Abstract
Potentiometric sensors are the largest and most commonly used group of electrochemical sensors. Among them, ion-selective electrodes hold a prominent place. Since the end of the last century, their re-development has been observed, which is a consequence of the introduction of solid contact constructions, i.e., electrodes without an internal electrolyte solution. Research carried out in the field of potentiometric sensors primarily focuses on developing new variants of solid contact in order to obtain devices with better analytical parameters, and at the same time cheaper and easier to use, which has been made possible thanks to the achievements of material engineering. This paper presents an overview of new materials used as a solid contact in ion-selective electrodes over the past several years. These are primarily composite and hybrid materials that are a combination of carbon nanomaterials and polymers, as well as those obtained from carbon and polymer nanomaterials in combination with others, such as metal nanoparticles, metal oxides, ionic liquids and many others. Composite materials often have better mechanical, thermal, electrical, optical and chemical properties than the original components. With regard to their use in the construction of ion-selective electrodes, it is particularly important to increase the capacitance and surface area of the material, which makes them more effective in the process of charge transfer between the polymer membrane and the substrate material. This allows to obtain sensors with better analytical and operational parameters. Brief characteristics of electrodes with solid contact, their advantages and disadvantages, as well as research methods used to assess their parameters and analytical usefulness were presented. The work was divided into chapters according to the type of composite material, while the data in the table were arranged according to the type of ion. Selected basic analytical parameters of the obtained electrodes have been collected and summarized in order to better illustrate and compare the achievements that have been described till now in this field of analytical chemistry, which is potentiometry. This comprehensive review is a compendium of knowledge in the research area of functional composite materials and state-of-the-art SC-ISE construction technologies.
1. Introduction
Along with the development of technology and increasing processing of raw materials used in various industries, there is a growing demand for research aimed at monitoring the ongoing changes and the general condition of the natural environment. They are caused by both natural processes over which humans have no direct influence and by the activities of society. Devices that allow to quickly and easily determine the content of ions in various types of environmental samples are ion-selective electrodes (ISEs), which are currently the most popular potentiometric sensors and have been the subject of research for many scientists around the world continuously for many years, thanks to their numerous advantages [1,2]. The enormity of possibilities offered by modifications of ion-selective electrodes, including the development of wearable sensors [3,4] and multi-sensor platforms [5,6,7,8] for simultaneous monitoring of ion concentrations in a continuous mode in the in situ environment, causes that research on new constructions of electrodes to achieve better and better analytical and electrical parameters is still being published [9]. ISEs are currently used to determine the concentration of various types of ions (both inorganic and organic) in liquid samples in many areas of human life (agriculture [10,11], food industry [11,12,13] and pharmaceutical industry [14,15], process control or clinical diagnostics [16,17,18,19]). ISEs can be used to determine the content of selected ions in natural water samples, both surface waters (rivers, lakes and seas) and groundwater, as well as in tap water and sewage [20,21,22,23].
There are many review articles available in the scientific literature focusing on the collection and comparison of groups of substances and/or materials, their properties (including mechanical and electrical) and their applications in various industries. Nanocomposites consisting of polymers and carbon nanomaterials [24,25,26], as well as nanocomposites of polymers and ionic liquids [27], are widely described. The variety of composite materials and the number of possibilities of synthesis and modifying their composition or/and type of components can be a reason for their great popularity among scientists. Depending on the desired properties of materials and their future applications, it is possible to obtain composites that will be characterized by similar parameters, or most often even much better than the parameters of their individual components. Many review articles focus mainly on the method of synthesis of new materials and their parameters measured by multiple research methods. This article focuses on the use of nanocomposite materials in the construction of solid contact electrodes, in which they act as an ion–electron transducer (or sometimes even as an active component) and allow to obtain sensors with very good electrical and analytical parameters. Taking into account the type of materials used in the synthesis of nanocomposites, they can be divided into three main groups: composite materials obtained from conductive polymers and carbon nanomaterials; materials based on carbon nanomaterials and conductive polymers in combination with other materials, i.e., nanoparticles of metals and metal oxides, ionic liquids, molecular organic materials or zeolites. Selected analytical parameters of the electrodes described in the literature are summarized in the form of a table, which enables a quick comparison between the types of electrodes constructed in recent years, depending on the ions they determine and their capabilities.
2. Potentiometry and Ion-Selective Electrodes
Potentiometry is a highly selective and relatively cheap method that allows achieving low detection limits and a very wide dynamic range of sensors (up to eight orders of units) [28,29]. The principle of the method is to measure the electromotive force (EMF) of a cell made of two types of electrodes: a reference electrode whose potential has to necessarily be constant regardless of the composition and concentration of the sample, and an indicator (working) electrode whose potential changes depending on the activity of the main ion present in the sample solution to which the ion-selective membrane is sensitive [30]. A potentiometric response is obtained, which is dependent on potential changes in real time [31]. In most cases, no steps are necessary to prepare the sample for measurement. Moreover, the wide range of linearity of the method allows to avoid dilution or concentration of the sample solution, as is often necessary using ASA or HPLC methods. Most often (if necessary), it is limited to a small addition of ionic strength buffer and/or other substances to the sample solution, immediately before measurement and mixing. Sometimes it can also be necessary to ensure the correct pH of the environment when the pH of the test sample is not in the range, where the electrodes can work without interference. However, in most cases, this range is wide enough to allow the determination of ions directly in the collected sample. The great advantage of ISEs is the ability to test colored or muddy solutions, because both the color and the presence of solid particles do not interfere with the tests.
Among the various constructions of ISEs, a special place is held by electrodes without an internal electrolyte solution, the so-called ion-selective electrodes with solid contact (SCISEs). SCISEs are much more convenient during use, transport and storage, and they are easier to miniaturize and modify their shape and also more mechanically resistant. In addition, they can work in any position and in conditions of increased pressure and temperature, which results from the elimination of the internal solution present in classic electrodes, which acts as a link between the discharge electrode and the ion-selective membrane [13]. For electrodes of this type, it is very important to properly select the material that will function as an ion–electron transducer, thus enabling the correct operation of the electrodes by ensuring appropriate stability and reproducibility of the potential. When developing a new type of SCISEs, there are two main ways to improve their analytical parameters. The first is the use of a new active substance which as a membrane component is responsible for the appropriate selectivity of the sensors towards the selected main ion (especially important in the study of complex samples containing interferences that may interfere with the proper measurement), and the second is the search for new electroactive materials that can be successfully used as solid contact. The use of solid contact is additionally aimed at obtaining a satisfactory stability of the sensor potential, which will allow doing measurements for a longer period of time (weeks, months), often without the need for frequent calibration [9]. Electrodes used for this purpose should also be resistant to changes in measurement conditions (temperature, lighting and the presence of gases) [32]. Due to the common trend towards miniaturization of devices (also due to much smaller amounts of materials used for their construction and compatibility with small volumes of samples necessary to perform measurements) and the desire to use them directly in the natural environment, it is necessary to obtain sensors characterized by sufficiently good values of analytical parameters and, at the same time, easy to use and mechanically resistant [9]. Reliable measurement results can only be achieved with electrodes exhibiting a specific set of characteristics.
In the case of SCISEs, potential stability, both short-term and long-term, is particularly important. Long-term stability can be determined by systematically measuring the potential of the sensors at constant intervals over an extended period of time (e.g., days, weeks and months). This parameter is usually characterized as the change in potential E0 over time. With regard to short-term stability, two approaches are possible. The first one consists of continuous measuring of the electrode potential in a relatively short time (1–3 h, that is the time needed to perform calibration and a series of measurements) in no-current conditions and determining the potential drift as ΔEMF/Δt (Figure 1). The second faster way uses the chronopotentiometry method (CP) and the measurement of the changing electrode potential in time (for example, 60 s) after applying a short-term electrical impulse (the most often in nA) to the electrode. The potential drift, which is a measure of stability, is determined from a rectilinear section of the chronopotentiometric curve from the dE/dt relationship. Based on the determined potential drift, the capacitance of the electrodes can be determined according to the equation C = i/(dE/dt). The chronopotentiometry technique can also be used to determine the resistance of the electrode, which often changes as a result of adding a solid contact material (Figure 2) [33]. Another technique commonly used to assess the effectiveness of a solid contact material is electrochemical impedance spectroscopy (EIS), an advanced method for studying electrode processes. In the case of ISEs, this method allows the determination of the membrane resistance, electric capacitance and charge transfer resistance between the ion-sensitive membrane and the internal electronic conductor [34,35,36]. These data are determined on the basis of the analysis of the impedance spectrum determined in a wide frequency range of 0.1 Hz–100 kHz by the semicircle method or by fitting an equivalent electrical circuit (Figure 3).
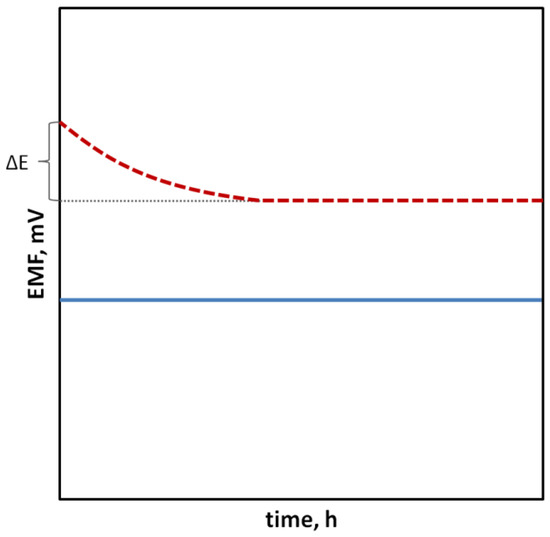
Figure 1.
Change in the electrode potential immersed in the solution of the main ion in time, indicating electrode with more stable (⸺) and less stable (- - -) potential.
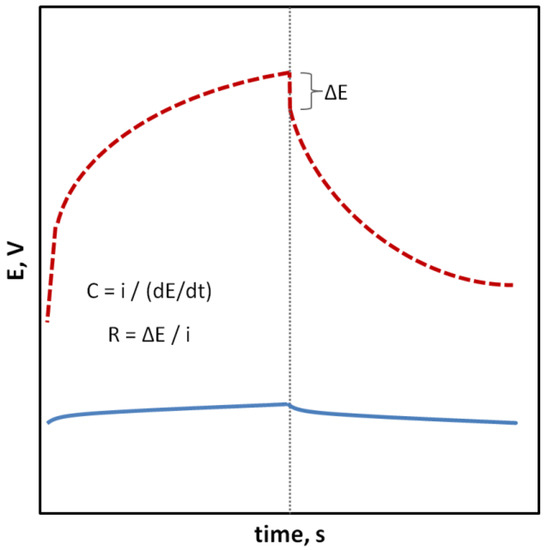
Figure 2.
Chronopotentiogram representing an electrode with a stable (⸺) and less stable (- - -) potential; formulas for determining the electric capacitance (C) and total resistance (R), where potential drift (dE/dt), ΔE—potential jump as a result of changing the direction of the electric current (i).
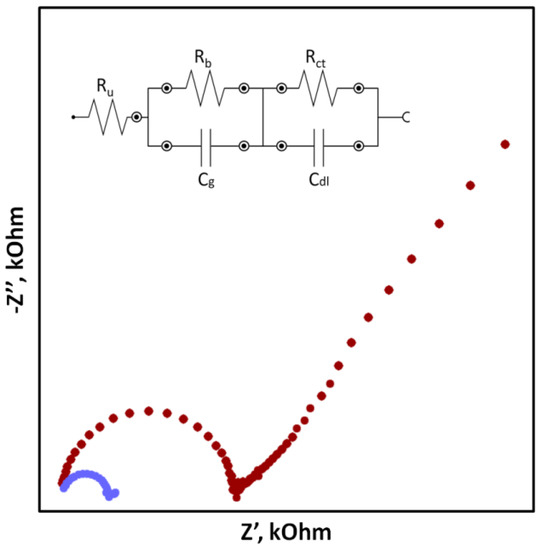
Figure 3.
Exaplary impedance spectra for coated disc electrode without solid contact (red dots) and solid contact electrode (blue dots) with an electrical circuit (where Ruuncompensated series resistance; Rb-bulk resistance; Cg-geometric capacitance; Rct-charge transfer resistance; Cdllow-frequency layer capacitance).
SCISEs are tested for the formation of a water layer, which is formed as a result of water uptake and transport through the ion-selective membrane material [1]. The composition of the water layer may change during the modification of the sample solution composition in which the electrode is immersed, due to the penetration of interfering ions inside the layer, which is the reason for the drift of the measured potential [37]. This is a particularly undesirable process; therefore, water layer tests are performed (usually in accordance with the procedure proposed by Fibbioli et al. [37]) consisting in the use of high-concentration solutions of the main ion and interfering ion, and the observation of the potential drift caused by the change of solutions (in the order: main ion → interfering ion → main ion). Electrodes without a tendency to form a water layer maintain a constant potential after reaching a certain value after immersing the sensor in the interfering ion solution and quickly return to the initial potential value previously obtained in the solution of the main ion to which the membrane is sensitive (Figure 4).
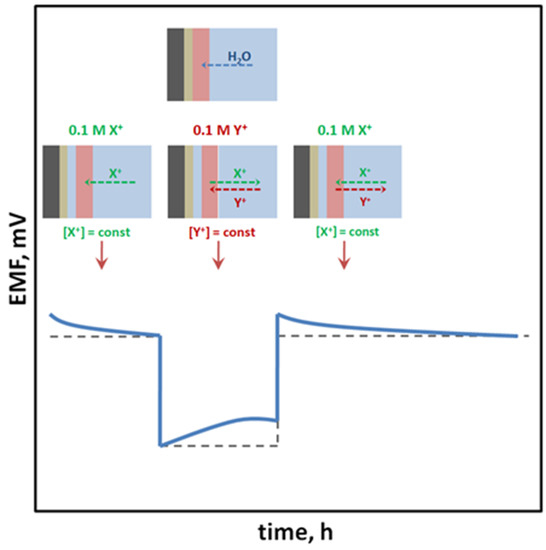
Figure 4.
Results of the water layer test obtained for (⸺) electrodes with a tendency to form an undesirable water layer and (- - -) electrodes without this tendency.
3. Statistics
The high popularity of potentiometric methods can be confirmed by the results of searching for scientific articles in databases. In the case of the SCOPUS database with the “search within article title, abstract, keywords” option, after entering the entries “ion-selective” and “potentiometry”, 3105 results appeared (as of 21 May 2023). Since the turn of the 20th and 21st centuries, articles on this subject appear regularly, often in excess of 100 papers per year, and the vast majority of these papers focus on SCISEs. The first mentions of “solid contact” appeared in 1974, and since the 1990s, the number of articles on this subject has been steadily increasing. In recent years, the construction of SCISEs has been dominated by the use of composite and/or multi-component materials (Figure 5).
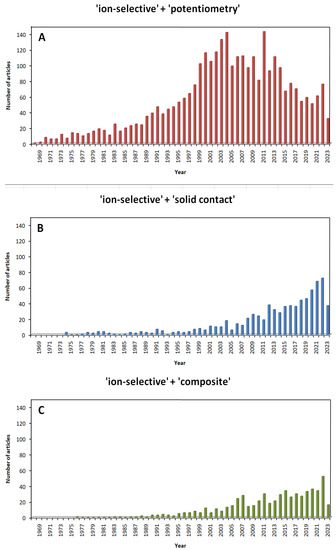
Figure 5.
Results based on the SCOPUS database for scientific articles available after entering selected keywords (contained in “article title, abstract and keywords”) depending on the year of publication (access date: 21 May 2023): (A) ■ “ion-selective” + “potentiometry”; (B) ■ “ion-selective” + “solid contact” and (C) ■ “ion-selective” + “composite”.
4. Solid Contact Materials
SCISEs are simple and easy to use and do not require complicated and expensive equipment. The production of electrodes is also, in most cases, not a complicated or very expensive process, but the cost depends on the type of selected internal electrodes and the market price of materials used in the construction or the synthesis of these materials. In order to improve the analytical parameters of sensors, many materials have been used till now as solid contact. In most cases, this affected mainly the electrical parameters of the electrodes, in particular, increasing the capacitance of the double layer and reducing the membrane resistance. An important change resulting from the additional modification of the electrode construction is a significant improvement in the stability of their potential compared to unmodified electrodes [38,39]. Sometimes, analytical parameters are also improved, the range of linearity of the calibration curve can be extended [40,41] or the detection limit can be lowered [40,42]. Selection of the appropriate conductive material is particularly important due to the significant impact of the type of intermediate layer also on the stability of the composition of the ion-selective membrane [43].
Newly developed materials which could be used as solid contact should meet a number of requirements. Firstly, they must be materials with a high volumetric capacity ensuring potential stability and chemically stable, not generating any side reactions in the ion–electron transduction process. In addition, the conversion process between ionic and electronic conductivity should be reversible. It is also important that the material is sufficiently hydrophobic so that no water layer forms between the inner electrode material and the ion-selective membrane [44]. The choice of the material used as SC should be made taking into account many requirements and parameters, for example, based on the method and cost of their synthesis, their mechanical resistance, durability and analytical effectiveness and lifetime [31]. The way of applying the solid contact material is twofold. Most often, it is used as an intermediate layer between the electrode surface and the ion-sensitive membrane, which is applied to the substrate by drop casting or electrochemical deposition. The second way is to use a solid contact material as a component of the ion-selective membrane (Figure 6).
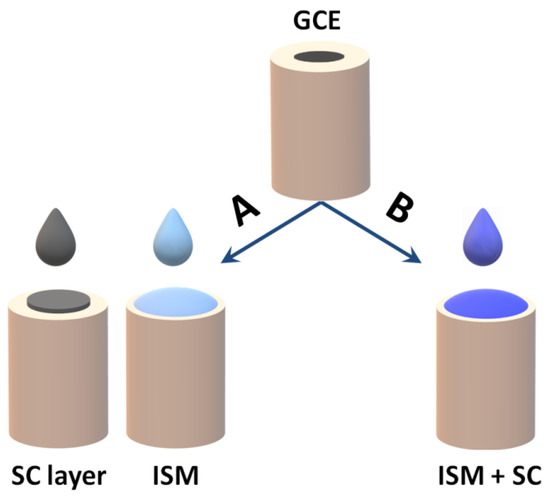
Figure 6.
Scheme of SCISEs preparation: (A) 2 steps—the application of an SC layer and then covering it with an ISM layer and (B) 1 step—the direct application of ISM with SC additive.
4.1. Conductive Polymers
The first materials used as solid contact were conductive polymers (CPs). From this group of compounds, polypyrrole (PPy) [45,46,47] was used for this purpose already in the 1990s, then also poly(3,4-ethylenedioxythiophene) (PEDOT) [48,49,50], poli(3-octylothiophen) (POT) [51,52,53,54,55], poly(N-methylpyrrole) (PNMP) [56] or polyaniline (PANI) [57,58,59]. Various types of conductive nanostructured polymers mainly based on polyaniline were also used for the construction of the sensors: in the form of nanotubes [60], nanofibers [61], nanoparticles [62] or nanowires [63,64].
The wide application of CPs is due to their numerous advantages: simplicity of synthesis and variety of possible modifications using other materials, high environmental stability, attractive price and unique chemical structures [65,66]. These compounds are characterized by double conductivity (ionic and electronic) and therefore can be successfully used as ion–electron transducers placed in the form of an intermediate layer of solid contact in ion-selective electrodes or as an additional component dissolved in the membrane or in a functionalized form as ready-made membranes [67].
The first sensors using CPs had significantly improved membrane conductivity but often were sensitive to changing environmental conditions (light, the presence of oxygen and carbon dioxide in the sample solution and changes in pH) as a result of side electrochemical reactions [66]. These materials also often did not show sufficiently high hydrophobicity, which resulted in the formation of a water layer between the membrane and the inner electrode. Therefore, the search for materials with similar properties, but differing in the method of synthesis, structure or composition and more hydrophobic, was started in order to improve the adhesion of the ion-selective membrane and reduce water absorption [9].
4.2. Carbon Nanomaterials
Carbon nanomaterials (CNs) are another group of materials with unique properties that have so far been commonly used in the construction of ion-selective electrodes. They are defined as materials with at least one dimension less than 100 nm [68]. They are particularly popular mainly due to their unique chemical, physical and electrical properties, i.e., mechanical resistance, hydrophobicity, good electrical conductivity and high electrical capacity [44,69]. The very high surface-to-volume ratio resulting from the small size of the nanostructures favors their interaction with neighboring materials, and their unique electrical properties improve potential stability, which makes them particularly attractive materials that can be used as ion–electron transducers in SCISEs [29]. The properties of the synthesized nanomaterials and their subsequent possible applications depend to a large extent on the method used for their synthesis [70,71]. In addition, these nanomaterials can be subjected to various types of modifications, e.g., by attaching selected functional groups to them in the process of derivatization in order to change their properties. In this way, it is possible to obtain nanomaterials characterized, for example, by better solubility or chemical reactivity compared to the starting materials [72,73,74]. Among the most popular carbon nanomaterials used in SCISEs are carbon nanotubes (CNTs) invented by Iijima in 1991. Numerous review articles refer to them [75,76,77,78,79,80,81,82,83,84], which describe methods of obtaining and purifying CNTs and their extensively studied properties, possible functionalization reactions and their use in the synthesis of nanocomposites. There are also many articles focusing on their use in electrochemistry [85,86,87,88].
Scientists have already described the results of research on the use of various types of carbon nanomaterials as a solid contact in the construction of electrodes: SWCNTs [69,89,90,91], MWCNTs [92,93,94,95,96], fullerenes [97,98], nanohorns [99], graphene [100,101] or colloid-imprinted mesoporous carbon (CIM) [39], amorphous carbon in the form of carbon black [102], three-dimensionally ordered microporous carbon (3DOM) [42,103] or chemically and electrochemically reduced graphene oxide (CRGO [104] and ERGO [105]).
4.3. Metal and Metal Oxide Nanoparticles
Currently, there are more and more studies on various types of nanoparticles (by definition, materials with at least two dimensions below 100 nm [68]) (mainly metal and metal oxide nanoparticles), their properties and possible applications [106]. One such application is the use of metal nanoparticles to improve the analytical parameters and electrical potentiometric sensors, which is possible due to their high redox activity and very large surface area. So far, metal nanoparticles have already been used for this purpose: gold [107,108,109,110], silver [111] and platinum nanoparticles [112,113,114]; metal oxides: copper(II) oxide [115], cerium oxide [116], ruthenium oxide [117] and iridium oxide nanoparticles [118], as well as bimetallic [119] or metal–nonmetal nanoparticles [120]. In addition to typical studies on the influence of the presence of nanoparticles on the direct operation of sensors, the use of silver nanoparticles with known bactericidal and antimicrobial properties [121,122] to extend the life of electrodes used to study complex environmental samples exposed to the presence of microorganisms and biofilms has also been described [123].
Metal oxides (PtO2, IrO2, RuO2, OsO2, Ta2O5 and TiO2) have been used in the construction of pH electrodes almost 40 years ago [124]. It has been confirmed that the modification of sensors with nanoparticles has a positive effect on the potential stability as a result of low resistance values and high capacitances achieved [116,117].
4.4. Nanocomposites
By definition, composites are materials that were created from at least two components different from each other, in order to significantly improve selected parameters characterizing the material and/or obtain new materials properties. The effective use of many single-component materials was the starting point for research on the development and use of composite materials in the field of ion-selective electrodes. Composites of carbon nanomaterials and polymers are particularly popular [24]. Addition, among others carbon nanotubes to polymers, makes it possible to obtain composite materials with improved mechanical, thermal, electrical, optical and chemical properties [24,25,125,126]. Composites obtained from polymers and carbon nanomaterials in combination with other materials, such as metal nanoparticles, metal oxides, ionic liquids and many others, are widely described [127,128,129,130]. Using various methods, scientists produce more and more composite materials that are a mixture of various types of components, for which, after research using a number of analytical methods (including Scanning and Transmission Electron Microscopy, Photoluminescence, UV-Vis and IR Spectroscopy, Raman Scattering, Diffuse Reflectance Spectroscopy, Energy-Dispersive X-Ray Spectroscopy, X-Ray Photoelectron Spectroscopy or X-Ray Diffraction), new applications are sought. These applications also include the use of newly synthesized materials as a solid contact material in SCISEs.
It is worth mentioning that nanocomposites based on conductive polymers with the addition of other materials or subjected to appropriate reactions are also used as an active substance added to the membrane mixture acting as a cation exchanger (often still in the case of electrodes with liquid contact, not described in detail in this review), among others PMMA-CeMoO4 nanocomposite [131], PANI-ZrI nanocomposite [132], [N,N′-bis(1-hydroxynaphthalene-2-carbaldehyde)-o-phenylenediamine] [133] and N,N’-bis(salicylaldehyde)phenylenediamine (salophen) [134].
Many examples of the use of composite materials as solid contact in ISEs have been described in the literature so far. An overview of such applications described in the literature in recent years is presented in the following subsections.
4.4.1. Composites Based on Polymers and Carbon Nanomaterials
Composites made of various types of carbon nanomaterials and polymers have recently become very popular due to the advantages resulting from the combination of materials with significantly different properties. There are many methods to obtain this type of two-component nanocomposite, which can show much better mechanical resistance and increase electrical conductivity even by several orders of magnitude compared to its components separately [25]. This group of composites is dominated by materials obtained on the basis of conductive polymers and carbon nanotubes.
Nanocomposite obtained during the electrochemical synthesis of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT), where MWCNTs were used as dopants, was described in [135]. The electroactive film, PEDOT(CNT) was used as solid contact in K+-SCISEs. The electrical parameters of the electrodes were examined and sensors were also tested for sensitivity to changing measurement conditions. It was observed that the PEDOT(CNT) composite layer itself was sensitive to the presence of CO2, but after covering it with a polymer membrane, this sensitivity was no longer observed. The electrodes with the nanocomposite layer showed a low potential drift (12.0 µV s−1, i ± 1 nA), which was lower than the drift determined for the electrode with the Cl– ion-doped PEDOT layer (17.6 µV s−1, i ± 1 nA). The effect of differences in low-frequency capacitance values was also noticeable (83 µF for ISE with PEDOT(CNT) and 57 µF for ISE with PEDOT(Cl)).
Electrodes sensitive to Ag+ ions using modified polypyrrole–multiwalled carbon nanotubes composite as SC with newly synthesized lariat ether as ionophore were described in publication [136]. Very good analytical parameters were obtained: a wide range of linearity 1.0 × 10−7–1.0 × 10−1 M, low detection limit—9.3 × 10−8 M and good selectivity of the sensors. In addition, they operated in a wide range of pH (1.6–7.7) for a minimum period of 50 days. Their practical application was tested on samples of silver sulphadiazine as a burning cream.
In article [137], Athavale et al. proposed sensors for the determination of ammonium ions in the in situ environment (eutrophic lakes). For the construction of the electrodes, composite polyvinyl chloride membrane impregnated with carbon nanotubes (CNT-PVC) and plasticizer-free methyl methacrylate–decyl methacrylate copolymer (MMA-DMA) was used. Profiles of the content of NH4+ ions were made depending on the depth of the water reservoir. The measurement platform, apart from potentiometric sensors, also had other sensors whose purpose was to measure additional parameters useful from the point of view of environmental research: conductivity, temperature, oxygen and photosynthetically active radiation.
A superhydrophobic polymer–carbon nanocomposite composed of a combination of carbon black with a highly porous acrylic-fluorinated graphene copolymer was proposed [138]. The material was characterized by a high capacitance of 1410 mF and a highly developed surface area, and the sensors constructed using it showed a Nernst slope of 59.1 mVdec−1 in the linearity range of 3.16 × 10−7–1 × 10−1 M and a low detection limit of 2.0 × 10−7 M. The use of numerous research methods (CP, EIS, cyclic voltammetry (CV), wettability measurements and tests of potential sensitivity to changing environmental conditions) confirmed that the use of the nanocomposite significantly improved the analytical parameters of the electrodes (potassium in this case).
A composite consisting of exfoliated graphene and polyaniline was used in Ca2+-SCISEs (PANI-graphene). The material was characterized by much better hydrophobicity than PANI (30° higher contact angle with water). The measured redox capacitance of the electrodes with the composite layer was 11 µF and with the PANI layer was 8 µF. Sensors with good stability and potential reproducibility, low detection limit (5.0 × 10−8 M) with no tendency to form a water layer between the substrate material and the ISM were obtained [101].
In publication [139], carbon paste electrodes with graphite-oxide-imprinted polymer composite for the determination of Cu2+ ions were described. The authors made a number of electrodes differing in the qualitative and quantitative composition of the mixture and selected the one for which the best analytical parameters were obtained (20.0% ionophore (Cu2+-ion-imprinted polymer), 10.0% paraffin oil, 5.0% MWCNTs and 65.0% graphite oxide). These sensors had a slope of calibration curve equal to 26.1 dec−1 in the linearity range of 1.0 × 10−6–1.0 × 10−1 M and an LoD of 4.0 × 10−7 M. The sensors worked fast (response time ~ 3 s) in the pH range of 4.0–8.0. While the range of linearity was not very wide, the authors ensured that the lifetime of the electrodes was more than 1 year, which is very promising in the case of potentiometric sensors. The obtained electrodes were used to determine the content of copper(II) ions in water samples (spiked river, dam and tap water) and were also used in potentiometric titration.
Nanocomposite of POT-MWCNTs (poly(3-octylthiophene-2,5-diyl) and multiwalled carbon nanotubes) with high capacitance, electrical conductivity and lipophilicity (in which POT acted as a dispersant) was used as SC in potassium all-solid-state electrodes [140]. Using numerous methods (contact angle and fluorometric measurements, Raman spectroscopy, etc.) the authors examined the properties of the nanocomposite and confirmed that the nanocomposite is characterized by a large contact angle and high conductivity, and its use prevents the unwanted process of POT transfer to the membrane phase, which eliminates the risk of changes in analytical parameters due to uncontrolled changes in the composition of the membrane. The slope of the obtained sensors was 56.3 mVdec−1 in the range of linearity 1 × 10−6–1 × 10−1 M (LoD ~ 1.6 × 10−7 M).
A nanocomposite membrane with the addition of MWCNTs was used in ISEs sensitive to Pb2+ ions based on gold disc electrodes [141]. The preparation of the electrodes was a simple one-step process, which consisted in dispersing MWCNTs in a plasticized ion-selective membrane in the ultrasonication process without the use of surfactants. Sensors with a slope of 29.0 mVdec−1, a linearity range of 2.0 × 10−3–2.0 × 10−9 M and a low detection limit of 4.0 × 10−10 M were obtained. In addition to typical potentiometric measurements, the contact angle test and chronopotentiometric measurements were also performed. The modification of the electrodes significantly reduced the potential drift of the electrodes (from 533.25 µV s−1 to 19.8 µV s−1, i ± 1 nA). According to the authors, the electrodes had very good long-term potential stability and no water layer and were also resistant to changing environmental conditions. The effectiveness of the sensors was checked on the basis of tests of tap water samples.
The planar sensors for the determination of NH4+ ions were described in [142]. The solid contact composite material in which graphite particles were embedded in a polyvinyl butyral matrix was used. The sensors operated in the pH range of 2.5–8.5 and reached a slope of 57.3 mVdec−1. Measurements for the electrodes were made in static and flow mode, and according to the authors, they can be successfully used for measurements in the in situ environment.
Calcium electrodes with a newly synthesized ionophore (4,7-diaza-2,3,8,9-dibenzo-15-crown-5) were described in [143]. A composite of carbon nanotubes and PVC was used as a solid contact. Studies were performed to optimize the composition of the ion-selective membrane, obtaining a low detection limit (9.1 × 10−8 M). The electrodes were used for potentiometric titration and testing of horticulture drain water samples using AAS as a comparative method.
In paper [144], ion-selective electrodes sensitive to chloride ions were described, in which a nanocomposite obtained from MWCNTs and PANINFs-Cl (polyaniline nanofibers doped with chloride ions) was used as the SC. Initial measurements of the electrical parameters (using CP and EIS) of the electrodes modified with an intermediate layer consisting of nanocomposites with different mass ratios of components and a layer containing only MWCNTs or PANINFs-Cl were made. It was confirmed that the nanocomposites showed better electrical parameters (lower resistance, higher capacitance) than their components separately. The best among the tested electrodes turned out to be sensors with an intermediate layer of PANINFs-Cl:MWCNTs nanocomposite in a weight ratio of 2:1. Comparing the SC layers, the layers containing (2:1) PANINFs-Cl:MWCNTs showed a capacitance almost 4 times higher than PANINFs and more than 10 times higher than MWCNTs. In the case of sensors, the electrodes with this layer of nanocomposites were characterized by almost 4 times lower membrane resistance and over 200 times lower charge transfer resistance with a simultaneous almost 200 times increase in the capacity of the double layer, as a result of much faster processes of diffusion and charge transport at the membrane/GCE. In addition, electrodes with a nanocomposite intermediate layer (2:1) PANINFs-Cl:MWCNTs showed a slope of characteristic equal to –61.3 mVdec−1, linearity range of the calibration curve 5 × 10−6–1 × 10−1 M and the lowest detection limit (2.3 × 10−6 M) and the best potential stability (0.03 mVh−1) compared to the other modified electrodes and the unmodified electrode. All sensors were resistant to changes in external conditions and maintained a constant potential in the pH range of 4–9.
GCE-based potentiometric sensors sensitive to cesium ions were described in publication [145]. The SC material was described as magnetic multiwalled carbon nanotubes/cesium-ion-imprinted polymer composite (MWCNTs@Cs-IIP). SEM and TEM images of both the nanocomposite and carbon nanotubes were presented. The process of optimizing the composition of ion-selective membranes with different types of plasticizers (DBP and NPOE) and different content of PVC and other components was performed. The electrode with the optimal composition showed a low detection limit of 4.0 × 10−8 M, but a relatively narrow measuring range of 1 × 10−7 to 1 × 10−4 M. The practical application of the sensors was tested on samples of river water, industrial wastewater and brine.
A multi-component combination of materials was proposed by Niemiec et al. [146] in the work about the modification of various carbon materials (electrospun carbon nanofibers (eCNFs), electrospun carbon nanofibers with embedded cobalt nanoparticles (eCNF-Co) and hierarchical nanocomposite with the nanoparticles of cobalt and nickel as a catalyst for the growth of carbon nanotubes (eCNF/CNT-NiCo)) using a POT polymer. Materials with an interesting structure were obtained, which, as a result of POT modification, were characterized by increased hydrophobicity. In the case of eCNFs and eCNF-Co, a significant increase in capacitance was obtained: from 1.62 to 7.87 mFcm−2 for eCNFs and from 2.67 to 4.37 mFcm−2 for eCNFs-Co, while for eCNF/CNT-NiCo effect was opposite and capacity was reduced. These materials placed as an intermediate layer allowed to obtain potassium sensors with low detection limits (3.2 × 10−6 M) and excellent potential stability (0.03 mVh−1 for the composite with eCNFs).
An interesting solution was presented by the authors in article [147], proposing ternary nanocomposites consisting of three types of materials: conductive polymer (poly(3-octylthiophene-2,5-diyl)), carbon nanomaterials (carbon black and nanotubes) and metal oxide (hydrated iridium dioxide). The obtained materials were characterized by high hydrophobicity (contact angle up to 180°). The obtained electrodes were characterized by very good long-term stability (potential drift: 43 µV h−1 for the NT-based electrodes and 79 μV h−1 for the CB-based electrodes) and high capacitance (1.5 and 0.9 mF, respectively).
In article [116], the same authors described the use of hydrous cerium dioxide nanoparticles and their combination with carbon nanotubes and poly(3-octylthiophene-2,5-diyl) as SC in potassium electrodes. The measured contact angles obtained for the tested materials confirmed that the combination of hydrophilic CeO2 nanoparticles (contact angle 17°) resulted in the formation of superhydrophobic composites (100° and 120° for hCeO2-NTs and hCeO2-POT) with a large surface area. The material with the highest capacitance turned out to be hCeO2-NTs (800 ± 10 µF), which also contributed to achieving the best potential stability by the sensors that were constructed using it. The proposed K+-SCISEs are able to work in variable external conditions and in a very wide range of pH (2.0–11.5).
4.4.2. Composites Based on Polymers with Other Materials
The self-plasticizing triblock copolymer polystyrene-block-polybutadiene-block-polystyrene (PS-PB-PS, SBS), and a composite of this material and an ionic liquid (BMImPF6), was used in the construction of electrodes sensitive to a number of cations (Na+, K+, Ca2+, Cu2+ and Pb2+) on the gold electrode surface [148]. The authors confirmed that the membrane containing SBS is more hydrophobic than the PVC membrane, thanks to which the electrode does not show a tendency to form a water layer. Plasticizer-free electrodes with characteristic slopes similar to Nernst were obtained.
Poly(3,4-ethylenedioxythiophene) doped with poly(4-sodium styrene sulfonate), i.e., PEDOT(PSS), was used as SC in studies on the change in standard potential of electrodes (E0) [149]. Three types of ISM were applied on the layer of SC put previously on GCE: valinomycin-based potassium-selective membranes with and without ETH-500 (the lipophilic salt: tetradocedylammoniumtetrakis(4-clorophenyl)borate) and membrane without ionophore. Based on the results, it was found that the E0 of electrodes containing a conductive polymer as an ion–electron transducer can be moved by applying low-current pulses (nA) or by applying a potential that differs from the open-circuit potential of the sensor in the selected electrolyte solution. According to the authors, the ability to control E0 and its adjustment in a way that can be predicted or repeated can be used in the future in practical measurements.
SCISEs sensitive to ammonium ions were described in publication [58]. The obtained sensors were characterized by an immediate response time (<1 s) and a long lifetime (3 months). The electrodes were able to work in a very wide range of pH (2.6–10.1). Wastewater samples were tested using them and the obtained results were verified by comparing them with the results obtained by means of titration and the colorimetric method.
In publication [150], the authors proposed solution-casted chitosan/Prussian blue nanocomposite (ChPBN) as a solid contact in ISEs sensitive to sodium ions. Thorough morphological studies of the material confirmed that it has the structure of a macroporous chitosan network containing Prussian blue nanoparticles inside. The nanocomposite was placed as an intermediate layer between the ion-selective membrane and the screen-printed carbon electrode. The electrodes were characterized by the slope of the calibration curve of 52.4 mVdec−1 in the range of only 1 × 10−4–1 M, while the electric capacitance estimated by CP was 154 ± 4 μF and potential stability (potential drift of 1.3 μV h−1) was promising. Due to the high volumetric capacity of the synthesized material and its highly porous structure, a large interfacial surface was obtained in relation to the outer layer of the selective sodium membrane, which improved the stability of the electrode potential. The electrodes containing the nanocomposite layer showed better properties than both the unmodified electrodes (which is obvious) and the electrodes in which single components of the nanocomposite were used as SCs.
The next composites that were used as SCs were made by combining polypyrrole and zeolites with different SiO2:Al2O3 ratios, i.e., 23, 80 and 280 (PPy/H-ZSM composites) [151]. SEM pictures of the materials from above and in the cross-section position were taken, which showed the presence of zeolite both on the surface and inside the composite, and the anionic groups of the zeolite acted as counterions. Based on the contact angle tests, it was estimated that the hydrophobicity of the obtained composites decreased with the decrease in the Al2O3 content in the composite structure. Both the analytical and electrical parameters of the potassium electrodes made using them have been extensively studied. The slopes of the electrode calibration curves were in the range of 52.1–53.1 mVdec−1 and were comparable to the values obtained for electrodes based on PPy-Cl; however, for electrodes with a composite, lower detection limits were obtained.
The intermediate layers of SC deposited on GCEs composed of MOMs (molecular organic materials) such as tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ), tetrathiafulvalene (TTF) and its chloride (TTFCl) with/without carbon black were described by Pięk et al. [152]. SEM pictures of the materials and CV tests were taken. All modified chloride electrodes showed a slope in the range of −58.18 and −59.63 mVdec−1. The improvement in the electrical parameters of the electrodes was confirmed by CP and EIS measurements.
K+-SCISEs with SC of a nanocomposite composed of ruthenium dioxide and poly(3-octylthiophene-2,5-diyl) (RuO2+POT) have been described [153]. SEM images and contact angle measurements were taken for the nanocomposite layers (centrifuged ~149° and noncentrifuged ~110°), and much higher values were obtained than for its components separately (RuO2 ~18° and POT ~91°). The parameters of sensors with different SC layers were tested. The electrodes with the nanocomposite layer showed a resistance of 273.7 ± 0.5 kΩ and a capacitance of 1.167 ± 0.028 mF and were resistant to changes in external conditions and the formation of a water layer.
A highly hydrophobic material (with a water contact angle of 104°) prepared by electrospinning e-PANI-PS (hydrophobic polyaniline-polystyrene microfiber films) was used as a solid contact in Pb2+-SCISEs [154]. Electrodes with a very low detection limit (5 × 10−9 M) and a Nernst slope of the characteristic curve (29.1 mVdec−1) were obtained. The sensors showed a much lower resistance and a higher electric capacity compared to the sensors containing only PANI microfibers, and thanks to the high hydrophobicity of the SC material, they were not susceptible to the formation of a water layer. The content of Pb2+ ions in tap water samples was determined and compared with the results obtained using the graphite furnace atomic absorption spectrometry method (GFAAS). The results obtained at the concentration level of 10−8 M were similar to those obtained by the comparative method.
In article [155], the authors proposed miniaturized all-solid-state electrodes (5 mm diameter), created from the coating of Au electrodes with poly(3-octyl-thiophene) and molybdenum disulfide nanocomposite (POT-MoS2), which can be successfully used to monitor nitrate content in soil samples. Small-size reference electrodes coupled with miniaturized ISEs have also been constructed, which consist of a screen-printed Ag/AgCl electrode covered with a protonated Nafion layer (to prevent chloride ions (Cl−) leaching during measurements). The practical application of the construction was tested by immersing the sensor in soil suspension for almost a month, where the nitrate concentration was measured continuously.
In the construction of Pb2+-SCISEs, polyaniline doped with titanium dioxide (PANI-TiO2) was used as an intermediate layer of SC [156]. Using X-ray diffraction (XRD), the structure of the materials was investigated. A very low detection limit of 7.9 × 10−10 M was achieved. The obtained electrodes were resistant to environmental changes (presence of gases, change of lighting) and were with very good selectivity, which is particularly important in the possible testing of environmental samples. As part of the analytical application of SCISEs, three samples of tap water were tested, and the obtained results were compared with those obtained using an atomic absorption spectrometry (AAS) method.
In addition, the combination of poly(3,4-ethylenedioxythiophene) backbone with hydroquinone pendant groups (PEDOT-HQ) was used as solid contact in K+-SCISEs [157]. A number of tests of the stability of the sensors in variable external conditions (gases, light) were carried out. A low detection limit of 2.0 × 10−7 M and a close to the theoretical slope of the calibration curve of 60.9 mVdec–1 were obtained.
A new nanocomposite material—ruthenium dioxide and poly(3,4-ethylenedio xythiophene) polystyrenesulfonate composite—was used as SC in K+-SCISEs [158]. The nanocomposite was characterized by a very high capacitance (~17.5 mF), as was the electrode with a layer of this material (~7.2 mF). SC layers of different thicknesses were examined by CP, and then electrodes with optimal SC thickness were made. Sensors with good potential stability, Nernst slope, resistance to changes in external conditions and the formation of an undesirable water layer were obtained.
Lead electrodes with SC made of silver nanoparticles and polyaniline were described in article [159]. Several methods were used to test the sensors, including XPS, CV and EIS. A very low detection limit (6.3 × 10−10 M) and fast sensor response time (<5 s) were obtained. Tap water samples were analyzed using atomic absorption spectrometry (GFAAS) as a comparative method.
SCISEs for the determination of anions: Cl− and NO3− with a short response time were constructed using an electrodeposited composite layer of manganese dioxide and poly(allylamine) [160]. A number of studies were performed including X-ray diffraction (XRD), voltammetry, EIS and CP. Modified sensors were characterized by much better parameters than sensors with ISM applied directly to the Pt electrode material.
Application of the synthesized material EDOT-S (3,4-ethylenedioxythiophene and 4-(2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl-methoxy)-1-butanesulfonic acid, sodium salt) in the construction of SCISEs sensitive to potassium and calcium ions on a substrate with gold disk electrodes was described in [161]. Sensors with a slope of the calibration curve close to Nernst’s (57.2 and 28.5 mVdec–1 for K+ and Ca2+, respectively) were obtained. The obtained electrodes were used to determine the content of appropriate ions in the water sample from the stream, and the obtained results were compared with the values obtained by other analytical methods: ICP-OES (for K+) and colorimetry (for Ca2+). Promising results were obtained: 28.1 ± 1.05 and 28.0 ± 0.71 µM K+ and 257 ± 7.27 and 262 ± 1.5 µM Ca2+ (where the first result was obtained potentiometrically, and the second by comparative method).
The biocompatible and stable conductive material used, as the authors suggested, in bioelectronics was poly(3,4-ethylenedioxythiophene)-polyethylene glycol (PEDOT:PEG), which was used as SC in NO3−-SCISEs based on carbon (C110) screen-printed electrodes (SPEs) [162]. A simple fabrication method was employed, which involved applying a PEDOT:PEG mixture onto the surface of the working electrode, drying it, immersing the electrode in a solution of the selected salt (in order to carry out the ion-exchange process of the originally present ClO4– ions) and subsequently applying the ISM. After drying and conditioning, sensors were obtained with good selectivity and a slope of the calibration curve maintained for a period of 72 days (change from −55.8 to −53.3 mVdec−1). In order to check the practical applicability of the electrodes, the content of nitrate ions was determined in agricultural medium samples, which had not been subjected to any pre-treatment. Satisfactory results were obtained (less than ±2% logarithmic deviation compared to the comparative method).
4.4.3. Composites Based on Carbon Nanomaterials with Other Materials
A composite material in the form of carbon black supporting platinum nanoparticles (PtNPs-CB) was used as an SC in nitrate electrodes [163]. SEM and TEM images were taken and the size and distribution of Pt nanoparticles were estimated. Tests for the formation of a water layer of the sensors were also carried out, and the dependence of their potential on the redox potential of the sample solutions was examined. Low detection limits were obtained (7.9 × 10−7 M for K+-SCISEs and 5.0 × 10−7 M for NO3−-SCISEs). The authors confirmed that the long-term stability of the sensors with PtNPs-CB was much better than for electrodes in which the components of the composite were used as a solid contact separately.
The combination of nanomaterials (SWCNTs and graphene) with an ionic liquid (tetradodecylammoniumtetrakis (4-chlorophenyl) borate, ETH 500) was used as SC in electrodes sensitive to Cu2+ and Pb2+ ions [164]. Electrodes with very low detection limits (4.0 × 10−9 and 1.8 × 10−9 M, respectively) and with good selectivity were obtained. Impedance measurements and water layer tests were also performed.
SCISEs for the continuous measurements of the H2PO4− ions concentration were described in publication [165]. The obtained polymer–MWCNTs nanocomposite was characterized by high hydrophobicity and, when added to the polymer membrane, increased its hydrophobicity. The contact angle of the membrane with the composite increased by 10° compared to the membrane without the composite and was 92.8 ± 0.3°. The electrodes made using the nanocomposite showed the Nernstian slope of the calibration curve and were not prone to the formation of a water layer. The sensors were used to study samples of eutrophicated water, using spectrophotometry as a comparative method. Very similar results were obtained: for SCISEs (504 ± 0.05 µM) and for UV-VIS (502 ± 0.08 µM) H2PO4− ions.
The SCISEs with graphene–tetrathiafulvalene nanocomposite (graphene-TTF/TTF+) were described in article [166]. This material showed high capacity, both double layer and redox capacitance (which was confirmed by CP) and high hydrophobicity. For a better study of the effect of the addition of the nanocomposite, electrodes with graphene as SC were also constructed. The electrodes containing the nanocomposite were characterized by better analytical parameters compared to the electrodes with graphene: the slope −59.14 instead of –58.3 mVdec–1 and the linearity range 1 × 10−6–1 × 10−1 M instead of 3 × 10−6–1 × 10−1 M.
In article [167], a number of different types of solid contact layers were investigated, which included various combinations containing, among others, graphene and 7,7,8,8-tetracyanoquinodimethane (TCNQ) and its copper salt (TCNQ-Cu), including nanocomposites made of these ingredients. Both the analytical and electrical parameters of electrodes based on composite materials were better compared to electrodes based on TCNQ and TCNQ-Cu alone. For the electrode with the GR, TCNQ and TCNQ-Cu nanocomposite, the detection limit was 2.5 × 10−9 M and the linearity range was 1.0 × 10−8–1.0 × 10−2 M. In terms of electrical parameters, compared to the basic electrode without SC, the resistance of the proposed electrode decreased almost 8 times (from 1251 to 161 kΩ), capacitance increased almost 200 times (from 2.14 to 396 µF), while potential drift decreased over 180 times (from 4660 to 25.2 µV s−1). The obtained sensors were successfully used to determine the content of copper(II) ions in water samples and packaging of food products, determining recovery and using voltammetry as a comparative method.
As proposed by Li et al., the three-dimensional porous graphene-mesoporous platinum nanoparticles composite (3D PGR-MPN) material was characterized by high hydrophobicity, conductivity and capacity of the double layer (1.4 mF) [168]. The obtained composite was also examined using SEM (scanning electron microscopy), HRTEM (high-resolution transmission electron microscopy), EDS (energy dispersive spectroscopy) and XPS (X-ray powder diffraction). It was used to construct electrodes sensitive to Cd2+ ions, which were additionally investigated by CV and EIS. Electrodes with a very low detection limit (1.6 × 10–9 M) were obtained. The electrodes showed no tendency to form a water layer and were insensitive to changes in lighting and gas content in solutions.
In paper [113], a two-fold application of graphene supporting platinum nanoparticles was described. This composite was used as a solid contact in K+-SCISEs, which were characterized by a Nernst slope of the calibration curve (59.1 mVdec–1) and good stability and potential reversibility, and in voltammetric sensors for paracetamol determination in the concentration range from 0.02 to 2.2 μM.
Bimetallic nanoparticles (AuCu) coupled with multiwalled carbon nanotubes (MWCNTs) were also used as a solid contact in ISEs [119]. This time in two types of ion-sensitive electrodes (Ca2+ and SO42– ions) for which the slope of the calibration curves was 29.0 and 27.0 mVdec−1, respectively, and the linearity ranges were 1.0× 10−6–1.0 × 10−1 and 1.0 × 10−5–1.0 × 10−1 M. The electrodes were also characterized by fast charge transfer (resistance 2.22 and 0.46 MΩ) and high double-layer capacitance (54 and 105 µF). The sensors were then used in practical determinations of ions in water and milk samples.
The nanocomposite MWCNTs:THTDPCl (ionic liquid: trihexyltetradecylphosphonium chloride) was used for the construction of GC-based electrodes sensitive to nitrate(V) ions [169]. The interaction of the cations of the ionic liquid with the surface of the MWCNTs ensures electrostatic and steric stabilization, which enables the formation of a homogeneous nanocomposite material that is easily dispersed in the polymer membrane material. In research nanocomposites obtained from various types of carbon nanotubes of different sizes (length and diameter) were used. As a comparative system, unmodified electrodes (ion-selective membrane without the addition of nanocomposite) and electrodes with solid contact in the form of an intermediate layer of MWCNTs were tested. It was found that the type of nanotubes in the nanocomposite had a significant impact on the parameters of the obtained electrodes, and the best results were obtained for the nanocomposite with MWCNTs, which were the shortest and most homogeneous among all others. The result of this was the synthesis of the most homogeneous membrane. Compared to the unmodified sensors, the electrodes containing the nanocomposite in the membrane were characterized by an order of magnitude greater linearity (1 × 10−6–1 × 10−1 M), a lower limit of detection (5 × 10−7 M) and also a higher slope of the calibration curve (−57.1 mVdec−1). The conducted water layer test showed that when the solutions were changed, the potential drift of the modified electrodes was much smaller than that of the unmodified electrode. It was also shown that the use of nanotubes in the form of a nanocomposite as a component of ISM is more effective than using them as an intermediate layer between the membrane and GCE. An additional advantage of the use of the nanocomposite was a simpler one-step method of electrode preparation.
The next publication on copper electrodes focused on the influence of the type of solid contact on the sensors’ behavior. The main object of interest in these studies was the nanocomposite MWCNTs:BMImPF6 (ionic liquid: 1-butyl-3-methylimidazolium hexafluorophosphate) in a weight ratio of 1:5 [170]. The interaction of the components of the composite results in its steric and electrostatic stabilization. This prevents aggregation of composite nanostructures and allows for uniform dispersion in the polymer phase of the ion-selective membrane. The sensors with the nanocomposite content in the membrane equal to 0, 2, 4, 6 and 8 wt.% were prepared. The modified electrodes were characterized by a higher slope and lower detection limits compared to the unmodified electrode and the electrode with liquid contact. The addition of 6% of the nanocomposite turned out to be the best in this case. The response time of the modified electrode was shorter, and the reversibility and stability of the potential were significantly improved. The potential drift decreased from 0.16 to 0.046 mVmin–1. The water layer test and the tests of potential stability depending on the change in the redox potential or the presence of gases were performed. After three months of use, the slope of the calibration curve slightly decreased (from 29.8 to 28.6 mVdec–1). The modification of the membrane with the addition of a nanocomposite resulted in improved analytical parameters. The determined membrane resistance of the sensors decreased more than 10 times (from 309 to 0.36 kΩ), and the capacitance in the low-frequency range increased more than 35 times (from 1.29 to 45.7 µF).
Nanocomposite materials consisting of ruthenium oxide and carbon nanomaterials (single-layer graphene, carbon black and multiwalled carbon nanotubes) with high capacitance (up to about 14 mF for MWCNTs + RuO2) and very low hydrophilicity were used in the construction of potassium electrodes [171]. The properties of the obtained nanocomposites as well as unmodified carbon nanomaterials were examined, confirming that the addition of RuO2 improves the electrical parameters of the materials. Sensors with very good stability and potential reversibility were obtained, with linearity in the range of 10−6–10−1 M, resistant to the formation of an undesirable water layer.
Studies on the construction of Fe2+-SCISEs using MWCNTs–gemifloxacin composite were described in publication [172]. The resulting sensor had a wide range of linearity (1 × 10−2–1 × 10−8 M) with a slope of 30.37 ± 0.3 mVdec−1 and a fast response time (5 s). Practical determination was carried out in samples of water, milk and multivitamin tablets, obtaining comparable results.
Comparative studies of materials that can be used as SCs in SCISEs have been described also in [173]. The study involved, among others, electrospun carbon nanofibers with/without incorporated cobalt nanoparticles and hierarchical nanocomposites of carbon nanotubes deposited on nanofibers with different cobalt/nickel nanoparticles. Both SEM pictures of the materials were taken and contact angles were measured to determine their degree of hydrophobicity. Electrodes with good potential stability, no tendency to form a water layer, resistance to changes in lighting and operating in a wide range of pH (2.0–10.5) were obtained. The best parameters were for SCISEs containing a layer of carbon nanofibers with high-density NiCo nanoparticles: detection limit of 5 × 10−7 M, linear range of 1 × 10–6–1 ×10−1 M and the highest potential capacity and reproducibility among all electrodes. The highest hydrophobicity was found in the layer of carbon nanofibers with carbon nanotubes and cobalt nanoparticles (contact angle 168°), and the electrodes with this SC layer showed the best reversibility and potential stability. The sensors were used to determine the content of potassium ions in the tomato juice samples and the recovery percentage was determined.
A smartphone-compatible portable system capable of real-time determination of Ca2+ ions in biological fluids using polystyrene-graphite nanoplatelets and carbon black as a solid contact was described in [174]. A series of SEM images of the tested materials and optimization of the qualitative and quantitative composition of the ion-selective membrane were taken. The electrical parameters of the electrodes (capacity 47.5 µF) were also measured, and tests of sensitivity to gases (CO2 and O2) and changes in lighting were performed. According to the authors, the production cost of the platform is low (<$25), but it is possible to test samples with very small volumes (<10 µL) in a short time (response time < 5 s). Tests in urine, artificial serum and artificial cerebrospinal fluid were successfully performed.
The highly stretchable 3D graphene oxide–carbon nanotubes composite was used in the construction of electrodes sensitive to ammonium ions with a slope of 59.6 mVdec−1, good potential reversibility and no tendency to form a water layer [175]. The nanocomposite layer was deposited on the electrode as a result of the electrodeposition process. The main application of the sensors is the determination of NH4+ ions in sweat to monitor the health of patients.
An interesting solution was presented in article [176], which describes fully flexible and wireless detection systems for Na+ ions, which integrates gold–carbon nanotube–gold sensors (Au/CNT/Au), with the use of which the calibration curve slope of 55.5 ± 0.3 mVdec−1 was obtained. This device is a good response to the miniaturization trend that has been popular in recent years. The production is a combination of the drop-casting method and electrochemical deposition method, and in addition to the ISE, an integrated miniaturized reference electrode was also prepared. The thin-film nanocomposite sensor showed high stability and mechanical strength.
Application as solid contact composite aerogel fabricated of cellulose fibers and carbon nanotubes in a homogeneous dispersion process was described in article [177]. The authors investigated the properties of the resulting composites using a number of analytical methods, including FTIR, SEM and thermal analysis. Sensors sensitive to potassium ions showed a good slope of the calibration curve equal to 52.0 mVdec–1, unfortunately in the range of only 1 × 10−4–1 × 10−1 M.
Pb2+-SCISEs electrodes obtained by adding a nanocomposite of carbon nanofibers (CNFs with a 100 nm diameter and a 20–200 µm length) and 1-hexyl-3-methylimidazolium hexafluorophosphate (ionic liquid: HMInPF6) to a polymer membrane were presented in article [178]. Studies were carried out to optimize the content of the nanocomposite in the ion-selective membrane (0–9% w/w) in two types of substrate electrodes: a platinum wire and a glassy carbon disc electrode. The analytical and electrical parameters of the electrodes containing the nanocomposite in the membrane were significantly improved compared to the unmodified electrodes. The best results were obtained for the Pt-based electrode with a membrane containing 6% of the nanocomposite addition. The determined value of bulk resistance decreased from 762 to 46.5 kΩ and charge transfer resistance from 9832 to 28.6 kΩ as a result of the addition of the nanocomposite. Electrodes with excellent selectivity (significantly better than electrodes without nanocomposite) and low detection limit (6 × 10−9 M) were obtained, with the use of which it was possible to successfully determine the content of lead ions in the certified reference material of wastewater (CRM SPS-WW1 Batch 113) with a result of 98.3 ± 3.8 µgL−1 for a given value of 100 ± 0.5 µg L−1.
In work [179], a nanocomposite of copper(II) oxide nanoparticles and multiwalled carbon nanotubes was used as solid contact of electrodes sensitive to Cu2+ ions. The sensors were also constructed using the components of the nanocomposite separately. The solid contact material was placed both as an intermediate layer and as a component of the membrane mixture. The electrodes with the nanocomposite in the membrane showed the lowest detection limit among all the tested electrodes (1.5 × 10−8 M) and the best short-term (132.0 µV h−1) and long-term potential stability (slope change during 8 weeks, 30.05 ± 0.09 mVdec−1). Based on the EIS measurements, it was found that the low-frequency capacitance of electrodes with SC material in the membrane was significantly higher for the addition of nanocomposite (92.5 µF) than for nanoparticles and carbon nanotubes (8.4 and 45.9 µF, respectively). The sensors were used to determine the content of copper ions in tap and mineral water samples.
4.4.4. Other Composites
Ammonium electrodes constructed using SiO2/ZrO2/phosphate-NH4+ composite were described in [180]. The preparation of the composite was quite time-consuming, but low detection limits (1.6 × 10−7 M) were obtained. The electrodes were used to measure NH4+ ions in natural water samples.
Au and Pt nanostructures deposited by the electrochemical method (according to the authors, a process lasting only 6 min) played the role of SC in electrodes sensitive to Li+ ions [181]. The SC layer consisted of Au nanocorals and Pt nanoflowers together and separately. The finished materials were characterized by high hydrophobicity, high double-layer capacity and developed surface.
The material made from tetrathiafulvalene and tetracyanoquinodimethane (TTF-TCNQ) was described in article [182]. It was used as the SC in K+-SCISEs and NO3–-SCISEs, and slopes close to Nernst’s were obtained (58.52 and 58.47 mVdec−1, respectively). The solid contact material was examined using additional techniques, such as SEM or UV-VIS spectroscopy, and the electrode potential in solutions with different redox potentials was measured. The electrical parameters of the sensors were estimated using the CP method as well. High capacitance (255 and 629 µF) and low resistance (166 and 89 kΩ) were obtained for them. These parameters were significantly improved compared to SC-free ISEs.
The structures of potassium and nitrate electrodes were described in [183]. Conductive metal-organic frameworks (MOFs) were used as an SC, thanks to which sensors with high capacitance (204 µF) and good potential stability (drift of potential 11.1 µA h–1) were obtained. The electrodes did not show a tendency to form a water layer.
Electrodes with SC, resistant to changes in external factors and to the formation of a water layer, in which MoS2/Fe3O4 composite was used as SC, were also described in article [184]. For the construction of the electrodes, GCEs were used, on which a nanocomposite suspension obtained by the solvothermal method was dripped and then covered with a layer of membrane mixture. The resistance estimated for the electrode with the nanocomposite layer was 0.36 MΩ and was lower than for the electrodes with the MoS2 layer (0.47 MΩ) and also electrodes without the SC layer (0.52 mΩ). The potential drift rates were 2.9, 60.0 and 457.1 µV s–1 (for i = ±1 nA), and as expected, the electrodes with a MoS2/Fe3O4 layer exhibited the smallest drift.
In article [185], the authors proposed the use of the CoNiFe2O4 magnetic nanocomposite in Ce3+-SCISEs. X-ray diffraction and FTIR analysis methods were used to investigate the properties of the nanocomposite, such as purity and crystallization. A very wide range of linearity of the curve (1.0 × 10−8–1.0 × 10−1 M) and a low limit of detection (7 × 10−9 M) were obtained at the unfortunately quite low slope of the electrode characteristics (17.5 mVdec−1). However, the sensors can operate in a very wide pH range of 2–10. According to the authors, the electrodes were prepared from graphite rods of batteries, on which membrane mixtures with the addition of a nanocomposite suspension in THF were dripped. Sensors containing benzo-15-crown-5 as an active substance were characterized by very good selectivity. They were used to determine samples of water, milk and Coca-Cola, estimating recovery (at the level of 85–95%).
5. Comparative Studies
In Table 1, basic analytical parameters for electrodes prepared with composite materials are presented. One of the parameters significantly influenced by the appliance of solid contact is the stability of the potential. As mentioned in the introduction, the stability of the SCISEs’ potential can be assessed in two ways: potentiometric under the no-current condition and constant-current chronopotentiometry under polarizing conditions. The method of determining the potential drift is marked in brackets with the current value. As can be seen in Table 1, composite-based electrodes showed good potential stability (small value of potential drift). It is difficult to reliably compare the effectiveness of the described composites in improving the potential stability because the reported drift values were determined under different conditions (different measurement time, different ion concentrations in the solution for no-current conditions) and different current values in the case of constant-current chronopotentiometry. Among the data determined for i = 0, the lowest potential drift values of several dozen µVh−1 were obtained for electrodes based on ChPBN nanocomposite [150], graphene-TTF/TTF [166], PtNPs-CB [163], MOFs [183] and oAuCuNPs-MWCNTs [119]. Apart from the improved potential stability, the authors very often report the lowering of the detection limit and the extension of the measurement range as a result of the use of composite materials. Similar results have also been reported for electrodes based on single-component SC.

Table 1.
Summary of analytical parameters for electrodes described in the literature with a solid contact, ordered according to the type of ion.
6. Conclusions
This has been a review of the use of composite materials in the construction of all solid-state ion-selective electrodes. Based on the summary presented in Figure 5, which shows the number of articles over time, it can be concluded that the interest in composite materials in this area has increased significantly in recent years. Based on the overview of more than 60 different composite materials, it can be observed that the overwhelming majority of them have been used as a solid contact in PVC-based polymeric membrane electrodes. Many new composite materials have been developed, based on materials used in the construction of electrodes separately, i.e., conductive polymers, carbon nanomaterials and others. Composites combine the desired properties of ingredients, which allows to obtain a material with better parameters (higher electric capacitance, lower resistance, larger surface area and more hydrophobic character). Thanks to this, the use of a composite is more effective than a single component. Many authors demonstrated in their works that composites show significantly better electrical parameters, i.e., higher electric capacitance and lower resistance. For some materials, very high capacitance values of the order of mF [158] are quoted. Interestingly, there is no simple proportionality between capacitance and SCISE potential stability. Thus, electrodes significantly different in capacitance show similar potential stability [144,158]. This proves that capacitance is an important parameter affecting the stability of the electrode potential, but other factors such as the composition of the membrane, the type of ion to which the electrode is sensitive and the substrate material also affect this parameter. Composites, such as single-component materials, are used in two ways, as an intermediate layer placed between the ion-selective membrane (ISM) and the electrode substrate (this method is most common) or as a component of the membrane. First of all, they perform the basic function as an ion to electron transducer, and additionally, other benefits are obtained. In most cases, the electrodes containing the composite material showed a lower limit of detection and a better slope of the characteristic. Nanocomposites based on carbon nanomaterials and ionic liquids improved the electrode selectivity [170,178]. Moreover, such composites show a much lower tendency to agglomeration than CNs and do not require the use of additional dispersants [169,170,178]. A similar effect was observed for MWCNTS-POT [140] and MWCNTs-CuONPs [179]. In some cases, the composite acts as an active potential-creating component, and it is simply added to an ion-selective membrane [145] or acts as an active component in paste electrodes [172,180]. Taking into account the type of ion to which obtained electrodes with the use of composites were sensitive, it can be seen that much more articles have been devoted to ISEs sensitive to cations. This is in line with the general trend for all ion-selective electrodes. In the group of cationic electrodes, K+-ISEs have been described the most, while NO3–-ISEs dominate among anion-sensitive electrodes. This is understandable, because when examining a new electrode material, it is justified to use a model membrane with a well-known response mechanism, as is the case with valinomycin-based ISM sensitive to K+ and TDMANO3-based ISM sensitive to NO3−. In summary, it can be said that the addition of composite materials was another significant step in the development of potentiometric sensors. This area will certainly continue to develop towards multi-component materials designed with specific properties in mind. This is facilitated by the continuous progress in materials engineering.
Author Contributions
Conceptualization, C.W. and K.P.; methodology and formal analysis, M.G.; investigation, K.P., K.M. and M.G.; data curation, K.P. and K.M.; writing—original draft preparation, K.P.; writing—review and editing, C.W.; literature survey, K.P., K.M. and M.G.; supervision, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindner, E.; Gyurcsányi, R.E. Quality Control Criteria for Solid-Contact, Solvent Polymeric Membrane Ion-Selective Electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Bieg, C.; Fuchsberger, K.; Stelzle, M. Introduction to Polymer-Based Solid-Contact Ion-Selective Electrodes—Basic Concepts, Practical Considerations, and Current Research Topics. Anal. Bioanal. Chem. 2017, 409, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon Nanotube-Based Ion Selective Sensors for Wearable Applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Mousavi, Z.; Granholm, K.; Sokalski, T.; Lewenstam, A. All-Solid-State Electrochemical Platform for Potentiometric Measurements. Sens. Actuators B Chem. 2015, 207, 895–899. [Google Scholar] [CrossRef]
- Ozer, T. Low-Cost Pencil-Graphite Multi-Electrodes for Simultaneous Detection of Iron and Copper. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Cuartero, M.; Ruiz, A.; Galián, M.; Ortuño, J.A. Potentiometric Electronic Tongue for Quantitative Ion Analysis in Natural Mineral Waters. Sensors 2022, 22, 6204. [Google Scholar] [CrossRef]
- Chango, G.; Palacio, E.; Cerdà, V. Potentiometric Chip-Based Multipumping Flow System for the Simultaneous Determination of Fluoride, Chloride, PH, and Redox Potential in Water Samples. Talanta 2018, 186, 554–560. [Google Scholar] [CrossRef]
- Zuliani, C.; Diamond, D. Opportunities and Challenges of Using Ion-Selective Electrodes in Environmental Monitoring and Wearable Sensors. Electrochim. Acta 2012, 84, 29–34. [Google Scholar] [CrossRef]
- Chaneam, S.; Taweetong, W.; Kaewyai, K.; Thienwong, P.; Takaew, A.; Chaisuksant, R. Fabrication of a Nitrate Selective Electrode for Determination of Nitrate in Fertilizers by Using Flow Injection Analysis System. Procedia Chem. 2016, 20, 73–75. [Google Scholar] [CrossRef]
- Lin, P.K.T.; Araujo, A.N.; Montenegro, M.C.B.S.M.; Pérez-Olmos, R. New PVC Nitrate-Selective Electrode: Application to Vegetables and Mineral Waters. J. Agric. Food Chem. 2005, 53, 211–215. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Wang, R.; Hu, G.; Miao, J.; Hai, K.; Lin, C. Determination of Copper(II) Ion in Food Using an Ionic Liquids-Carbon Nanotubes-Based Ion-Selective Electrode. J. Food Compos. Anal. 2017, 62, 63–68. [Google Scholar] [CrossRef]
- Chapman, B.R.; Goldsmith, I.R. Determination of Chloride, Sodium and Potassium in Salted Foodstuffs Using Ion-Selective Electrodes and the Dry Sample Addition Method. Analyst 1982, 107, 1014–1018. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.K.; Salem, M.Y. Ion Selective Electrode (in-Line Analyzer) versus UV-Spectroscopy (at-Line Analyzer); Which Strategy Offers More Opportunities for Real Time Monitoring of the Degradation Kinetics of Pyridostigmine Bromide. Sens. Actuators B Chem. 2015, 220, 255–262. [Google Scholar] [CrossRef]
- Numnuam, A.; Chumbimuni-Torres, K.Y.; Xiang, Y.; Bash, R.; Thavarungkul, P.; Kanatharana, P.; Pretsch, E.; Wang, J.; Bakker, E. Potentiometric Detection of DNA Hybridization. J. Am. Chem. Soc. 2008, 130, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R. Recent Developments in Potentiometric Biosensors for Biomedical Analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, L.; d’Angremont, E.; Olthuis, W. Solid Contact Potassium Selective Electrodes for Biomedical Applications—A Review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.T.; Wallace, G.; Diamond, D. Bio-Sensing Textile Based Patch with Integrated Optical Detection System for Sweat Monitoring. Sens. Actuators B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef]
- Lewenstam, A. Chapter 1 Clinical Analysis of Blood Gases and Electrolytes by Ion-Selective Sensors. Compr. Anal. Chem. 2007, 49, 5–24. [Google Scholar] [CrossRef]
- Cuartero, M.; Bakker, E. Environmental Water Analysis with Membrane Electrodes. Curr. Opin. Electrochem. 2017, 3, 97–105. [Google Scholar] [CrossRef]
- Slaveykova, V.I.; Wilkinson, K.J.; Ceresa, A.; Pretsch, E. Role of Fulvic Acid on Lead Bioaccumulation by Chlorella Kesslerii. Environ. Sci. Technol. 2003, 37, 1114–1121. [Google Scholar] [CrossRef]
- De Marco, R.; Clarke, G.; Pejcic, B. Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis 2007, 19, 1987–2001. [Google Scholar] [CrossRef]
- Crespo, G.A. Recent Advances in Ion-Selective Membrane Electrodes for in Situ Environmental Water Analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A Review of the Mechanical Properties of Carbon Nanotube-Polymer Composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon Nanotube-Polymer Composites: Chemistry, Processing, Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Scida, K.; Stege, P.W.; Haby, G.; Messina, G.A.; García, C.D. Recent Applications of Carbon-Based Nanomaterials in Analytical Chemistry: Critical Review. Anal. Chim. Acta 2011, 691, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Lindner, E.; Pendley, B.D. A Tutorial on the Application of Ion-Selective Electrode Potentiometry: An Analytical Method with Unique Qualities, Unexplored Opportunities and Potential Pitfalls; Tutorial. Anal. Chim. Acta 2013, 762, 1–13. [Google Scholar] [CrossRef]
- Düzgün, A.; Zelada-Guillén, G.A.; Crespo, G.A.; Macho, S.; Riu, J.; Rius, F.X. Nanostructured Materials in Potentiometry. Anal. Bioanal. Chem. 2011, 399, 171–181. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Polymer Membrane Ion-Selective Electrodes-What Are the Limits? Electroanalysis 1999, 11, 915–933. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Crespo, G.A. All-Solid-State Potentiometric Sensors: A New Wave for in Situ Aquatic Research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Tóth, K.; Gráf, E.; Horvai, G.; Pungor, E.; Buck, R.P. Plasticized Poly(Vinyl Chloride) Properties and Characteristics of Valinomycin Electrodes. 2. Low-Frequency, Surface-Rate, and Warburg Impedance Characteristics. Anal. Chem. 1986, 58, 2741–2744. [Google Scholar] [CrossRef]
- Suni, I.I. Impedance Methods for Electrochemical Sensors Using Nanomaterials. Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar] [CrossRef]
- Radu, A.; Anastasova-Ivanova, S.; Paczosa-Bator, B.; Danielewski, M.; Bobacka, J.; Lewenstam, A.; Diamond, D. Diagnostic of Functionality of Polymer Membrane—Based Ion Selective Electrodes by Impedance Spectroscopy. Anal. Methods 2010, 2, 1490–1498. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Jarvis, J.M.; Guzinski, M.; Pendley, B.D.; Lindner, E. Poly(3-Octylthiophene) as Solid Contact for Ion-Selective Electrodes: Contradictions and Possibilities. J. Solid State Electrochem. 2016, 20, 3033–3041. [Google Scholar] [CrossRef]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-Selective Electrodes with Colloid-Imprinted Mesoporous Carbon as Solid Contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef]
- Jiang, Z.; Xi, X.; Qiu, S.; Wu, D.; Tang, W.; Guo, X.; Su, Y.; Liu, R. Ordered Mesoporous Carbon Sphere-Based Solid-Contact Ion-Selective Electrodes. J. Mater. Sci. 2019, 54, 13674–13684. [Google Scholar] [CrossRef]
- Criscuolo, F.; Taurino, I.; Stradolini, F.; Carrara, S.; De Micheli, G. Highly-Stable Li+ Ion-Selective Electrodes Based on Noble Metal Nanostructured Layers as Solid-Contacts. Anal. Chim. Acta 2018, 1027, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Z.; Joyer, M.M.; Fierke, M.A.; Petkovich, N.D.; Stein, A.; Bühlmann, P. Subnanomolar Detection Limit Application of Ion-Selective Electrodes with Three-Dimensionally Ordered Macroporous (3DOM) Carbon Solid Contacts. J. Solid State Electrochem. 2009, 13, 123–128. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Piech, R.; Cabaj, L. The Influence of an Intermediate Layer on the Composition Stability of a Polymeric Ion-Selective Membrane. Electrochim. Acta 2012, 85, 104–109. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of Nanomaterials in Potentiometric Sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Cadogan, A.; Gao, Z.; Lewenstam, A.; Ivaska, A.; Diamond, D. All-Solid-State Sodium-Selective Electrode Based on a Calixarene Ionophore in a Poly(Vinyl Chloride) Membrane with a Polypyrrole Solid Contact. Anal. Chem. 1992, 64, 2496–2501. [Google Scholar] [CrossRef]
- Sutter, J.; Lindner, E.; Gyurcsányi, R.E.; Pretsch, E. A Polypyrrole-Based Solid-Contact Pb2+-Selective PVC-Membrane Electrode with a Nanomolar Detection Limit. Anal. Bioanal. Chem. 2004, 380, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Mosayebzadeh, Z. Construction of a New Solid-State U(VI) Ion-Selective Electrode Based on Polypyrrole Conducting Polymer. J. Radioanal. Nucl. Chem. 2014, 299, 1597–1605. [Google Scholar] [CrossRef]
- Vázquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of Oxygen and Carbon Dioxide on the Electrochemical Stability of Poly(3,4-Ethylenedioxythiophene) Used as Ion-to-Electron Transducer in All-Solid-State Ion-Selective Electrodes. Sens. Actuators B Chem. 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Lindfors, T. Light Sensitivity and Potential Stability of Electrically Conducting Polymers Commonly Used in Solid Contact Ion-Selective Electrodes. J. Solid State Electrochem. 2009, 13, 77–89. [Google Scholar] [CrossRef]
- Mendecki, L.; Fayose, T.; Stockmal, K.A.; Wei, J.; Granados-Focil, S.; McGraw, C.M.; Radu, A. Robust and Ultrasensitive Polymer Membrane-Based Carbonate-Selective Electrodes. Anal. Chem. 2015, 87, 7515–7518. [Google Scholar] [CrossRef]
- Bobacka, J.; McCarrick, M.; Lewenstam, A.; Ivaska, A. All Solid-State Poly(Vinyl Chloride) Membrane Ion-Selective Electrodes with Poly(3-Octylthiophene) Solid Internal Contact. Analyst 1994, 119, 1985–1991. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Plasticizer-Free All-Solid-State Potassium-Selective Electrode Based on Poly(3-Octylthiophene) and Valinomycin. Anal. Chim. Acta 1999, 385, 195–202. [Google Scholar] [CrossRef]
- Chumbimuni-Torres, K.Y.; Rubinova, N.; Radu, A.; Lauro, T.; Bakker, E. Solid Contact Potentiometric Sensors for Trace Level Measurements. Anal. Chem. 2006, 78, 1318–1322. [Google Scholar] [CrossRef]
- Sutter, J.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Solid-Contact Polymeric Membrane Electrodes with Detection Limits in the Subnanomolar Range. Anal. Chim. Acta 2004, 523, 53–59. [Google Scholar] [CrossRef]
- Rubinova, N.; Chumbimuni-Torres, K.; Bakker, E. Solid-Contact Potentiometric Polymer Membrane Microelectrodes for the Detection of Silver Ions at the Femtomole Level. Sens. Actuators B Chem. 2007, 121, 135–141. [Google Scholar] [CrossRef]
- Bomar, E.; Owens, G.; Murray, G. Nitrate Ion Selective Electrode Based on Ion Imprinted Poly(N-Methylpyrrole). Chemosensors 2017, 5, 2. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-Piece All-Solid-State Ion-Selective Electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Yin, T.; Jia, J.; Ding, Q.; Zheng, H.; Chen, C.T.A.; Ye, Y. A Novel All-Solid-State Ammonium Electrode with Polyaniline and Copolymer of Aniline/2,5-Dimethoxyaniline as Transducers. J. Electroanal. Chem. 2015, 741, 87–92. [Google Scholar] [CrossRef]
- Han, W.S.; Lee, Y.H.; Jung, K.J.; Ly, S.Y.; Hong, T.K.; Kim, M.H. Potassium Ion-Selective Polyaniline Solid-Contact Electrodes Based on 4′,4″(5″)-Di-Tert-Butyldibenzo-18-Crown-6-Ether Ionophore. J. Anal. Chem. 2008, 63, 987–993. [Google Scholar] [CrossRef]
- Das, P.; Mondal, S.; Malik, S. Fully Organic Polyaniline Nanotubes as Electrode Material for Durable Supercapacitor. J. Energy Storage 2021, 39, 102662. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, Y.; Wang, K.; Zhao, S.; Zhu, J.; Cheng, H. Two Kinds of Polyaniline Fiber Photo Sensor with Interdigital Electrode and Flexible Hydrogel. J. Appl. Polym. Sci. 2021, 138, 50628. [Google Scholar] [CrossRef]
- Lindfors, T.; Aarnio, H.; Ivaska, A. Potassium-Selective Electrodes with Stable and Geometrically Well-Defined Internal Solid Contact Based on Nanoparticles of Polyaniline and Plasticized Poly(Vinyl Chloride). Anal. Chem. 2007, 79, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yue, H.; Gao, X.; Yao, F.; Chen, H.; Lu, X.; Wang, Y.; Guo, X. A Novel Electrode Material of Polyaniline Nanowire Array/Three-Dimensional Hollow Graphene Balls-Graphene Oxide for Symmetric Supercapacitor. Ionics 2020, 26, 2063–2070. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Improving the Stability of Pb2+ Ion-Selective Electrodes by Using 3D Polyaniline Nanowire Arrays as the Inner Solid-Contact Transducer. Electrochim. Acta 2021, 384, 138414. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Zeng, G.; Liu, Y.; Shao, B.; Li, Z.; Liu, Y.; Zhang, W.; He, Q. Polyaniline-Based Adsorbents for Removal of Hexavalent Chromium from Aqueous Solution: A Mini Review. Environ. Sci. Pollut. Res. 2018, 25, 6158–6174. [Google Scholar] [CrossRef]
- Mei-Rong, H.; Guo-Li, G.U.; Yong-Bo, D.; Xiao-Tian, F.U.; Rong-Gui, L. Advanced Solid-Contact Ion Selective Electrode Based on Electrically Conducting Polymers. Chin. J. Anal. Chem. 2012, 40, 1454–1460. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in Environment: Behavior, Fate, Bioavailability, and Effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4665. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Xie, S.; Li, W.; Pan, Z.; Chang, B.; Sun, L. Mechanical and Physical Properties on Carbon Nanotube. J. Phys. Chem. Solids 2000, 61, 1153–1158. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A. Carbon Nanotubes in Analytical Sciences. Microchim. Acta 2006, 152, 157–174. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. The Electrochemistry of Carbon Nanotubes: Fundamentals and Applications. Chem. A Eur. J. 2009, 15, 4970–4978. [Google Scholar] [CrossRef]
- Sinnott, S.B.; Andrews, R. Carbon Nanotubes: Synthesis, Properties, and Applications. Crit. Rev. Solid State Mater. Sci. 2001, 26, 145–249. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable Carbon Materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Unwin, P.R.; MacPherson, J.V. Electrochemistry at Carbon Nanotubes: Perspective and Issues. Chem. Commun. 2009, 7345, 6886–6901. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for Carbon Nanotubes Synthesis—Review. J. Mater. Chem. 2011, 21, 15872. [Google Scholar] [CrossRef]
- Sgobba, V.; Guldi, D.M. Carbon Nanotubes—Electronic/Electrochemical Properties and Application for Nanoelectronics and Photonics. Chem. Soc. Rev. 2009, 38, 165–184. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Characterization of Carbon Nanotubes and Analytical Methods for Their Determination in Environmental and Biological Samples: A Review. Anal. Chim. Acta 2015, 853, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Riu, J.; Pingarrón, J.M.; Rius, F.X. Electrochemical Sensing Based on Carbon Nanotubes. Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; Del Valle, M.; Alegret, S. New Materials for Electrochemical Sensing VI: Carbon Nanotubes. Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Najafi, M.; Maleki, L.; Rafati, A.A. Novel Surfactant Selective Electrochemical Sensors Based on Single Walled Carbon Nanotubes. J. Mol. Liq. 2011, 159, 226–229. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-Selective Electrodes Using Carbon Nanotubes as Ion-to-Electron Transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef]
- Ampurdanés, J.; Crespo, G.A.; Maroto, A.; Sarmentero, M.A.; Ballester, P.; Rius, F.X. Determination of Choline and Derivatives with a Solid-Contact Ion-Selective Electrode Based on Octaamide Cavitand and Carbon Nanotubes. Biosens. Bioelectron. 2009, 25, 344–349. [Google Scholar] [CrossRef]
- Parra, E.J.; Crespo, G.A.; Riu, J.; Ruiz, A.; Rius, F.X. Ion-Selective Electrodes Using Multi-Walled Carbon Nanotubes as Ion-to-Electron Transducers for the Detection of Perchlorate. Analyst 2009, 134, 1905–1910. [Google Scholar] [CrossRef]
- Crespo, G.A.; Gugsa, D.; Macho, S.; Rius, F.X. Solid-Contact PH-Selective Electrode Using Multi-Walled Carbon Nanotubes. Anal. Bioanal. Chem. 2009, 395, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Montazerozohori, M.; Sahraei, R. Comparison of the Influence of Nanomaterials on Response Properties of Copper Selective Electrodes. J. Ind. Eng. Chem. 2013, 19, 1356–1364. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Anthis, A.H.C.; Ghahraman Afshar, M.; Pankratova, N.; Cuartero, M.; Crespo, G.A.; Bakker, E. All-Solid-State Potentiometric Sensors with a Multiwalled Carbon Nanotube Inner Transducing Layer for Anion Detection in Environmental Samples. Anal. Chem. 2015, 87, 8640–8645. [Google Scholar] [CrossRef]
- Fouskaki, M.; Chaniotakis, N. Fullerene-Based Electrochemical Buffer Layer for Ion-Selective Electrodes. Analyst 2008, 133, 1072–1075. [Google Scholar] [CrossRef]
- Li, J.; Yin, T.; Qin, W. An All-Solid-State Polymeric Membrane Pb2+-Selective Electrode with Bimodal Pore C60 as Solid Contact. Anal. Chim. Acta 2015, 876, 49–54. [Google Scholar] [CrossRef]
- Jiang, C.; Yao, Y.; Cai, Y.; Ping, J. All-Solid-State Potentiometric Sensor Using Single-Walled Carbon Nanohorns as Transducer. Sens. Actuators B Chem. 2019, 283, 284–289. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-Solid-State Potassium-Selective Electrode Using Graphene as the Solid Contact. Analyst 2012, 137, 618–623. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Lindfors, T. Few-Layer Graphene and Polyaniline Composite as Ion-to-Electron Transducer in Silicone Rubber Solid-Contact Ion-Selective Electrodes. Sens. Actuators B Chem. 2016, 224, 624–631. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. All-Solid-State Selective Electrodes Using Carbon Black. Talanta 2012, 93, 424–427. [Google Scholar] [CrossRef]
- Fierke, M.A.; Lai, C.Z.; Bühlmann, P.; Stein, A. Effects of Architecture and Surface Chemistry of Three-Dimensionally Ordered Macroporous Carbon Solid Contacts on Performance of Ion-Selective Electrodes. Anal. Chem. 2010, 82, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an All-Solid-State Potassium Ion-Selective Electrode Using Graphene as the Solid-Contact Transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of Electrochemically Reduced Graphene Oxide on Screen-Printed Ion-Selective Electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Woźnica, E.; Wójcik, M.M.; Wojciechowski, M.; Mieczkowski, J.; Bulska, E.; Maksymiuk, K.; Michalska, A. Dithizone Modified Gold Nanoparticles Films for Potentiometric Sensing. Anal. Chem. 2012, 84, 4437–4442. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An All-Solid-State Nitrate Ion-Selective Electrode with Nanohybrids Composite Films for in-Situ Soil Nutrient Monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef]
- Jaworska, E.; Wójcik, M.; Kisiel, A.; Mieczkowski, J.; Michalska, A. Gold Nanoparticles Solid Contact for Ion-Selective Electrodes of Highly Stable Potential Readings. Talanta 2011, 85, 1986–1989. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Z.; Liu, P. An All-Solid-State NO3- Ion-Selective Electrode with Gold Nanoparticles Solid Contact Layer and Molecularly Imprinted Polymer Membrane. PLoS ONE 2020, 15, e0240173. [Google Scholar] [CrossRef]
- Yin, T.; Han, T.; Li, C.; Qin, W.; Bobacka, J. Real-Time Monitoring of the Dissolution of Silver Nanoparticles by Using a Solid-Contact Ag+-Selective Electrode. Anal. Chim. Acta 2020, 1101, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Platinum Nanoparticles Intermediate Layer in Solid-State Selective Electrodes. Analyst 2012, 137, 5272–5277. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Piech, R.; Wardak, C.; Cabaj, L. Application of Graphene Supporting Platinum Nanoparticles Layer in Electrochemical Sensors with Potentiometric and Voltammetric Detection. Ionics 2018, 24, 2455–2464. [Google Scholar] [CrossRef]
- Jaworska, E.; Kisiel, A.; Maksymiuk, K.; Michalska, A. Lowering the Resistivity of Polyacrylate Ion-Selective Membranes by Platinum Nanoparticles Addition. Anal. Chem. 2011, 83, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Khun, K.; Ibupoto, Z.H.; Willander, M. Urea Assisted Synthesis of Flower like CuO Nanostructures and Their Chemical Sensing Application for the Determination of Cadmium Ions. Electroanalysis 2013, 25, 1425–1432. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Hydrous Cerium Dioxide-Based Materials as Solid-Contact Layers in Potassium-Selective Electrodes. Membranes 2022, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Optimization of Ruthenium Dioxide Solid Contact in Ion-Selective Electrodes. Membranes 2020, 10, 182. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Wyrwa, J.; Paczosa-Bator, B. Potassium-Selective Solid-Contact Electrode with High-Capacitance Hydrous Iridium Dioxide in the Transduction Layer. Membranes 2021, 11, 259. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yan, R.; Gao, Y.; Wang, P. Bimetallic AuCu Nanoparticles Coupled with Multi-Walled Carbon Nanotubes as Ion-to-Electron Transducers in Solid-Contact Potentiometric Sensors. Electrochim. Acta 2020, 331, 135370. [Google Scholar] [CrossRef]
- Gupta, D.K.; Neupane, S.; Yadav, H.C.; Subedi, V.; Singh, S.; Yadav, R.J.; Das, A.K.; Yadav, B.; Nakarmi, K.B.; Karki, N.; et al. Trace Level Monitoring of Cu(II) Ion Using CuS Particles Based Membrane Electrochemical Sensor. Heliyon 2021, 7, e07167. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, T.; Liang, R.; Qin, W. Polymeric Membrane Ion-Selective Electrodes with Anti-Biofouling Properties by Surface Modification of Silver Nanoparticles. Sens. Actuators B Chem. 2021, 328, 129014. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic Semiconducting Oxides as PH Sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Omidi, M.; Hossein Rokni, D.T.; Milani, A.S.; Seethaler, R.J.; Arasteh, R. Prediction of the Mechanical Characteristics of Multi-Walled Carbon Nanotube/Epoxy Composites Using a New Form of the Rule of Mixtures. Carbon 2010, 48, 3218–3228. [Google Scholar] [CrossRef]
- Jackson, E.M.; Laibinis, P.E.; Collins, W.E.; Ueda, A.; Wingard, C.D.; Penn, B. Development and Thermal Properties of Carbon Nanotube-Polymer Composites. Compos. Part B Eng. 2016, 89, 362–373. [Google Scholar] [CrossRef]
- Strakhov, I.S.; Rodnaya, A.I.; Mezhuev, Y.O.; Korshak, Y.V.; Vagramyan, T.A. Enhancement of the Strength of a Composite Material Based on ED-20 Epoxy Resin by Reinforcement with a Carbon Fiber Modified by Electrochemical Deposition of Poly(o-Phenylenediamine). Russ. J. Appl. Chem. 2014, 87, 1918–1922. [Google Scholar] [CrossRef]
- Shahnazi, A.; Nabid, M.R.; Sedghi, R. Synthesis of Surface Molecularly Imprinted Poly-o-Phenylenediamine/TiO2/Carbon Nanodots with a Highly Enhanced Selective Photocatalytic Degradation of Pendimethalin Herbicide under Visible Light. React. Funct. Polym. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Baibarac, M.; Daescu, M.; Matei, E.; Nastac, D.; Cramariuc, O. Optical Properties of Composites Based on Poly (o-Phenylenediamine), Poly (Vinylenefluoride) and Double-Wall Carbon Nanotubes. Int. J. Mol. Sci. 2021, 22, 8260. [Google Scholar] [CrossRef]
- Ekinci, E.; Erdogdu, G.; Karagozler, A.E. Preparation, Optimization, and Voltammetric Characteristics of Poly (o-Phenylenediamine) Film as a Dopamine-Selective Polymeric Membrane. J. Appl. Crystallogr. 2001, 79, 327–332. [Google Scholar] [CrossRef]
- Khan, A.A.; Paquiza, L. Synthesis and Characterization of in Situ Polymerized Poly(Methyl Methacrylate)-Cerium Molybdate Nanocomposite for Electroanalytical Application. J. Appl. Polym. Sci. 2013, 127, 3737–3748. [Google Scholar] [CrossRef]
- Arfin, T.; Tarannum, A. Rapid Determination of Lead Ions Using Polyaniline-Zirconium(IV) Iodate -Based Ion Selective Electrode. J. Environ. Chem. Eng. 2019, 7, 102811. [Google Scholar] [CrossRef]
- Kaur, K.; Aulakh, J.S.; Malik, A.K. Fabrication of Zn(II) Selective Polyvinyl Chloride Membrane Electrode Based on N,N’-Bis(1-Hydroxynaphthalene-2-Carbaldehyde)-o-Phenylenediamine as an Ionophore: Experimental and Theoretical Approaches. J. Anal. Chem. 2019, 74, 134–142. [Google Scholar] [CrossRef]
- Mirzaei, M.; Pili, H.B. Potentiometric Determination of Cadmium Using Coated Platinum and PVC Membrane Sensors Based on N,N′-Bis(Salicylaldehyde)Phenylenediamine (Salophen). J. Anal. Chem. 2015, 70, 731–737. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Poly(3,4-Ethylenedioxythiophene) (PEDOT) Doped with Carbon Nanotubes as Ion-to-Electron Transducer in Polymer Membrane-Based Potassium Ion-Selective Electrodes. J. Electroanal. Chem. 2009, 633, 246–252. [Google Scholar] [CrossRef]
- Abbaspour, A.; Tashkhourian, J.; Ahmadpour, S.; Mirahmadi, E.; Sharghi, H.; Khalifeh, R.; Shahriyari, M.R. Construction of a New Selective Coated Disk Electrode for Ag (I) Based on Modified Polypyrrole-Carbon Nanotubes Composite with New Lariat Ether. Mater. Sci. Eng. C 2014, 34, 326–333. [Google Scholar] [CrossRef]
- Athavale, R.; Kokorite, I.; Dinkel, C.; Bakker, E.; Wehrli, B.; Crespo, G.A.; Brand, A. In Situ Ammonium Profiling Using Solid-Contact Ion-Selective Electrodes in Eutrophic Lakes. Anal. Chem. 2015, 87, 11990–11997. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. Ion-Selective Electrodes with Superhydrophobic Polymer/Carbon Nanocomposites as Solid Contact. Carbon 2015, 95, 879–887. [Google Scholar] [CrossRef]
- Topcu, C.; Lacin, G.; Yilmaz, V.; Coldur, F.; Caglar, B.; Cubuk, O.; Isildak, I. Electrochemical Determination of Copper(II) in Water Samples Using a Novel Ion-Selective Electrode Based on a Graphite Oxide–Imprinted Polymer Composite. Anal. Lett. 2018, 51, 1890–1910. [Google Scholar] [CrossRef]
- Kałuza, D.; Jaworska, E.; Mazur, M.; Maksymiuk, K.; Michalska, A. Multiwalled Carbon Nanotubes-Poly(3-Octylthiophene-2,5-Diyl) Nanocomposite Transducer for Ion-Selective Electrodes: Raman Spectroscopy Insight into the Transducer/Membrane Interface. Anal. Chem. 2019, 91, 9010–9017. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Gao, Y.; Wang, P. A General Approach to One-Step Fabrication of Single-Piece Nanocomposite Membrane Based Pb2+-Selective Electrodes. Sens. Actuators B Chem. 2019, 281, 705–712. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Ressler, A.; Kassal, P. Fabrication of an All-Solid-State Ammonium Paper Electrode Using a Graphite-Polyvinyl Butyral Transducer Layer. Chemosensors 2021, 9, 333. [Google Scholar] [CrossRef]
- Kumar, P. All-Solid-State CNT Composite Calcium(II)-Selective Potentiometric Sensor Based on 4,7-Diaza-2,3,8,9-Dibenzo-15-Crown-5 Ionophore. Anal. Bioanal. Electrochem. 2022, 14, 179–190. [Google Scholar]
- Pietrzak, K.; Morawska, K.; Malinowski, S.; Wardak, C. Chloride Ion-Selective Electrode with Solid-Contact Based on Polyaniline Nanofibers and Multiwalled Carbon Nanotubes Nanocomposite. Membranes 2022, 12, 1150. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhou, C.; Sun, C. Determination of Cesium Ions in Environmental Water Samples with a Magnetic Multi-Walled Carbon Nanotube Imprinted Potentiometric Sensor. RSC Adv. 2021, 11, 10075–10082. [Google Scholar] [CrossRef]
- Niemiec, B.; Piech, R.; Paczosa-Bator, B. Modification of Carbon Nanomaterials by Association with Poly(3-Octylthiophene-2,5-Diyl) as a Method of Improving the Solid-Contact Layer in Ion-Selective Electrodes. Membranes 2022, 12, 1275. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Carbon Nanomaterials—Poly(3-Octylthiophene-2,5-Diyl)—Hydrous Iridium Dioxide Triple Composite Materials as Superhydrophobic Layers for Ion-Selective Electrodes. J. Electrochem. Soc. 2022, 169, 127508. [Google Scholar] [CrossRef]
- Xie, L.; Qin, Y.; Chen, H.Y. Preparation of Solid Contact Potentiometric Sensors with Self-Plasticizing Triblock Polymer and Ionic Liquid-Polymer Composites. Sens. Actuators B Chem. 2013, 186, 321–326. [Google Scholar] [CrossRef]
- Vanamo, U.; Bobacka, J. Electrochemical Control of the Standard Potential of Solid-Contact Ion-Selective Electrodes Having a Conducting Polymer as Ion-to-Electron Transducer. Electrochim. Acta 2014, 122, 316–321. [Google Scholar] [CrossRef]
- Ghosh, T.; Chung, H.J.; Rieger, J. All-Solid-State Sodium-Selective Electrode with a Solid Contact of Chitosan/Prussian Blue Nanocomposite. Sensors 2017, 17, 2536. [Google Scholar] [CrossRef]
- Yu, K.; He, N.; Kumar, N.; Wang, N.X.; Bobacka, J.; Ivaska, A. Electrosynthesized Polypyrrole/Zeolite Composites as Solid Contact in Potassium Ion-Selective Electrode. Electrochim. Acta 2017, 228, 66–75. [Google Scholar] [CrossRef]
- Pięk, M.; Paczosa-Bator, B.; Smajdor, J.; Piech, R. Molecular Organic Materials Intermediate Layers Modified with Carbon Black in Potentiometric Sensors for Chloride Determination. Electrochim. Acta 2018, 283, 1753–1762. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R.; Królicka, A. Poly(3-Octylthiophene-2,5-Diyl)—Nanosized Ruthenium Dioxide Composite Material as Solid-Contact Layer in Polymer Membrane-Based K+-Selective Electrodes. Electrochim. Acta 2019, 322, 134718. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, C.; Zhao, Y.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. A Solid-Contact Pb2+ -Selective Electrode Based on a Hydrophobic Polyaniline Microfiber Film as the Ion-to-Electron Transducer. Synth. Met. 2019, 248, 94–101. [Google Scholar] [CrossRef]
- Ali, M.A.; Wang, X.; Chen, Y.; Jiao, Y.; Mahal, N.K.; Moru, S.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. Continuous Monitoring of Soil Nitrate Using a Miniature Sensor with Poly(3-Octyl-Thiophene) and Molybdenum Disulfide Nanocomposite. ACS Appl. Mater. Interfaces 2019, 11, 29195–29206. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, W.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Stable Pb2+ Ion-Selective Electrodes Based on Polyaniline-TiO2 Solid Contacts. Anal. Chim. Acta 2020, 1094, 26–33. [Google Scholar] [CrossRef]
- Ivanko, I.; Lindfors, T.; Emanuelsson, R.; Sjödin, M. Conjugated Redox Polymer with Poly(3,4-Ethylenedioxythiophene) Backbone and Hydroquinone Pendant Groups as the Solid Contact in Potassium-Selective Electrodes. Sens. Actuators B Chem. 2021, 329, 129231. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate. Materials 2021, 14, 1891. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, W.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Stable Pb(II) Ion-Selective Electrodes with a Low Detection Limit Using Silver Nanoparticles/Polyaniline as the Solid Contact. Microchim. Acta 2021, 188, 393. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Akatsuka, T.; Abe, Y.; Komaba, S. Design of All-Solid-State Chloride and Nitrate Ion-Selective Electrodes Using Anion Insertion Materials of Electrodeposited Poly(Allylamine)-MnO2 Composite. Electrochim. Acta 2021, 389, 138749. [Google Scholar] [CrossRef]
- Bahro, C.; Goswami, S.; Gernhart, S.; Koley, D. Calibration-Free Solid-State Ion-Selective Electrode Based on a Polarized PEDOT/PEDOT-S-Doped Copolymer as Back Contact. Anal. Chem. 2022, 94, 8302–8308. [Google Scholar] [CrossRef] [PubMed]
- Neo, Z.H.; Seah, G.E.K.K.; Ng, S.H.; Safanama, D.; Seng, D.H.L.; Goh, S.S. Solution-Printable PEDOT Solid-Contact for Nitrate-Selective Electrodes: Enhanced Selectivity from Anion Dopant Exchange. Anal. Chem. 2022, 94, 15956–15963. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Potentiometric Sensors with Carbon Black Supporting Platinum Nanoparticles. Anal. Chem. 2013, 85, 10255–10261. [Google Scholar] [CrossRef]
- Liang, R.; Yin, T.; Qin, W. A Simple Approach for Fabricating Solid-Contact Ion-Selective Electrodes Using Nanomaterials as Transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, T.; Zhu, J.; Qin, Y.; Jiang, D. Polymer-Multiwall Carbon Nanotubes Composites for Durable All Solid-Contact H2PO4--Selective Electrodes. Sens. Actuators B Chem. 2015, 219, 100–104. [Google Scholar] [CrossRef]
- Piȩk, M.; Piech, R.; Paczosa-Bator, B. All-Solid-State Nitrate Selective Electrode with Graphene/Tetrathiafulvalene Nanocomposite as High Redox and Double Layer Capacitance Solid Contact. Electrochim. Acta 2016, 210, 407–414. [Google Scholar] [CrossRef]
- Pięk, M.; Fendrych, K.; Smajdor, J.; Piech, R.; Paczosa-Bator, B. High Selective Potentiometric Sensor for Determination of Nanomolar Con-Centration of Cu(II) Using a Polymeric Electrode Modified by a Graphene/7,7,8,8-Tetracyanoquinodimethane Nanoparticles. Talanta 2017, 170, 41–48. [Google Scholar] [CrossRef]
- Li, J.; Yin, T.; Qin, W. An Effective Solid Contact for an All-Solid-State Polymeric Membrane Cd2+-Selective Electrode: Three-Dimensional Porous Graphene-Mesoporous Platinum Nanoparticle Composite. Sens. Actuators B Chem. 2017, 239, 438–446. [Google Scholar] [CrossRef]
- Pietrzak, K.; Wardak, C. Comparative Study of Nitrate All Solid State Ion-Selective Electrode Based on Multiwalled Carbon Nanotubes-Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Grabarczyk, M. Ionic Liquid-Multiwalled Carbon Nanotubes Nanocomposite Based All Solid State Ion-Selective Electrode for the Determination of Copper in Water Samples. Water 2021, 13, 2869. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. High Capacity Nanocomposite Layers Based on Nanoparticles of Carbon Materials and Ruthenium Dioxide for Potassium Sensitive Electrode. Materials 2021, 14, 1308. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.A. Novel Potentiometric Solid-Contact Electrode for the Determination of Fe2+ Ions via MWCNTs-Gemifloxacin Composite. Electroanalysis 2021, 33, 1283–1289. [Google Scholar] [CrossRef]
- Niemiec, B.; Zambrzycki, M.; Piech, R.; Wardak, C.; Paczosa-Bator, B. Hierarchical Nanocomposites Electrospun Carbon Nanofibers/Carbon Nanotubes as a Structural Element of Potentiometric Sensors. Materials 2022, 15, 4803. [Google Scholar] [CrossRef]
- Ozer, T. Carbon Composite Thermoplastic Electrodes Integrated with Mini-Printed Circuit Board for Wireless Detection of Calcium Ions. Anal. Sci. 2022, 38, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Guan, M.; Xia, L.; Chen, Y.; Mai, J.; Zhao, C.; Liao, C. Highly Stretchable and Robust Electrochemical Sensor Based on 3D Graphene Oxide-CNT Composite for Detecting Ammonium in Sweat. Biosensors 2023, 13, 409. [Google Scholar] [CrossRef]
- Lim, H.R.; Lee, Y.; Jones, K.A.; Kwon, Y.T.; Kwon, S.; Mahmood, M.; Lee, S.M.; Yeo, W.H. All-in-One, Wireless, Fully Flexible Sodium Sensor System with Integrated Au/CNT/Au Nanocomposites. Sens. Actuators B Chem. 2021, 331, 129416. [Google Scholar] [CrossRef]
- Gallego-Cerda, S.D.; Chanona-Perez, J.J.; Hernandez-Varela, J.D.; Lopez, M.C. Development of a Facile Aerogel-based Ion-selective Electrode Using Cellulose and Carbon Nanotube as Transducer Materials for Potentiometric Applications. J. Appl. Polym. Sci. 2023, 140, e53891. [Google Scholar] [CrossRef]
- Wardak, C.; Morawska, K.; Paczosa-Bator, B.; Grabarczyk, M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K. Nanocomposite of Copper Oxide Nanoparticles and Multi-Walled Carbon Nanotubes as a Solid Contact of a Copper-Sensitive Ion-Selective Electrode: Intermediate Layer or Membrane Component–Comparative Studies. Appl. Nanosci. 2023, 13, 409. [Google Scholar] [CrossRef]
- Coutinho, C.F.B.; Muxel, A.A.; Rocha, C.G.; De Jesus, D.A.; Alfaya, R.V.S.; Almeida, F.A.S.; Gushikemb, Y.; Alfaya, A.A.S. Ammonium Ion Sensor Based on SiO2/ZrO2/Phosphate- NH4+ Composite for Quantification of Ammonium Ions in Natural Waters. J. Braz. Chem. Soc. 2007, 18, 189–194. [Google Scholar] [CrossRef]
- Criscuolo, F.; Lobello, L.; Taurino, I.; Demarchi, D.; Carrara, S.; De Micheli, G. Mixed Gold and Platinum Nanostructured Layers for All-Solid-State Ion Sensors. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. TTF-TCNQ Solid Contact Layer in All-Solid-State Ion-Selective Electrodes for Potassium or Nitrate Determination. J. Electrochem. Soc. 2018, 165, B60–B65. [Google Scholar] [CrossRef]
- Mendecki, L.; Mirica, K.A. Conductive Metal-Organic Frameworks as Ion-to-Electron Transducers in Potentiometric Sensors. ACS Appl. Mater. Interfaces 2018, 10, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, T.; Song, C.; Fan, A.; Zhu, N.; Sun, B.; Yang, C. Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors 2021, 9, 155. [Google Scholar] [CrossRef]
- Sabeti Ghahfarokhi, F.; Ghaemi, A.; Mohammadzadeh Kakhki, R. Novel Polymeric Sensor for Ultra-Trace Determination of Cerium(III) Based on CoNiFe2O4 Nanocomposite. Inorg. Nano-Met. Chem. 2023; Ahead of print. 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).