Quantifying Hand Strength and Isometric Pinch Individuation Using a Flexible Pressure Sensor Grid
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval of Subjects
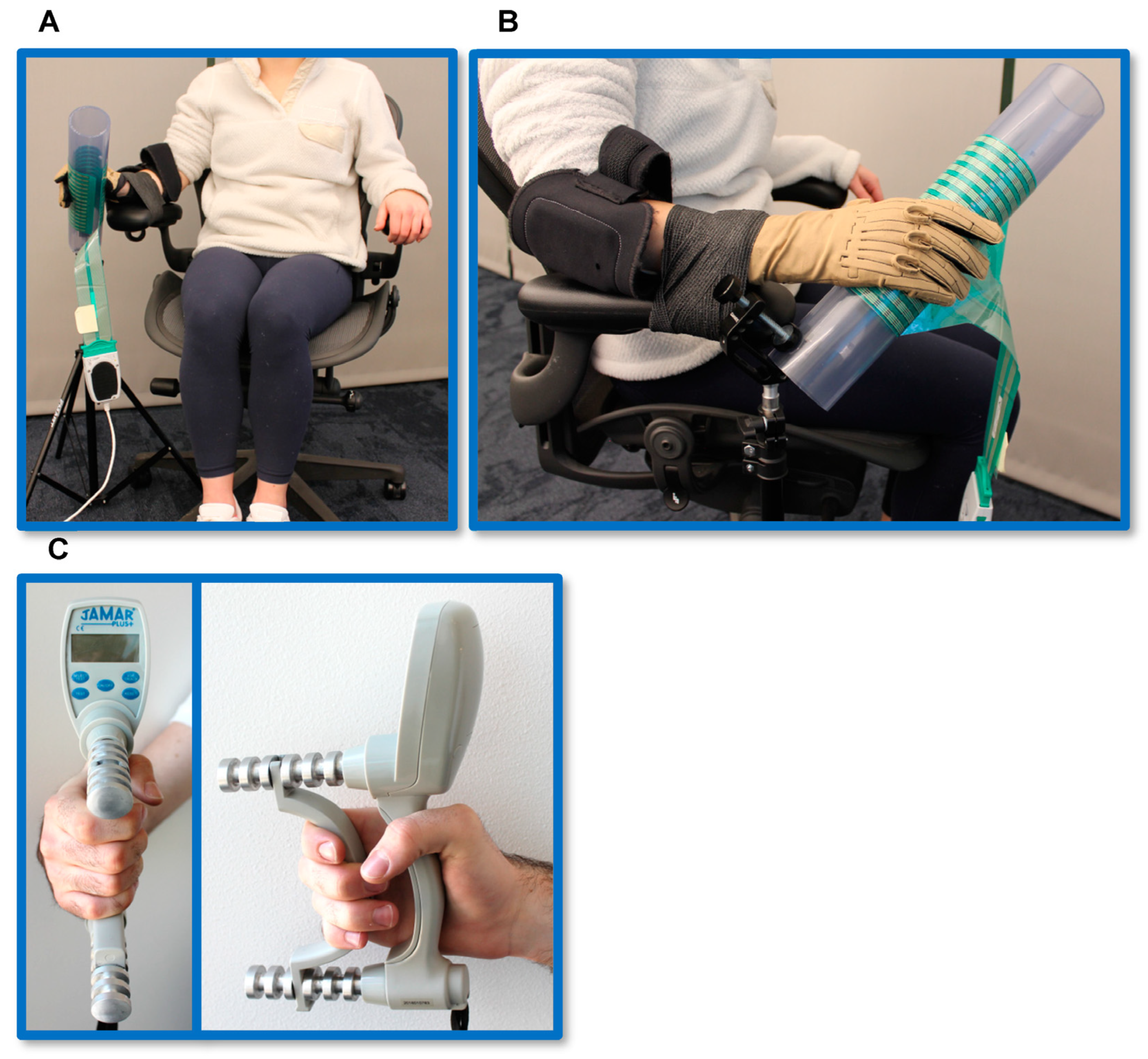
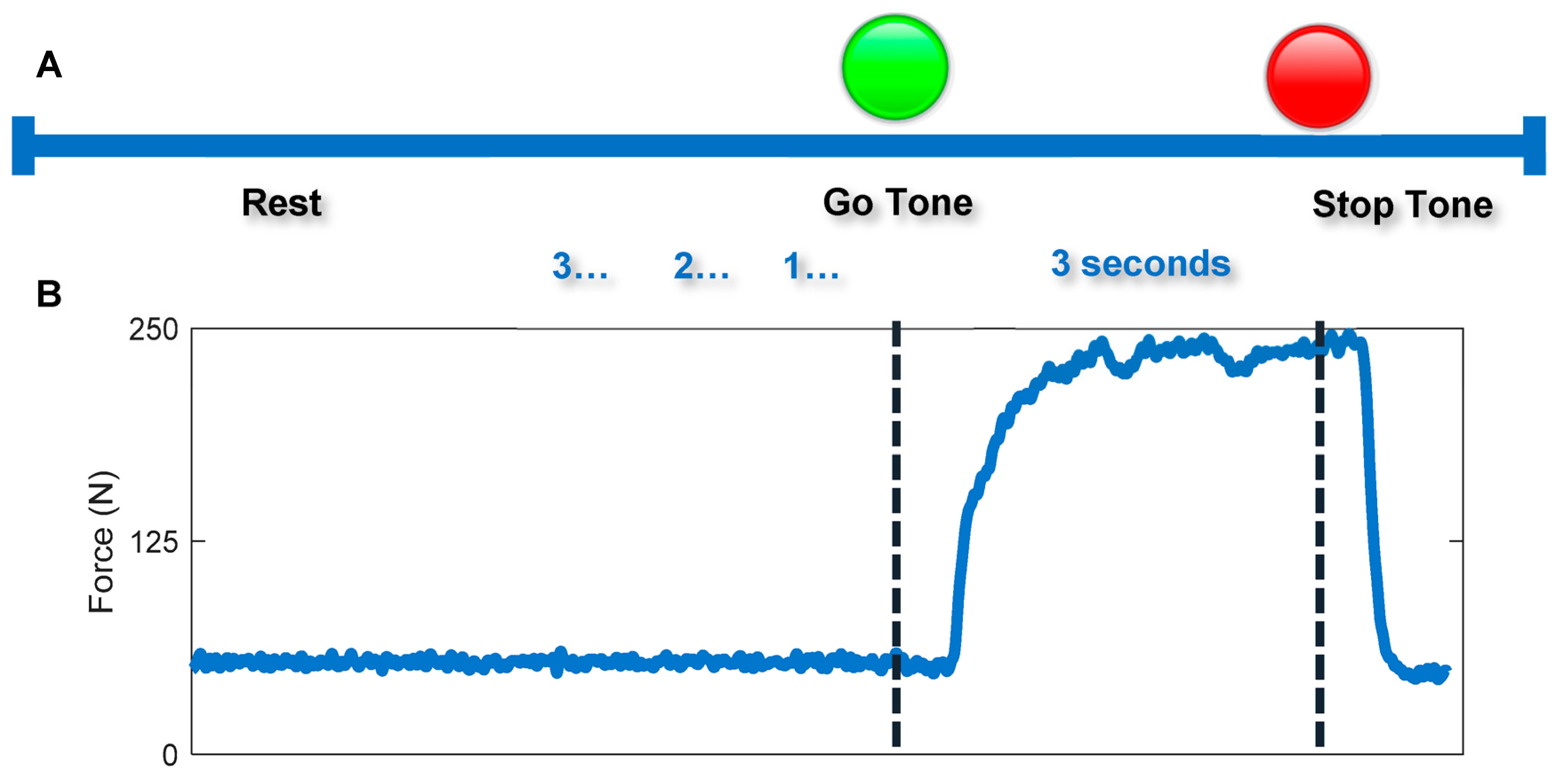
2.2. Isometric Pinch and Grasp Tasks
2.3. Data Analysis
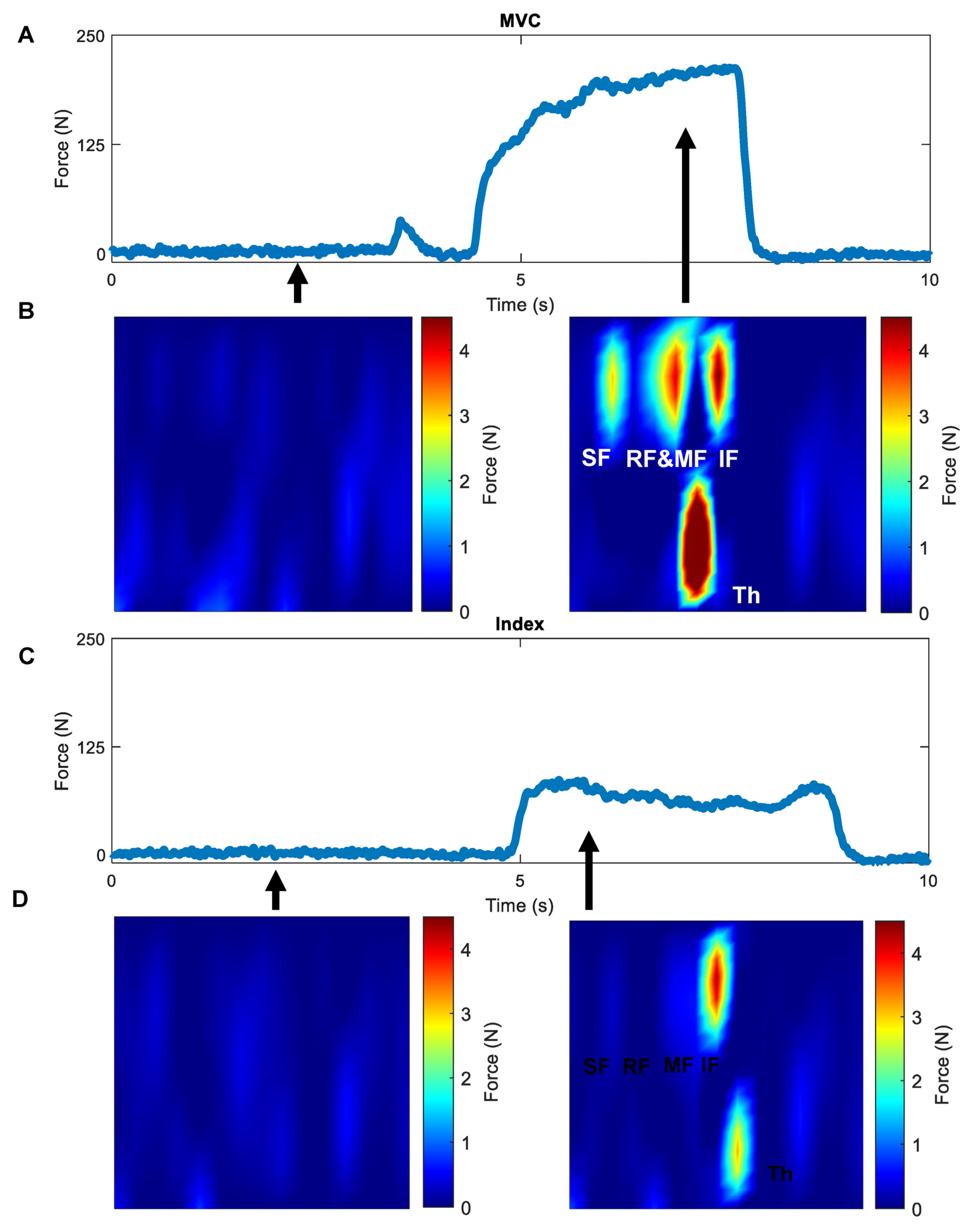
2.3.1. Whole-Hand Grasp Strength
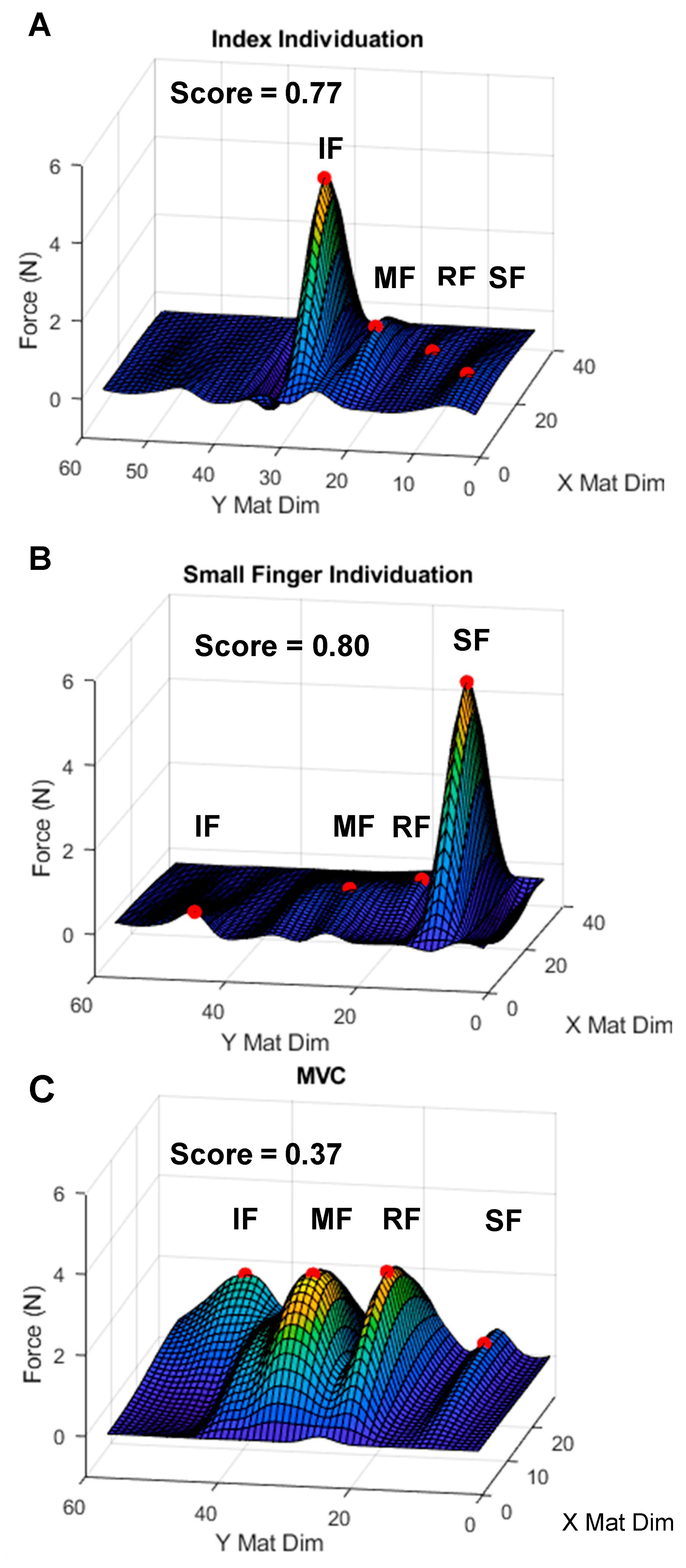
2.3.2. Isometric Pinch Individuation
- Isometric Individuation Score (IIS)
3. Results
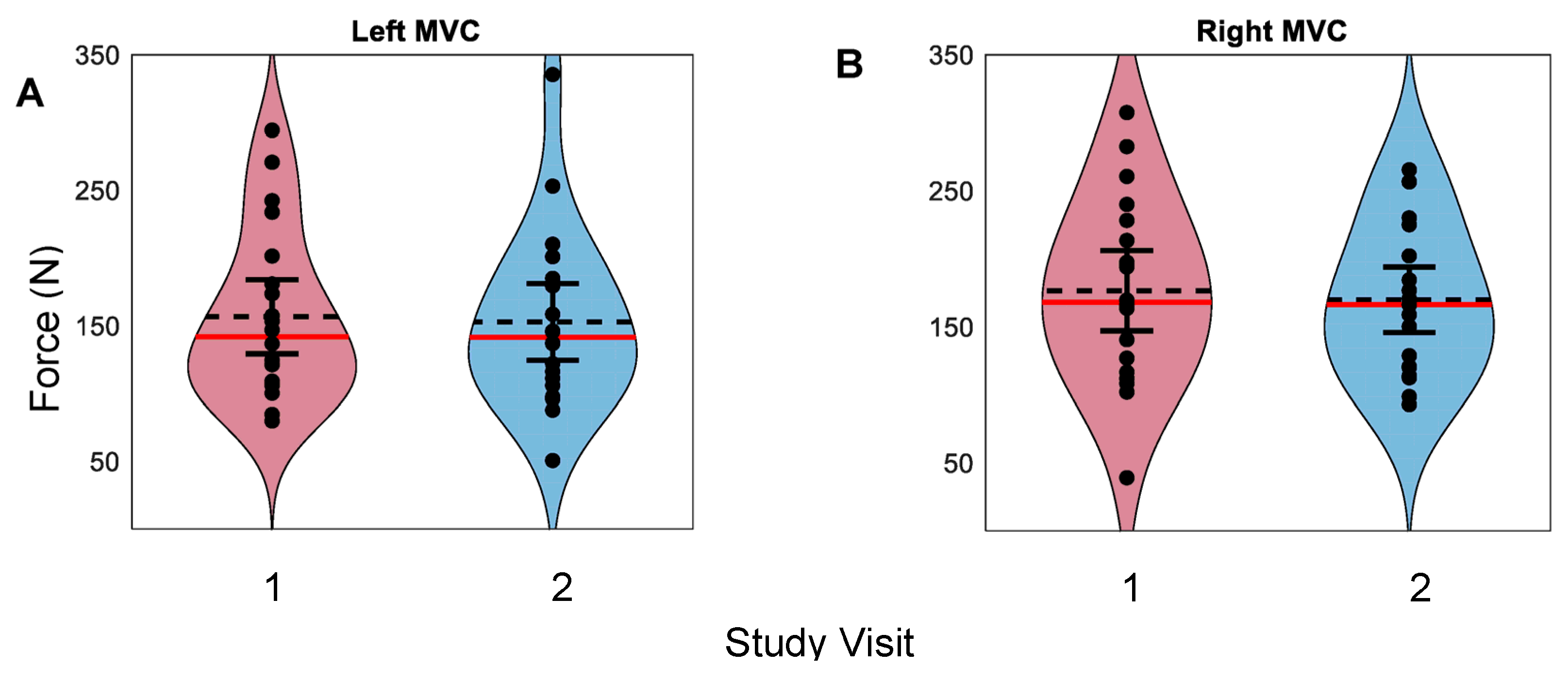
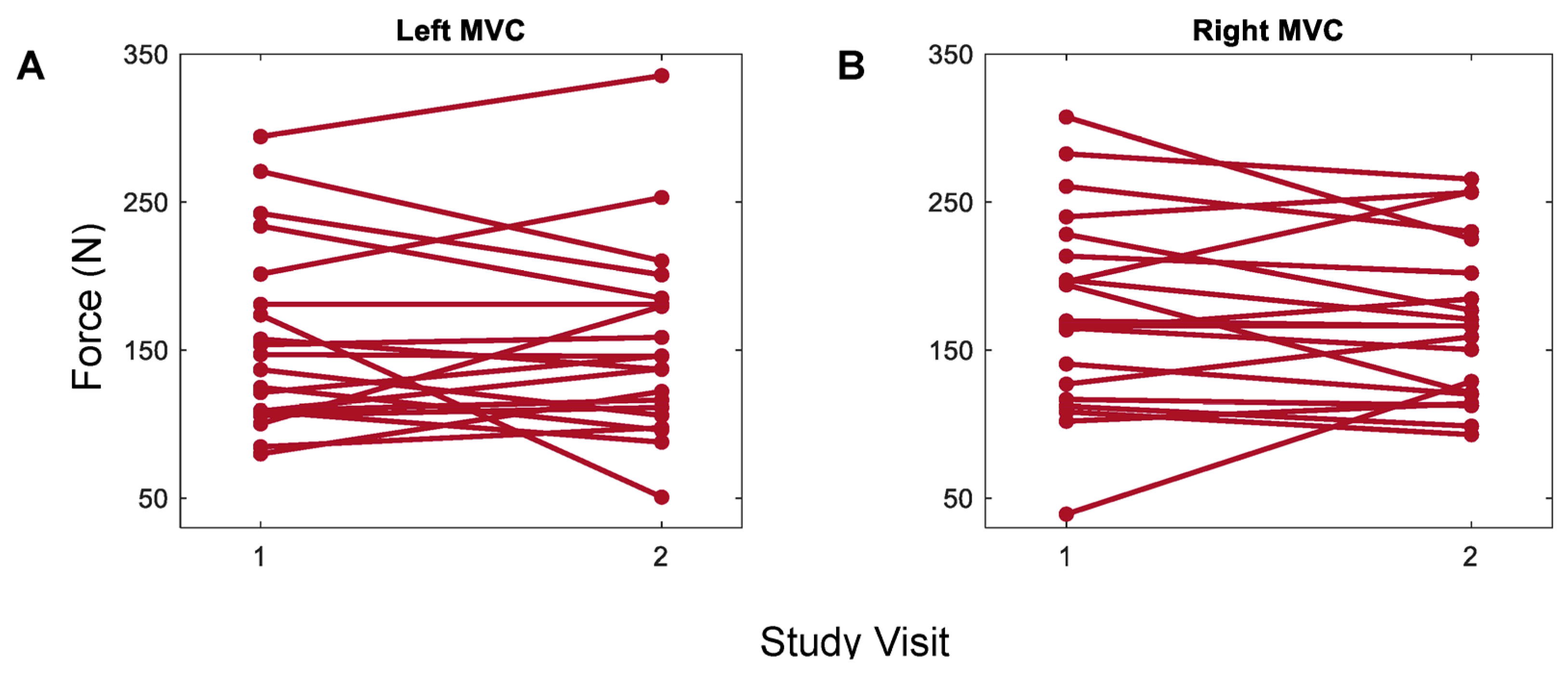
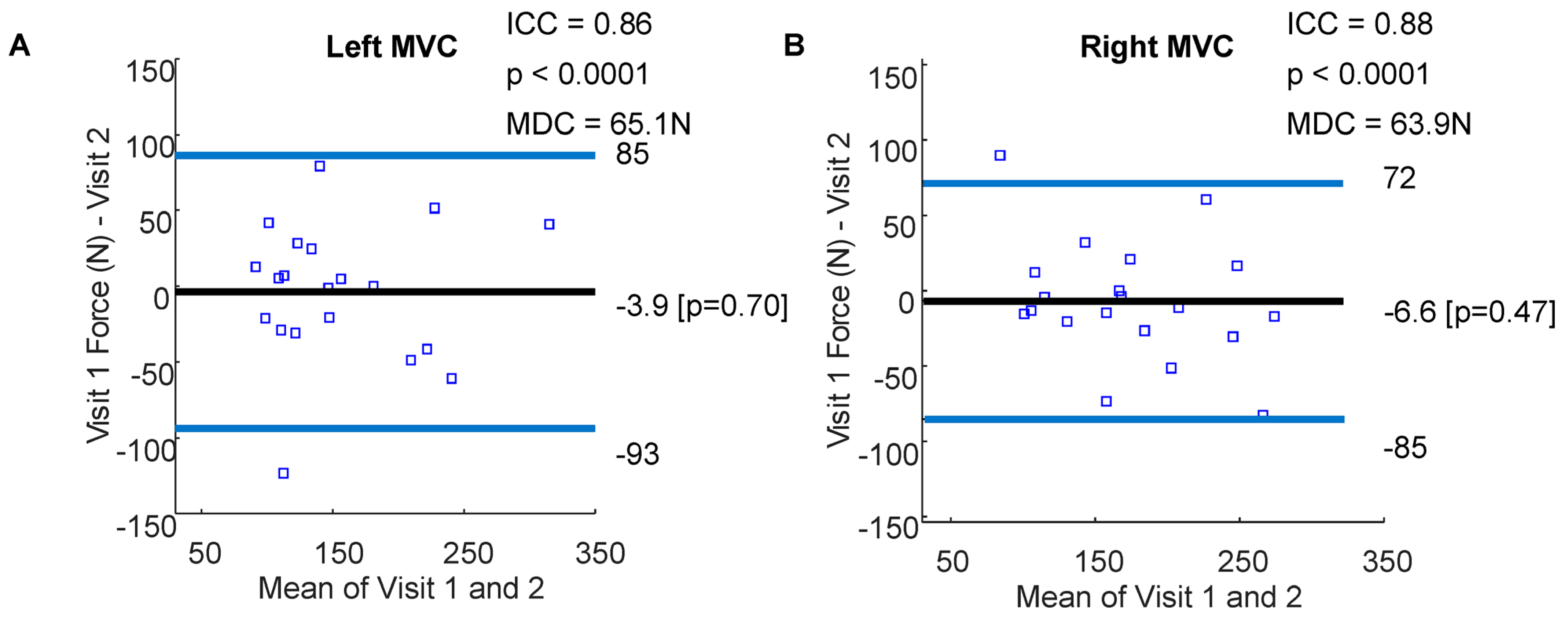
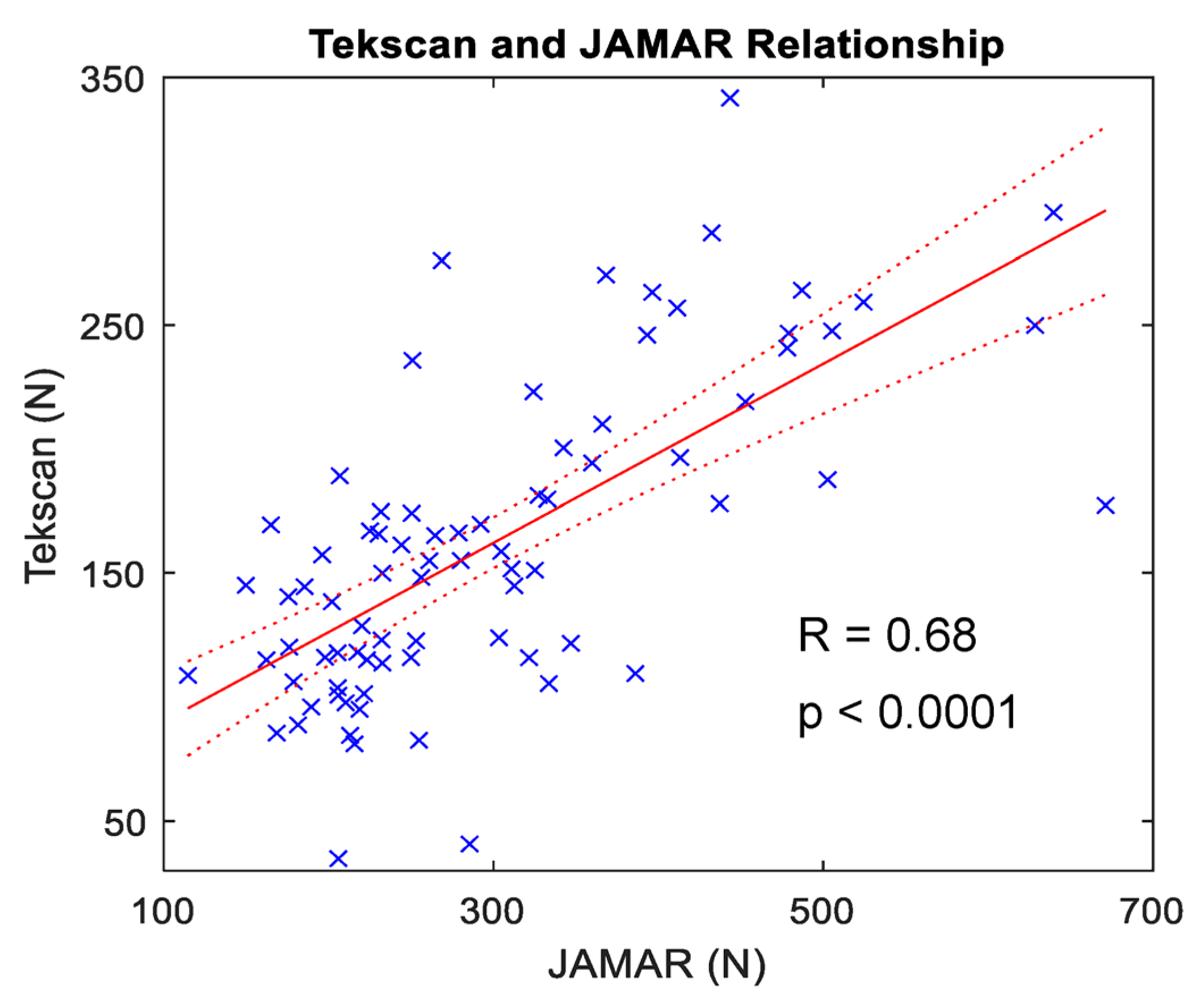
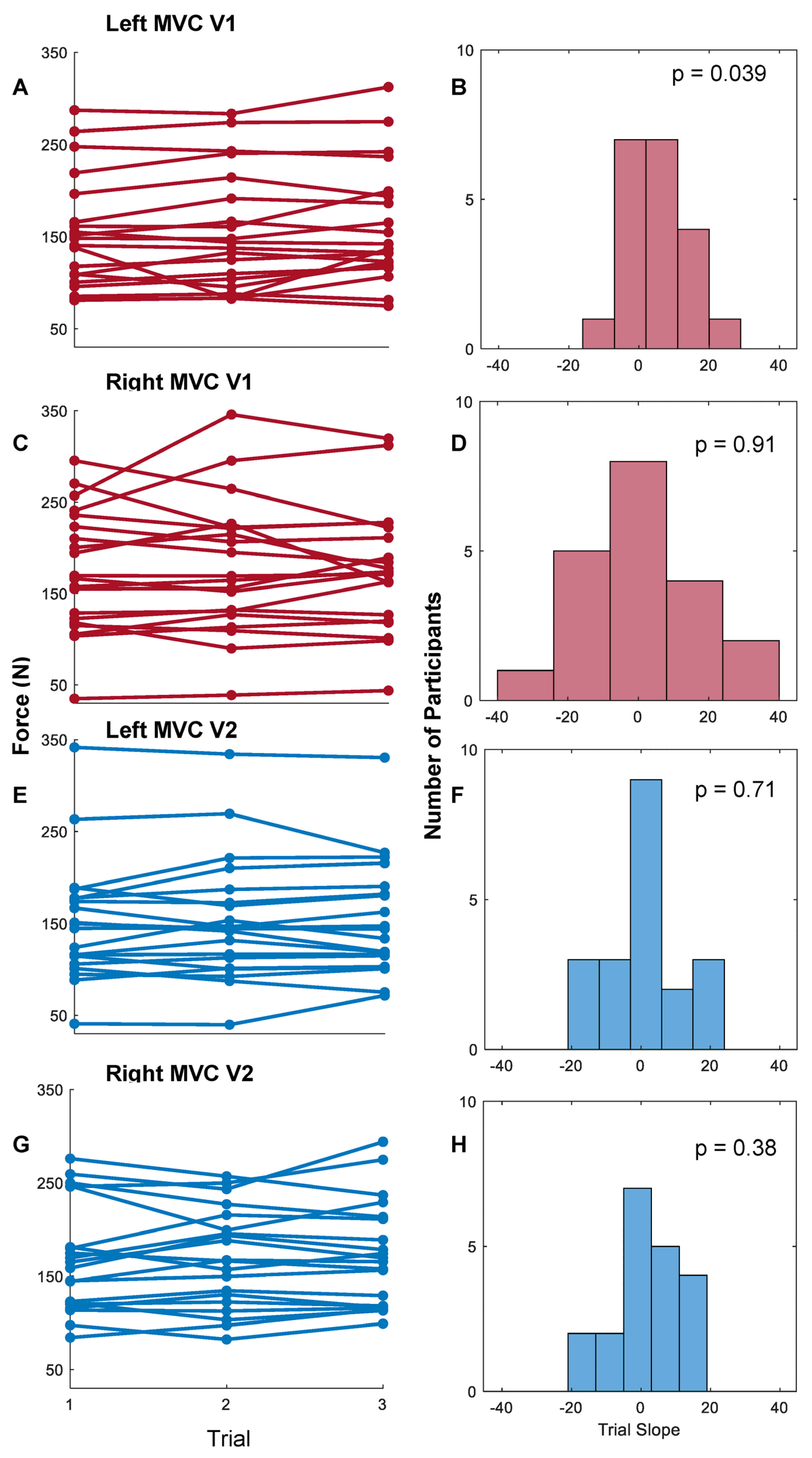
3.1. Whole-Hand Grasp Strength
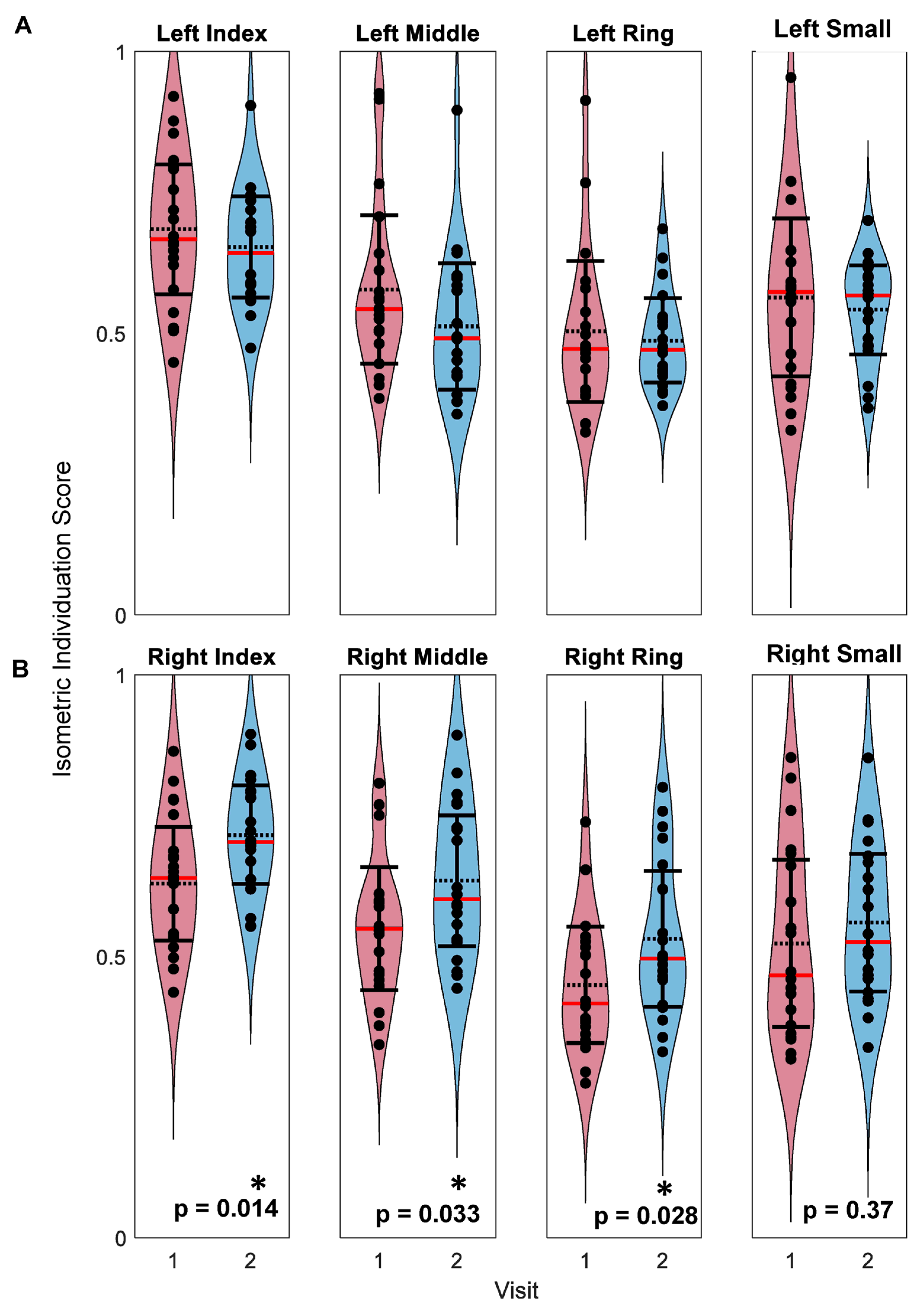
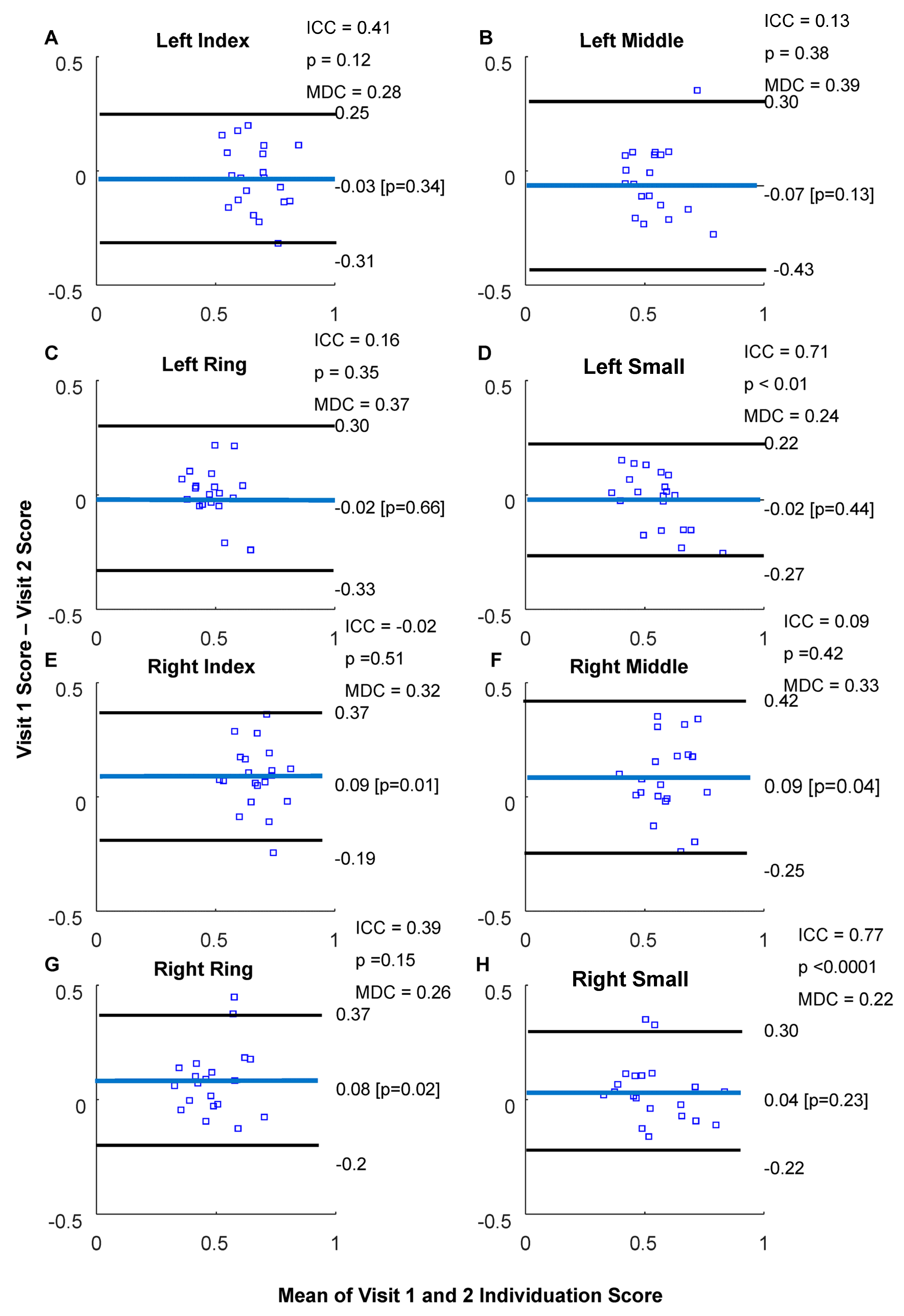
3.2. Isometric Pinch Individuation
4. Discussion
4.1. Maximum Voluntary Contraction Using a Flexible Pressure Grid
4.2. Isometric Individuation
4.3. Clinical and Research Applications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCall, J.V.; Hu, X.; Kamper, D.G. Exploring Kinetic and Kinematic Finger Individuation Capability in Children With Hemiplegic Cerebral Palsy. Percept. Mot. Ski. 2022, 130, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Wolbrecht, E.T.; Rowe, J.B.; Chan, V.; Ingemanson, M.L.; Cramer, S.C.; Reinkensmeyer, D.J. Finger strength, individuation, and their interaction: Relationship to hand function and corticospinal tract injury after stroke. Clin. Neurophysiol. 2018, 129, 797–808. [Google Scholar] [CrossRef]
- Xu, J.; Haith, A.M.; Krakauer, J.W. Motor Control of the Hand Before and After Stroke. In Clinical Systems Neuroscience; Springer: Tokyo, Japan, 2015; pp. 271–289. [Google Scholar] [CrossRef]
- Térémetz, M.; Colle, F.; Hamdoun, S.; Maier, M.A.; Lindberg, P.G. A novel method for the quantification of key components of manual dexterity after stroke. J. Neuroeng. Rehabil. 2015, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Latash, M.L.; Yue, G.H.; Siemionow, V.; Sahgal, V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin. Neurophysiol. 2003, 114, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Natraj, N.; Silversmith, D.B.; Chang, E.F.; Ganguly, K. Compartmentalized dynamics within a common multi-area mesoscale manifold represent a repertoire of human hand movements. Neuron 2021, 110, 154–174.e12. [Google Scholar] [CrossRef]
- Shibasaki, H.; Sadato, N.; Lyshkow, H.; Yonekura, Y.; Honda, M.; Nagamine, T.; Suwazono, S.; Magata, Y.; Ikeda, A.; Miyazaki, M.; et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 1993, 116, 1387–1398. [Google Scholar] [CrossRef]
- Aaronson, D.M.; Del Campo, E.M.; Boerger, T.F.; Conway, B.; Cornell, S.; Tate, M.; Mueller, W.M.; Chang, E.F.; Krucoff, M.O. Understanding Variable Motor Responses to Direct Electrical Stimulation of the Human Motor Cortex During Brain Surgery. Front. Surg. 2021, 8, 730367. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Burns, M.; Pei, D.; Vinjamuri, R. Decoding Synergy-Based Hand Movements using Electroencephalography. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 4816–4819. [Google Scholar] [CrossRef]
- Sivakumar, P.; Quinlan, D.J.; Stubbs, K.M.; Culham, J.C. Grasping performance depends upon the richness of hand feedback. Exp. Brain Res. 2021, 239, 835–846. [Google Scholar] [CrossRef]
- Boerger, T.F.; McGinn, L.; Wang, M.C.; Schmit, B.D.; Hyngstrom, A.S. Degenerative cervical myelopathy delays responses to lateral balance perturbations regardless of predictability. J. Neurophysiol. 2022, 127, 673–688. [Google Scholar] [CrossRef]
- Omori, M.; Shibuya, S.; Nakajima, T.; Endoh, T.; Suzuki, S.; Irie, S.; Ariyasu, R.; Unenaka, S.; Sano, H.; Igarashi, K.; et al. Hand Dexterity Impairment in Patients with Cervical Myelopathy: A New Quantitative Assessment Using a Natural Prehension Movement. Behav. Neurol. 2018, 5138234. [Google Scholar] [CrossRef]
- Jarque-Bou, N.J.; Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibáñez, V.; Roda-Sales, A. A calibrated database of kinematics and EMG of the forearm and hand during activities of daily living. Sci. Data 2019, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, K.; Thomas, E.; Hill, S.; Wilkie, R.; Peat, G.; Croft, P.R. The impact of musculoskeletal hand problems in older adults: Findings from the North Staffordshire Osteoarthritis Project (NorStOP). Rheumatology 2007, 46, 963–967. [Google Scholar] [CrossRef]
- Padovano, W.M.; Dengler, J.; Patterson, M.M.; Yee, A.; Snyder-Warwick, A.K.; Wood, M.D.; Moore, A.M.; Mackinnon, S.E. Incidence of Nerve Injury after Extremity Trauma in the United States. Hand 2020, 17, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Baud-Bovy, G.; Jörntell, H. Neural bases of hand synergies. Front. Comput. Neurosci. 2013, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, E.; Patish, H.; Coleman, R. The Aging Hand. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M146–M152. Available online: https://academic.oup.com/biomedgerontology/article/58/2/M146/593573 (accessed on 18 May 2023). [CrossRef]
- Smith, Z.A.; Barry, A.J.; Paliwal, M.; Hopkins, B.S.; Cantrell, D.; Dhaher, Y. Assessing hand dysfunction in cervical spondylotic myelopathy. PLoS ONE 2019, 14, e0223009. [Google Scholar] [CrossRef]
- Aslam, R.; van Bommel, A.C.M.; Hendriksz, C.J.; Jester, A. Subjective and objective assessment of hand function in mucopolysaccharidosis iva patients. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9, pp. 59–65. [Google Scholar] [CrossRef]
- Naqvi, U.; Sherman, A.L. Muscle Strength Grading; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing—Force Profiles and Their Reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef]
- Mafi, P.; Mafi, R.; Hindocha, S.; Griffin, M.; Khan, W. A Systematic Review of Dynamometry and its Role in Hand Trauma Assessment. Open Orthop. J. 2012, 6, 95–102. [Google Scholar] [CrossRef]
- Uysal, S.C.; Tonak, H.; Kitis, A. Validity, reliability and test-retest study of Grip strength measurement in two positions with two dynamometers: Jamar® Plus and K-Force® Grip. Hand Surg. Rehabil. 2022, 41, 305–310. [Google Scholar] [CrossRef]
- Steensgaard, R.; Kolbaek, R.; Jensen, J.B.; Angel, S. Action research as a catalyst for change: Empowered nurses facilitating patient participation in rehabilitation. Nurs. Inq. 2020, 28, e12370. [Google Scholar] [CrossRef] [PubMed]
- Beddaa, H.; Kably, B.; Marzouk, B.; Mouhi, I.; Marfak, A.; Azemmour, Y.; Alaoui, I.B.; Birouk, N. The effectiveness of the median nerve neurodynamic mobilisation techniques in women with mild or moderate bilateral carpal tunnel syndrome: A single-blind clinical randomised trial. S. Afr. J. Physiother. 2022, 78, 8. [Google Scholar] [CrossRef]
- Kasović, M.; Sagat, P.; Kalčik, Z.; Štefan, L.; Hubinák, A.; Krška, P. Allometric normalization of handgrip strength in older adults: Which body size parameter is the most appropriate? BMC Sports Sci. Med. Rehabil. 2023, 15, 18. [Google Scholar] [CrossRef]
- Sobinov, A.R.; Bensmaia, S.J. The neural mechanisms of manual dexterity. Nat. Rev. Neurosci. 2021, 22, 741–757. [Google Scholar] [CrossRef]
- Thielbar, K.; Lord, T.J.; Fischer, H.C.; Lazzaro, E.C.; Barth, K.C.; E Stoykov, M.; Triandafilou, K.M.; Kamper, D.G. Training finger individuation with a mechatronic-virtual reality system leads to improved fine motor control post-stroke. J. Neuroeng. Rehabil. 2014, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Mandonnet, E. The “onco-functional balance” in surgery for diffuse low-grade glioma: Integrating the extent of resection with quality of life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.S.; Barr, M.L.; Kim, D.; Jones, N.F. Tendon Transfers, Nerve Grafts, and Nerve Transfers for Isolated Radial Nerve Palsy: A Systematic Review and Analysis. Hand 2023. [Google Scholar] [CrossRef]
- Skie, M.C.; Parent, T.E.; Mudge, K.M.; Wood, V.E. Functional Deficit After Transfer of the Pronator Teres for Acquired Radial Nerve Palsy. J. Hand Surg. 2007, 32, 526–530. [Google Scholar] [CrossRef]
- Kilmarx, J.; Oblak, E.; Sulzer, J.; Lewis-Peacock, J. Towards a common template for neural reinforcement of finger individuation. Sci. Rep. 2021, 11, 1065. [Google Scholar] [CrossRef]
- Schieber, M.H. Individuated finger movements of rhesus monkeys: A means of quantifying the independence of the digits. J. Neurophysiol. 1991, 65, 1381–1391. [Google Scholar] [CrossRef]
- Lang, C.E.; Schieber, M.H. Human Finger Independence: Limitations due to Passive Mechanical Coupling Versus Active Neuromuscular Control. J. Neurophysiol. 2004, 92, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Schieber, M.H. Differential Impairment of Individuated Finger Movements in Humans After Damage to the Motor Cortex or the Corticospinal Tract. J. Neurophysiol. 2003, 90, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Häger-Ross, C.; Schieber, M.H. Quantifying the Independence of Human Finger Movements: Comparisons of Digits, Hands, and Movement Frequencies. J. Neurosci. 2000, 20, 8542–8550. [Google Scholar] [CrossRef] [PubMed]
- Sletten, I.N.; Winge, M.I.; Hellevuo, C.; Stavenes, A.B.; Bolstad, I.H.; Jokihaara, J. Validity and Reliability of the Thumb Grasp and Pinch Assessment for Children After Reconstruction of Congenital Hypoplastic Thumbs. J. Hand Surg. 2023. [CrossRef]
- Lang, C.E.; Beebe, J.A. Relating Movement Control at 9 Upper Extremity Segments to Loss of Hand Function in People with Chronic Hemiparesis. Neurorehabilit. Neural Repair 2007, 21, 279–291. [Google Scholar] [CrossRef]
- Conway, B.J.; Taquet, L.; Boerger, T.F.; Young, S.C.; Krucoff, K.B.; Schmit, B.D.; Krucoff, M.O. Quantitative assessments of finger individuation with an instrumented glove. J. Neuroeng. Rehabil. 2023, 20, 48. [Google Scholar] [CrossRef]
- Flint, R.D.; Tate, M.C.; Li, K.; Templer, J.W.; Rosenow, J.M.; Pandarinath, C.; Slutzky, M.W. The Representation of Finger Movement and Force in Human Motor and Premotor Cortices. Eneuro 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Flint, R.D.; Wang, P.T.; Wright, Z.A.; King, C.E.; Krucoff, M.O.; Schuele, S.U.; Rosenow, J.M.; Hsu, F.P.; Liu, C.Y.; Lin, J.J.; et al. Extracting kinetic information from human motor cortical signals. Neuroimage 2014, 101, 695–703. [Google Scholar] [CrossRef]
- Hyngstrom, A.S.; Kuhnen, H.R.; Kirking, K.M.; Hunter, S.K. Functional implications of impaired control of submaximal hip flexion following stroke. Muscle Nerve 2013, 49, 225–232. [Google Scholar] [CrossRef]
- Grosskopf, A.; Kuhtz-Buschbeck, J.P. Grasping with the left and right hand: A kinematic study. Exp. Brain Res. 2005, 168, 230–240. [Google Scholar] [CrossRef]
- Abolins, V.; Latash, M.L. The Nature of Finger Enslaving: New Results and Their Implications. Mot. Control 2021, 25, 680–703. [Google Scholar] [CrossRef] [PubMed]
- Roda-Sales, A.; Sancho-Bru, J.L.; Vergara, M.; Gracia-Ibáñez, V.; Jarque-Bou, N.J. Effect on manual skills of wearing instrumented gloves during manipulation. J. Biomech. 2020, 98, 109512. [Google Scholar] [CrossRef] [PubMed]
- Simone, L.K.; Sundarrajan, N.; Luo, X.; Jia, Y.; Kamper, D.G. A low cost instrumented glove for extended monitoring and functional hand assessment. J. Neurosci. Methods 2007, 160, 335–348. [Google Scholar] [CrossRef]
- Reuben, D.B.; Magasi, S.; McCreath, H.E.; Bohannon, R.W.; Wang, Y.C.; Bubela, D.J.; Rymer, W.Z.; Beaumont, J.; Rine, R.M.; Lai, J.S.; et al. Motor Assessment Using the NIH Toolbox. Neurology 2013, 80, S65–S75. [Google Scholar] [CrossRef]
- McNair, P.J.; Depledge, J.; Brettkelly, M.; Stanley, S.N. Verbal encouragement: Effects on maximum effort voluntary muscle: Action. Br. J. Sports Med. 1996, 30, 243–245. [Google Scholar] [CrossRef]
- Welch, A.S.; Tschampl, M. Something to Shout About: A Simple, Quick Performance Enhancement Technique Improved Strength in Both Experts and Novices. Appl. Sport Psychol. 2012, 24, 418–428. [Google Scholar]
- Ikai, M.; Steinhaus, A.H. Some factors modifying the expression of human strength. J. Appl. Physiol. 1961, 16, 157–163. [Google Scholar] [CrossRef]
- Salarian, A. Intra-Class Correlation Coefficient; Mathworks: Natick, MA, USA. Available online: https://www.mathworks.com/matlabcentral/fileexchange/22099-intraclass-correlation-coefficient-icc (accessed on 31 March 2023).
- Interp2; Mathworks: Natick, MA, USA. Available online: https://www.mathworks.com/help/matlab/ref/interp2.html (accessed on 31 March 2023).
- Vargas Aguilera, C.A. Extrema.m, Extrema2.m. MATLAB Central File Exchange; Mathworks: Natick, MA, USA. Available online: https://www.mathworks.com/matlabcentral/fileexchange/12275-extrema-m-extrema2-m (accessed on 31 March 2023).
- Klein, R. Bland-Altman and Correlation Plot; Mathworks: Natick, MA, USA. Available online: https://www.mathworks.com/matlabcentral/fileexchange/45049-bland-altman-and-correlation-plot (accessed on 31 March 2023).
- Hoffman, H. Violin Plot; Mathworks: Natick, MA, USA. Available online: https://www.mathworks.com/matlabcentral/fileexchange/45134-violin-plot (accessed on 31 March 2023).
- Shirley Ryan Ability Lab. Statistical Terms & Use; Shirley Ryan Ability Lab: Chicago, IL, USA. Available online: https://www.sralab.org/statistical-terms-use (accessed on 31 March 2023).
- Messick, S. Standards of Validity and the Validity of Standards in Performance Asessment. Educ. Meas. Issues Pr. 1995, 14, 5–8. [Google Scholar] [CrossRef]
- Liljequist, D.; Elfving, B.; Roaldsen, K.S. Intraclass correlation—A discussion and demonstration of basic features. PLoS ONE 2019, 14, e0219854. [Google Scholar] [CrossRef]
- Short, W.H.; Werner, F.W.; Fortino, M.D.; Palmer, A.K.; Mann, K.A. A dynamic biomechanical study of scapholunate ligament sectioning. J. Hand Surg. 1995, 20, 986–999. [Google Scholar] [CrossRef]
- Pirouzi, G.; Abu Osman, N.A.; Oshkour, A.A.; Ali, S.; Gholizadeh, H.; Abas, W.A.B.W. Development of an Air Pneumatic Suspension System for Transtibial Prostheses. Sensors 2014, 14, 16754–16765. [Google Scholar] [CrossRef]
- Lin, C.-L.; Yeh, M.-L.; Su, F.-C.; Wang, Y.-C.; Chiang, C.H.; Hong, C.-K.; Su, W.-R. Different suture anchor fixation techniques affect contact properties in humeral greater tuberosity fracture: A biomechanical study. BMC Musculoskelet. Disord. 2019, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Lupton-Smith, A.; Fourie, K.; Mazinyo, A.; Mokone, M.; Nxaba, S.; Morrow, B. Measurement of hand grip strength: A cross-sectional study of two dynamometry devices. S. Afr. J. Physiother. 2022, 78, 5. [Google Scholar] [CrossRef] [PubMed]
- Ketchum, L.D.; Thompson, D.; Pocock, G.; Wallingford, D. A clinical study of forces generated by the intrinsic muscles of the index finger and the extrinsic flexor and extensor muscles of the hand. J. Hand Surg. 1978, 3, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.P.; Hong, H.G.; Bush, T.R. Mapping Together Kinetic and Kinematic Abilities of the Hand. J. Biomech. Eng. 2019, 142. [Google Scholar] [CrossRef]
- Krucoff, M.; Miller, J.P.; Saxena, T.; Bellamkonda, R.; Rahimpour, S.; Harward, S.C.; Lad, S.P.; Turner, D.A. Toward Functional Restoration of the Central Nervous System: A Review of Translational Neuroscience Principles. Neurosurgery 2018, 84, 30–40. [Google Scholar] [CrossRef]
- Krucoff, M.O.; Rahimpour, S.; Slutzky, M.W.; Edgerton, V.R.; Turner, D.A. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci. 2016, 10, 584. [Google Scholar] [CrossRef]
- Sotelo, M.R.; Kalinosky, B.T.; Goodfriend, K.; Hyngstrom, A.S.; Schmit, B.D. Indirect Structural Connectivity Identifies Changes in Brain Networks After Stroke. Brain Connect. 2020, 10, 399–410. [Google Scholar] [CrossRef]
- Buretta, K.J.; Brat, G.A.; Christensen, J.M.; Ibrahim, Z.; Grahammer, J.; Furtmüller, G.J.; Suami, H.; Cooney, D.S.; Lee, W.P.A.; Brandacher, G.; et al. Near-infrared lymphography as a minimally invasive modality for imaging lymphatic reconstitution in a rat orthotopic hind limb transplantation model. Transpl. Int. 2013, 26, 928–937. [Google Scholar] [CrossRef]
- Rahman, M.; Abbatematteo, J.; De Leo, E.K.; Kubilis, P.S.; Vaziri, S.; Bova, F.; Sayour, E.; Mitchell, D.; Quinones-Hinojosa, A. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J. Neurosurg. 2017, 127, 123–131. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg. Focus 2010, 28, E1. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef]
- McGirt, M.J.; Mukherjee, D.; Chaichana, K.L.; Than, K.D.; Weingart, J.D.; Quinones-Hinojosa, A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 2009, 65, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Taquet, L.; Conway, B.J.; Boerger, T.F.; Young, S.C.; Schwartz, S.; Schmit, B.D.; Krucoff, M.O. Synchronization of kinetic and kinematic hand tasks with electrocorticography and cortical stimulation during awake craniotomies. PLoS ONE 2023, 18, e0283460. [Google Scholar] [CrossRef] [PubMed]


| Participant Demographics | Characteristics | Number of Participants (Percentage) |
|---|---|---|
| Sex | Male | 16 (80%) |
| Female | 4 (20%) | |
| Handedness | Right | 20 (100%) |
| Left | 0 (0%) | |
| Age | Mean ± Standard Deviation | 28.8 ± 2.5 |
| Median | 27.5 | |
| Minimum | 23 | |
| Maximum | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conway, B.J.; Taquet, L.; Boerger, T.F.; Young, S.C.; Krucoff, K.B.; Schmit, B.D.; Krucoff, M.O. Quantifying Hand Strength and Isometric Pinch Individuation Using a Flexible Pressure Sensor Grid. Sensors 2023, 23, 5924. https://doi.org/10.3390/s23135924
Conway BJ, Taquet L, Boerger TF, Young SC, Krucoff KB, Schmit BD, Krucoff MO. Quantifying Hand Strength and Isometric Pinch Individuation Using a Flexible Pressure Sensor Grid. Sensors. 2023; 23(13):5924. https://doi.org/10.3390/s23135924
Chicago/Turabian StyleConway, Brian J., Léon Taquet, Timothy F. Boerger, Sarah C. Young, Kate B. Krucoff, Brian D. Schmit, and Max O. Krucoff. 2023. "Quantifying Hand Strength and Isometric Pinch Individuation Using a Flexible Pressure Sensor Grid" Sensors 23, no. 13: 5924. https://doi.org/10.3390/s23135924
APA StyleConway, B. J., Taquet, L., Boerger, T. F., Young, S. C., Krucoff, K. B., Schmit, B. D., & Krucoff, M. O. (2023). Quantifying Hand Strength and Isometric Pinch Individuation Using a Flexible Pressure Sensor Grid. Sensors, 23(13), 5924. https://doi.org/10.3390/s23135924






