The Effects of mmW and THz Radiation on Dry Eyes: A Finite-Difference Time-Domain (FDTD) Computational Simulation Using XFdtd
Abstract
1. Introduction
2. Material and Methods
2.1. The Details of the Computational Model
2.1.1. Establishments of Anatomical Features of Simulation Model
2.1.2. Simulation Environment in Remcom XFdtd
2.1.3. Dielectric Properties
2.2. Exposure Scenario and Sensors
3. Results
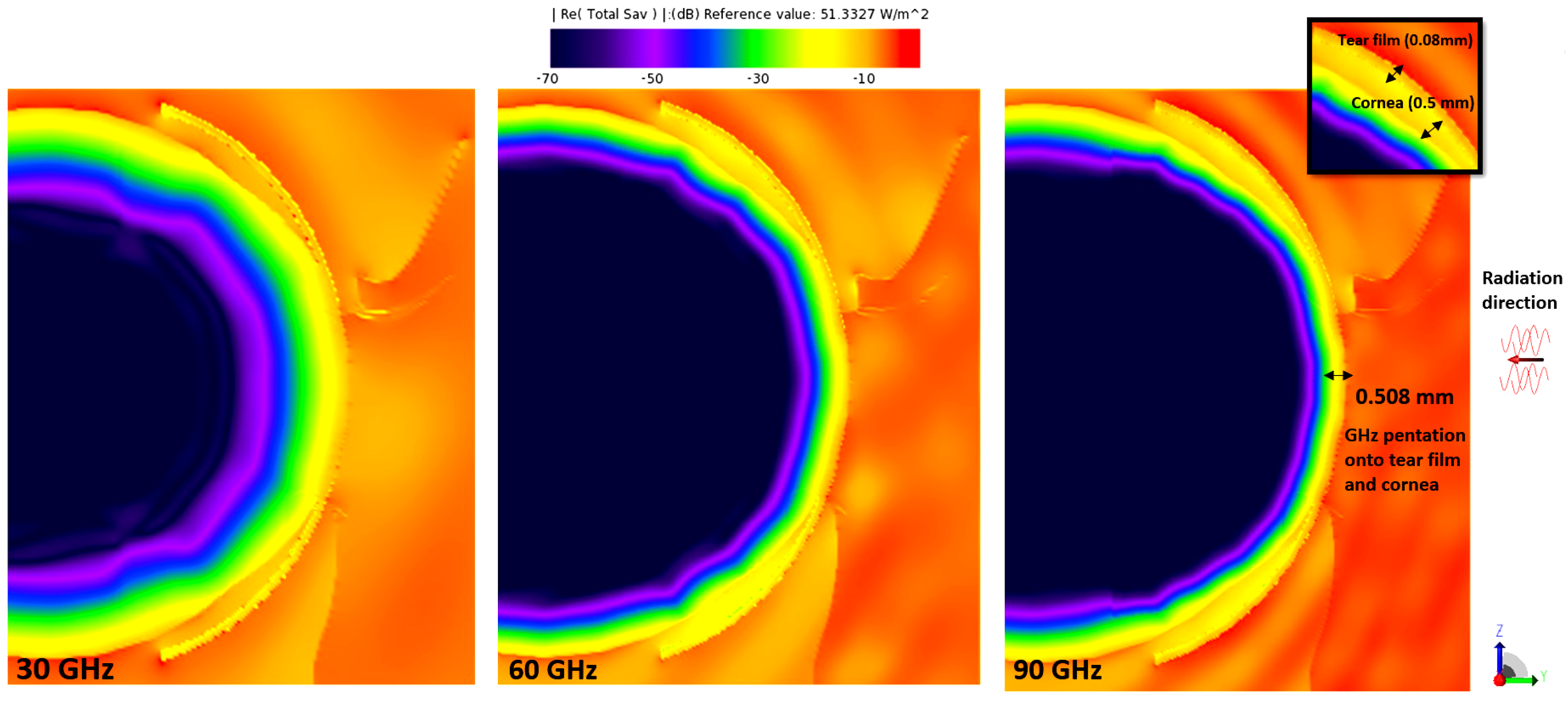
3.1. E-Field and Power Density Radiation Patterns of Plane Waves
3.2. Temperature Rise
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acharyya, A.; Biswas, A.; Inokawa, H. New Horizons in Millimeter-Wave, Infrared and Terahertz Technologies; Springer: Berlin/Heidelberg, Germany, 2022; Volume 953. [Google Scholar]
- Bauer, M.; Friederich, F. Terahertz and Millimeter Wave Sensing and Applications. Sensors 2022, 22, 9693. [Google Scholar] [CrossRef]
- Abbasi, Q.H.; Jilani, S.F.; Alomainy, A.; Imran, M.A. Antennas and Propagation for 5G and Beyond; Institution of Engineering and Technology: Stevenage, UK, 2020. [Google Scholar]
- Petrov, V.; Kurner, T.; Hosako, I. IEEE 802.15. 3d: First standardization efforts for sub-terahertz band communications toward 6G. IEEE Commun. Mag. 2020, 58, 28–33. [Google Scholar] [CrossRef]
- Kürner, T.; Mittleman, D.M.; Nagatsuma, T. THz Communications: Paving the Way Towards Wireless Tbps; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. Investigating the Impact of Synchrotron THz Radiation on the Corneal Hydration Using Synchrotron THz-Far Infrared Beamline. Sensors 2022, 22, 8261. [Google Scholar] [CrossRef]
- Karipidis, K.; Brzozek, C.; Mate, R.; Bhatt, C.R.; Loughran, S.; Wood, A.W. What evidence exists on the impact of anthropogenic radiofrequency electromagnetic fields on animals and plants in the environment: A systematic map. Environ. Evid. 2023, 12, 1–23. [Google Scholar] [CrossRef]
- Hirata, A.; Watanabe, S.; Fujiwara, O.; Kojima, M.; Sasaki, K.; Shiozawa, T. Temperature elevation in the eye of anatomically based human head models for plane-wave exposures. Phys. Med. Biol. 2007, 52, 6389. [Google Scholar] [CrossRef]
- Diao, Y.; Leung, S.W.; He, Y.; Sun, W.; Chan, K.H.; Siu, Y.M.; Kong, R. Detailed modeling of palpebral fissure and its influence on SAR and temperature rise in human eye under GHz exposures. Bioelectromagnetics 2016, 37, 256–263. [Google Scholar] [CrossRef]
- Flyckt, V.; Raaymakers, B.; Lagendijk, J. Modelling the impact of blood flow on the temperature distribution in the human eye and the orbit: Fixed heat transfer coefficients versus the Pennes bioheat model versus discrete blood vessels. Phys. Med. Biol. 2006, 51, 5007. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Xie, Y.; Wu, T. Dosimetric study on eye’s exposure to wide band radio frequency electromagnetic fields: Variability by the ocular axial length. Bioelectromagnetics 2014, 35, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I. Assessment of the computational uncertainty of temperature rise and SAR in the eyes and brain under far-field exposure from 1 to 10 GHz. Phys. Med. Biol. 2009, 54, 3393. [Google Scholar] [CrossRef] [PubMed]
- ARPANSA. The Radiation Protection Standard for Limiting Exposure to Radiofrequency Fields—100 kHz to 300 GHz- Radiation Protection Series S-1 (Rev.1); ARPANSA: Yallambie, Australia, 2021.
- Sah, A.K.; Aparna, S.; Ashwini, D. Impact of salivary stimulation on tear film function in dry eye subjects. Indian J. Ophthalmol. 2023, 71, 1643–1646. [Google Scholar] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. Tfos dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Barnes, F.S.; Greenebaum, B. Handbook of Biological Effects of Electromagnetic Fields-Two Volume Set; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Kojima, M.; Suzuki, Y.; Tsai, C.Y.; Sasaki, K.; Wake, K.; Watanabe, S.; Taki, M.; Kamimura, Y.; Hirata, A.; Sasaki, K.; et al. Characteristics of ocular temperature elevations after exposure to quasi-and millimeter waves (18–40 GHz). J. Infrared Millim. Terahertz Waves 2015, 36, 390–399. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Spathmann, O.; Schürmann, R.; Zang, M.; Streckert, J.; Hansen, V.; Saviz, M.; Clemens, M. Thermal impact on the human oral cavity exposed to radiation from biomedical devices operating in the terahertz frequency range. J. Infrared Millim. Terahertz Waves 2018, 39, 926–941. [Google Scholar] [CrossRef]
- Williams, R.; Schofield, A.; Holder, G.; Downes, J.; Edgar, D.; Harrison, P.; Siggel-King, M.; Surman, M.; Dunning, D.; Hill, S.; et al. The influence of high intensity terahertz radiation on mammalian cell adhesion, proliferation and differentiation. Phys. Med. Biol. 2012, 58, 373. [Google Scholar] [CrossRef] [PubMed]
- Heugen, U.; Schwaab, G.; Bründermann, E.; Heyden, M.; Yu, X.; Leitner, D.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Vilagosh, Z.; Lajevardipour, A.; Appadoo, D.; Ng, S.H.; Juodkazis, S.; Wood, A.W. Characterisation of Biological Materials at THz Frequencies by Attenuated Total Reflection: Lard. Appl. Sci. 2020, 10, 8692. [Google Scholar] [CrossRef]
- Vilagosh, Z.; Foroughimehr, N.; Lajevardipour, A.; Appadoo, D.; Juodkazis, S.; Wood, A. Estimating the dielectric parameters of water and gel using reflectance and transmission at 1.85 to 2.07 THz. In Biomedical Imaging and Sensing Conference 2021; SPIE: Philadelphia, PA, USA, 2021; Volume 11925, pp. 31–36. [Google Scholar]
- Taflove, A. Computational Electrodynamics: The Finite-Difference Time-Domain Method, 2nd ed.; Taflove, A., Hagness, S.C., Eds.; Artech House Antennas and Propagation Library; Artech House: Boston, MA, USA, 2000. [Google Scholar]
- Vilagosh, Z.; Lajevardipour, A.; Wood, A. Computational simulations of the penetration of 0.30 THz radiation into the human ear. Biomed. Opt. Express 2019, 10, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Vilagosh, Z.; Lajevardipour, A.; Wood, A.W. Computational phantom study of frozen melanoma imaging at 0.45 terahertz. Bioelectromagnetics 2019, 40, 118–127. [Google Scholar] [CrossRef]
- Vilagosh, Z.; Lajevardipour, A.; Wood, A.W. Computational absorption and reflection studies of normal human skin at 0.45 THz. Biomed. Opt. Express 2020, 11, 417–431. [Google Scholar] [CrossRef]
- Vilagosh, Z.; Foroughimehr, N.; Lajevardipour, A.; Wood, A.W. FDTD Simulations of Sweat Ducts and Hair at 0.45 THz. Dermato 2023, 3, 69–84. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Anderson, V.; McKenzie, R.J. A numerical evaluation of SAR distribution and temperature changes around a metallic plate in the head of a RF exposed worker. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2005, 26, 377–388. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Iskra, S.; Anderson, V. Significant RF-EMF and thermal levels observed in a computational model of a person with a tibial plate for grounded 40 MHz exposure. Bioelectromagnetics 2014, 35, 284–295. [Google Scholar] [CrossRef]
- Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. The Impact of Base Cell Size Setup on the Finite Difference Time Domain Computational Simulation of Human Cornea Exposed to Millimeter Wave Radiation at Frequencies above 30 GHz. Sensors 2022, 22, 5924. [Google Scholar] [CrossRef]
- Holland, E.J.; Mannis, M.J.; Lee, W.B. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film: Expert Consult-Online and Print; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Goto, E.; Matsumoto, Y.; Kamoi, M.; Endo, K.; Ishida, R.; Dogru, M.; Kaido, M.; Kojima, T.; Tsubota, K. Tear evaporation rates in Sjögren syndrome and non-Sjögren dry eye patients. Am. J. Ophthalmol. 2007, 144, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Freddo, T.F.; Chaum, E. Anatomy of the Eye and Orbit: The Clinical Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Willoughby, C.E.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function—A review. Clin. Exp. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- Sasaki, K.; Isimura, Y.; Fujii, K.; Wake, K.; Watanabe, S.; Kojima, M.; Suga, R.; Hashimoto, O. Dielectric property measurement of ocular tissues up to 110 GHz using 1 mm coaxial sensor. Phys. Med. Biol. 2015, 60, 6273. [Google Scholar] [CrossRef]
- Mizuno, M.; Kitahara, H.; Sasaki, K.; Tani, M.; Kojima, M.; Suzuki, Y.; Tasaki, T.; Tatematsu, Y.; Fukunari, M.; Wake, K. Dielectric property measurements of corneal tissues for computational dosimetry of the eye in terahertz band in vivo and in vitro. Biomed. Opt. Express 2021, 12, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, G.J.; Ibey, B.L.; Tongue, T.; Schulkin, B.; Laman, N.; Peralta, X.G.; Roth, C.C.; Cerna, C.Z.; Rivest, B.D.; Grundt, J.E.; et al. Development of a compact terahertz time-domain spectrometer for the measurement of the optical properties of biological tissues. J. Biomed. Opt. 2011, 16, 47006. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Wake, K.; Watanabe, S. Measurement of the dielectric properties of the epidermis and dermis at frequencies from 0.5 GHz to 110 GHz. Phys. Med. Biol. 2014, 59, 4739. [Google Scholar] [CrossRef]
- Sasaki, K.; Porter, E.; Rashed, E.A.; Farrugia, L.; Schmid, G. Measurement and image-based estimation of dielectric properties of biological tissues—past, present, and future—. Phys. Med. Biol. 2022, 67, 14TR01. [Google Scholar] [CrossRef]
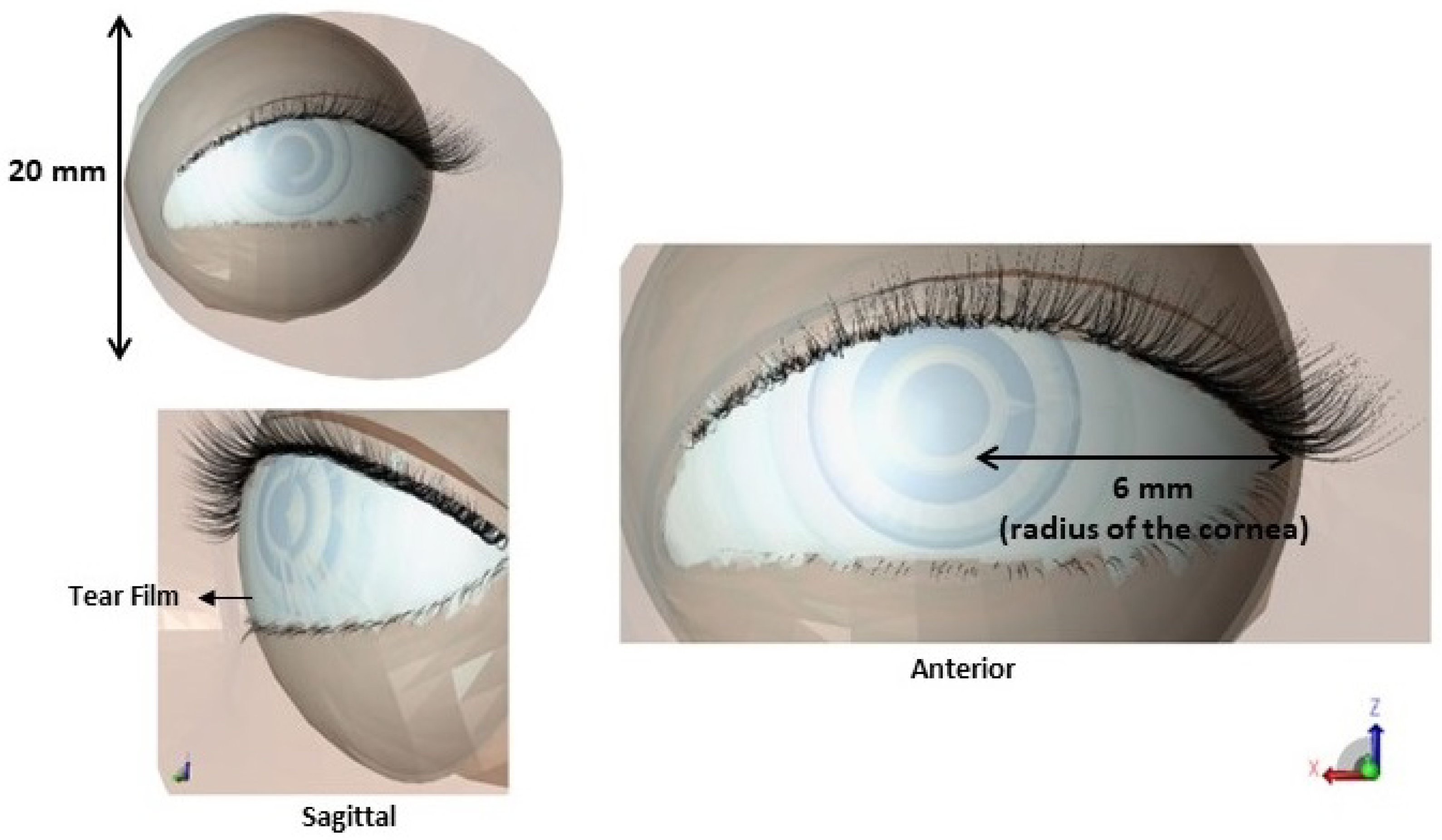


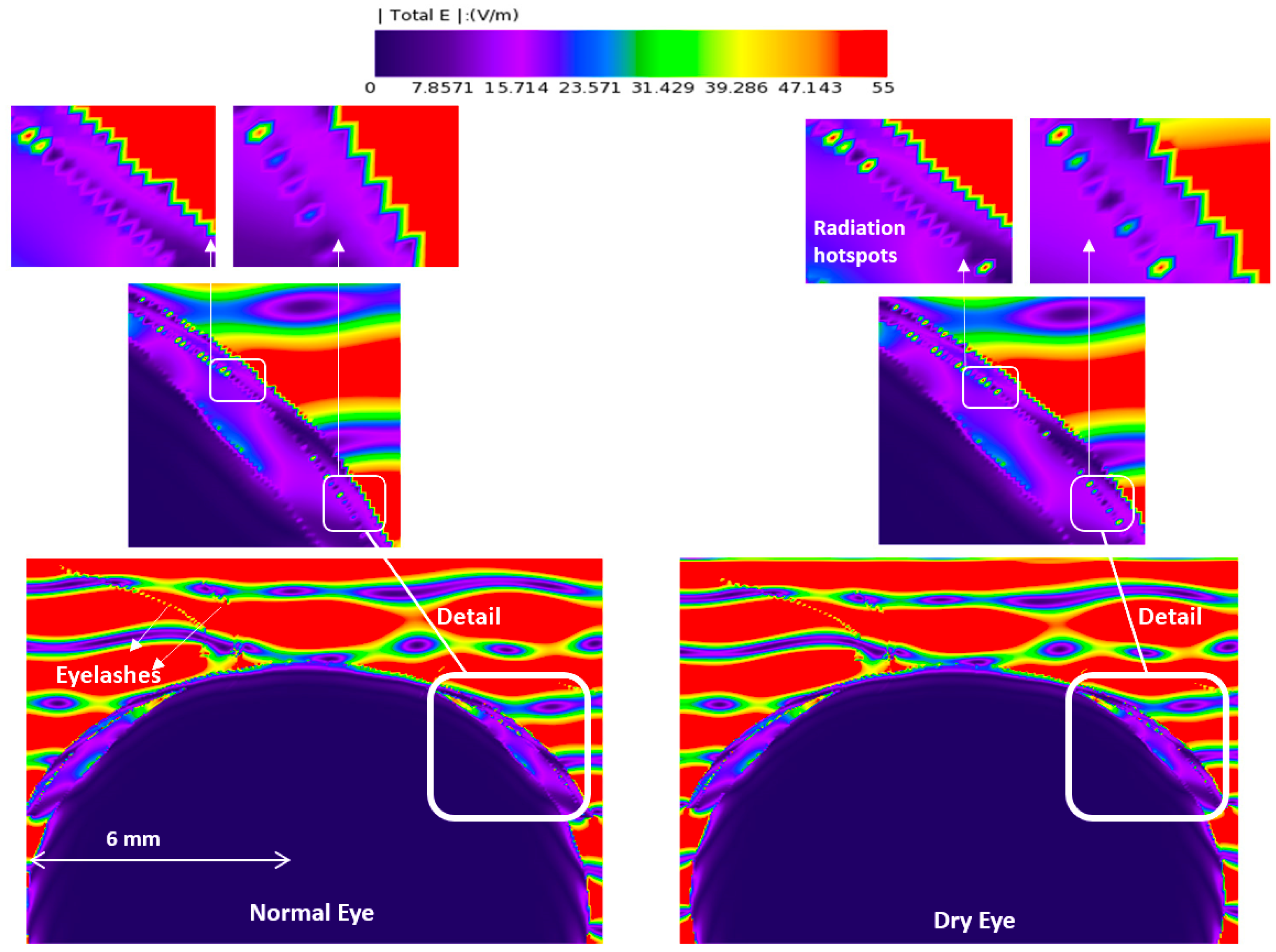
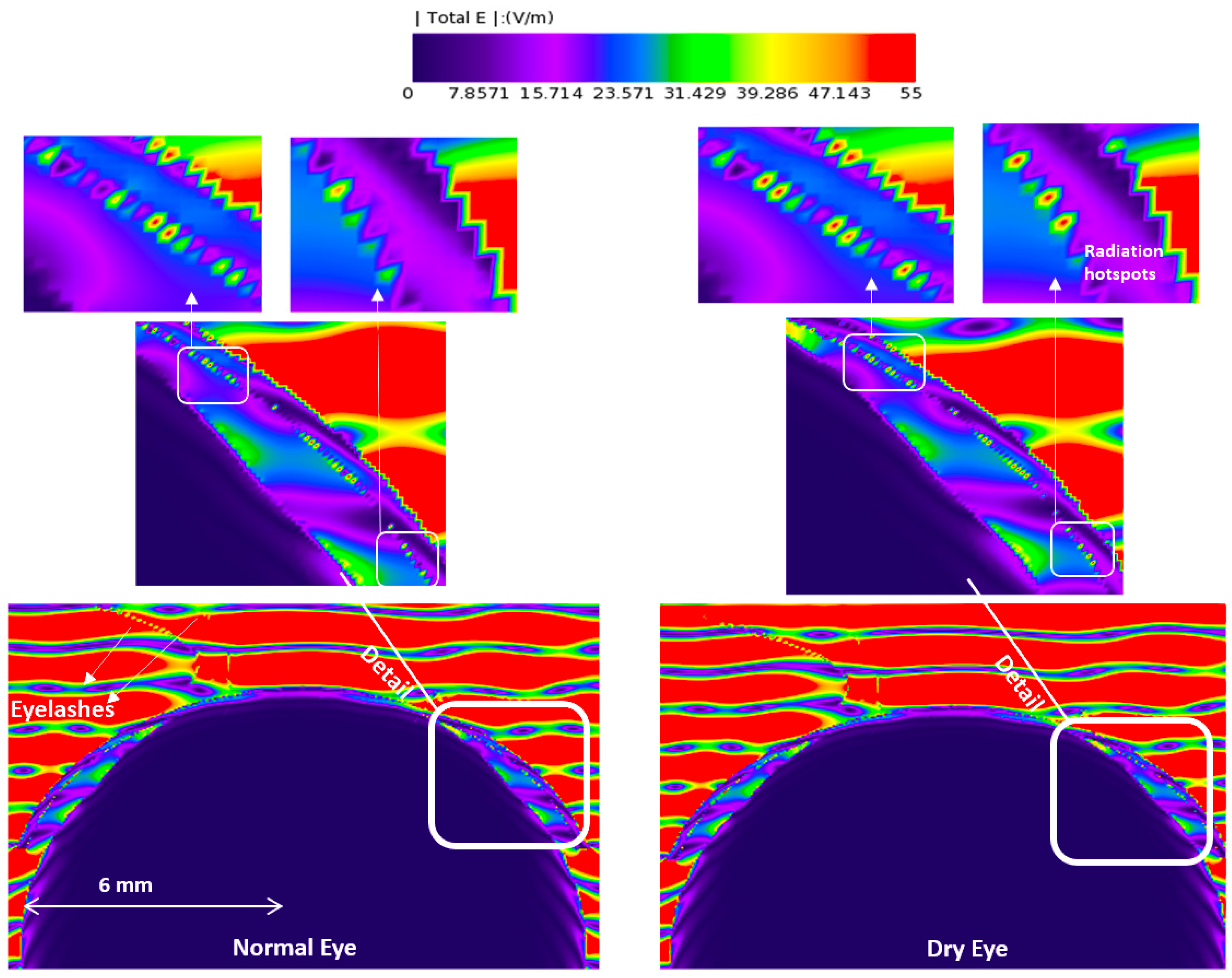
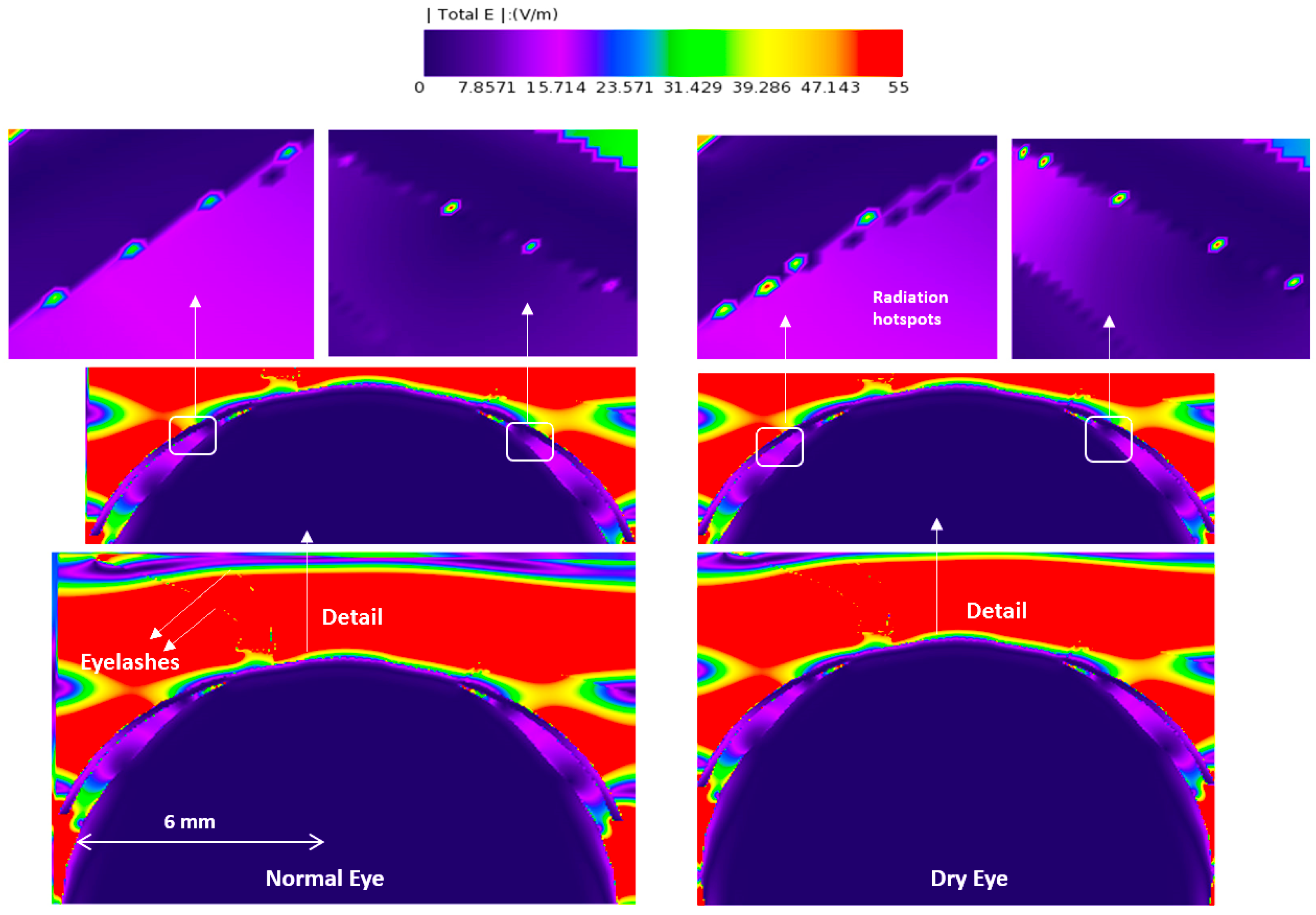

| Model | Thick Tear Film | Thin Tear Film | ||||
|---|---|---|---|---|---|---|
| Yee cell dimension | Yee cell dimension | |||||
| Frequency (GHz) | Time step (s) | Max cell size (mm) | Minimum cell size (mm) | Time step (s) | Maximum dimension | Minimum Dimension |
| 30 | 3.92157 × 10−14 | 0.393 × 0.295 × 0.297 | 0.015 × 0.041 × 0.021 | 3.92157 × 10−14 | 0.393 × 0.295 × 0.297 | 0.015 × 0.041 × 0.021 |
| 60 | 3.40136 × 10−14 | 0.245 × 0.245 × 0.245 | 0.013 × 0.041 × 0.019 | 3.40136 × 10−14 | 0.245 × 0.245 × 0.245 | 0.013 × 0.041 × 0.019 |
| 90 | 3.38753 × 10−14 | 0.153 × 0.166 × 0.159 | 0.013 × 0.041 × 0.019 | 3.38753 × 10−14 | 0.153 × 0.166 × 0.159 | 0.013 × 0.041 × 0.019 |
| 500 | 2.55232 × 10−14 | 0.058 × 0.058 × 0.059 | 0.013 × 0.013 × 0.013 | 2.55232 × 10−14 | 0.058 × 0.058 × 0.059 | 0.013 × 0.013 × 0.013 |
| Tissue | Thermal Conductivity W/(m·K) | Specific Heat Capacity C J/(kg·K) | Density (kg/m3) |
|---|---|---|---|
| Cornea | 0.58 | 4178 | 1050 |
| Aqueous Humour | 0.58 | 3997 | 996 |
| Iris | 0.52 | 3600 | 1050 |
| Lens | 0.4 | 3000 | 1000 |
| Eyelash | 0.37 | 1680 | 0.0013 |
| Eyelids | 0.36 | 3390 | 1109 |
| Normal Eye | Dry Eye | Temperature Rise Difference (%) | ||
|---|---|---|---|---|
| 30 GHz | 30 GHz | |||
| Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | |
| 20 | 0.02557 | 20 | 0.02575 | 0.70 |
| 100 | 0.1278 | 100 | 0.1288 | 0.78 |
| 60 GHz | 60 GHz | |||
| Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | |
| 20 | 0.02475 | 20 | 0.0252 | 1.82 |
| 100 | 0.1238 | 100 | 0.1261 | 1.86 |
| 90 GHz | 90 GHz | |||
| Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | Incident Power Density (Wm−2) | Maximum Temperature rise (°C) | |
| 20 | 0.01412 | 20 | 0.01439 | 1.91 |
| 100 | 0.07078 | 100 | 0.07216 | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroughimehr, N.; Vilagosh, Z.; Yavari, A.; Wood, A. The Effects of mmW and THz Radiation on Dry Eyes: A Finite-Difference Time-Domain (FDTD) Computational Simulation Using XFdtd. Sensors 2023, 23, 5853. https://doi.org/10.3390/s23135853
Foroughimehr N, Vilagosh Z, Yavari A, Wood A. The Effects of mmW and THz Radiation on Dry Eyes: A Finite-Difference Time-Domain (FDTD) Computational Simulation Using XFdtd. Sensors. 2023; 23(13):5853. https://doi.org/10.3390/s23135853
Chicago/Turabian StyleForoughimehr, Negin, Zoltan Vilagosh, Ali Yavari, and Andrew Wood. 2023. "The Effects of mmW and THz Radiation on Dry Eyes: A Finite-Difference Time-Domain (FDTD) Computational Simulation Using XFdtd" Sensors 23, no. 13: 5853. https://doi.org/10.3390/s23135853
APA StyleForoughimehr, N., Vilagosh, Z., Yavari, A., & Wood, A. (2023). The Effects of mmW and THz Radiation on Dry Eyes: A Finite-Difference Time-Domain (FDTD) Computational Simulation Using XFdtd. Sensors, 23(13), 5853. https://doi.org/10.3390/s23135853








