 20, 773–775. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holler, S.; Speck, M. Spatial Multiplexing of Whispering Gallery Mode Sensors. Sensors 2023, 23, 5925. https://doi.org/10.3390/s23135925
Holler S, Speck M. Spatial Multiplexing of Whispering Gallery Mode Sensors. Sensors. 2023; 23(13):5925. https://doi.org/10.3390/s23135925
Chicago/Turabian StyleHoller, Stephen, and Matthew Speck. 2023. "Spatial Multiplexing of Whispering Gallery Mode Sensors" Sensors 23, no. 13: 5925. https://doi.org/10.3390/s23135925
APA StyleHoller, S., & Speck, M. (2023). Spatial Multiplexing of Whispering Gallery Mode Sensors. Sensors, 23(13), 5925. https://doi.org/10.3390/s23135925






