Exploring the Applications of Indocyanine Green in Robot-Assisted Urological Surgery: A Comprehensive Review of Fluorescence-Guided Techniques
Abstract
1. Introduction
2. Indocyanine Green Overview
3. NIRF-Guided Robot-Assisted Renal Surgery
3.1. ICG-Guided Renal Mass Differential Fluorescence
3.2. ICG-Guided Renal Selective Perfusion Assessment
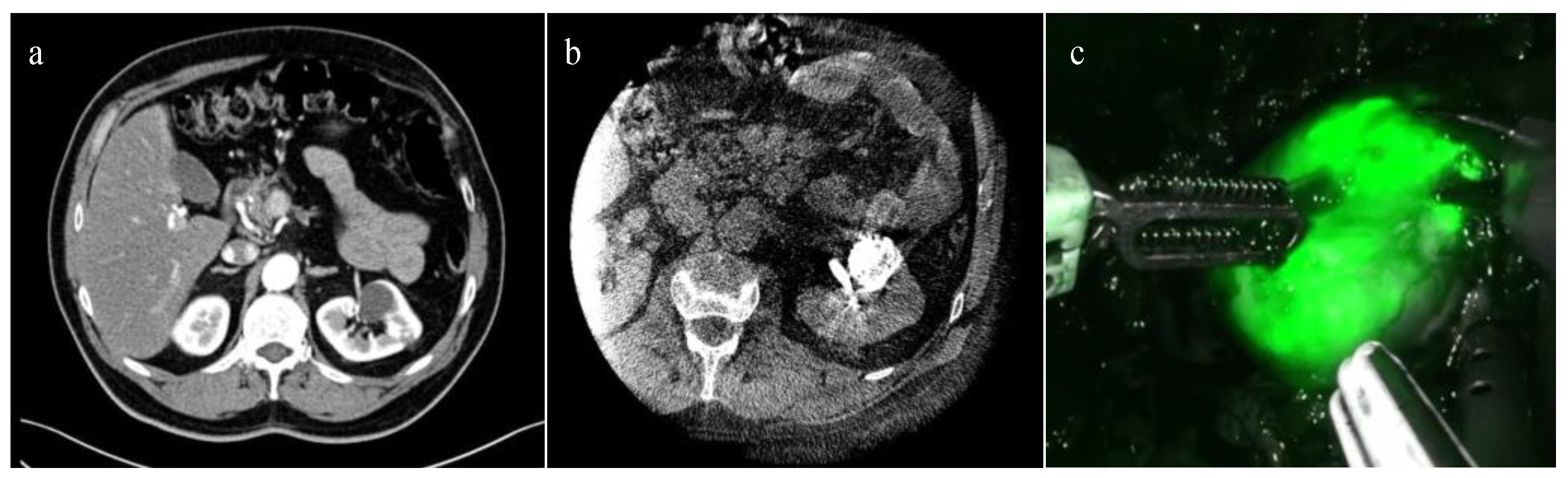
3.3. ICG-Guided Management of Endophytic Renal Tumors
4. NIRF-Guided Robot-Assisted Renal Transplant
| Robotic Procedure | Purpose | Potential Pros | Potential Limitations | ICG Administration |
|---|---|---|---|---|
| Partial Nephrectomy | Differential fluorescence to assess tumor margins [17,18,19,20,21,22] | Real-time guidance Maximal preservation of renal parenchyma | Doses of ICG outside an optimal range result in decreased contrast between the lesion and surrounding renal parenchyma Limited tissue penetration | Intravenous injection prior to arterial clamping |
| Perfusion assessment for selective arterial clamping or test clamping of main artery [24,25,26,27,28,29,30,31,32,33] | Useful in cases of challenging vascular anatomy or impaired renal function Monitoring segmental perfusion deficits after clamping | Limited assessment of deep devascularization | Intravenous injection after arterial clamping | |
| Assess kidney perfusion after resection and renorrhaphy [24,26,31,32,37] | Checking residual parenchyma blood supply and confirming absence of ischemic injury to healthy parenchyma | Lack of data about specific decision making and clinical impact | Intravenous injection after reperfusion | |
| Intraoperative identification and anatomical dissection of total endophytic renal masses [14,37,38,39] | Real-time guidance May improve preoperative resection strategy and intraoperative mass identification May promote nephron-sparing surgery | Needs preoperative renal mass marking No free dye application No benefit in case of avascular renal masses | Preoperative superselective catheterization of tertiary arterial branches feeding the tumor by interventional uroradiologist +\− embolization | |
| Renal Transplant | Assessment of graft perfusion before and after transplant [46,47] | Depicting graft microcirculation Evaluating ureteral reperfusion Useful for complex vasculature reconstruction | Preliminary experience No long-term outcomes evaluation | Intravenous injection Renal artery injection before the implantation |
5. NIRF-Guided Robot-Assisted Adrenal Surgery
| Robotic Procedure | Purpose | Potential Pros | Potential Limitations | ICG Administration |
|---|---|---|---|---|
| Partial and Radical Adrenalectomy | Tissue identification and dissection Identification of vasculature anatomy [49,50,51,52,53,54] | Better delineation between adrenal gland and retroperitoneal tissues | Multiple injections required Background liver fluorescence can interfere during right-sided posterior retroperitoneal approach | Intravenous injection |
| Tumor localization [49,52,53] | May promote adrenal-sparing surgery | Fluorescence pattern variability based on histological tumor origin | Intravenous injection |
6. NIRF-Guided Robot-Assisted Prostate Surgery
6.1. ICG-Guided Nerve-Sparing Approach during RARP
6.2. ICG-Guided Lymphadenectomy during RARP
6.3. ICG-Guided Urethra-Sparing Simple Prostatectomy
7. NIRF-Guided Robot-Assisted Penile Cancer Surgery
| Robotic Procedure | Purpose | Potential Pros | Potential Limitations | ICG Administration |
|---|---|---|---|---|
| Radical Prostatectomy | Identification of neurovascular bundle and landmark prostatic artery [57,58,59] | High identification rate May improve nerve-sparing quality | Only reports with low sample size Lack of evidence about postoperative functional outcome correlation | Intravenous injection |
| Lymphangiography and lymphadenectomy [62,63,64,65,66,67,68,69,70,71] | Simpler nodes identification Higher number of LND yield Can give information about prostatic lymphatic drainage routes, also outside standard templates Can be used with radiotracers to improve accuracy | Low sensitivity and specificity in detecting metastatic LN Not an alternative to ePLND Variable drainage related to tumor burden Lack of a standard injection protocol | Intraprostatic/intratumoral injection | |
| Simple Prostatectomy | Urethra identification and preservation [74] | Selective control of the intraprostatic urethra Identification of unintentional violation of the urinary system | Only exploratory report For urethra-sparing technique only | Retrograde injection through catheter |
| Inguinal lymphadenectomy for Penile cancer | Sentinel lymph node mapping [84,85,86] | May improve intraoperative optical SN detection rate | Only initial reports with low sample size Lack of a standard injection protocol | Intradermal injection at the base of the penis Subcutaneous injection below the tumor |
8. NIRF-Guided Robot-Assisted Upper Urinary Tract Surgery
9. NIRF-Guided Robot-Assisted Bladder Surgery
| Robotic Procedure | Purpose | Potential Pros | Potential Limitations | ICG Administration |
|---|---|---|---|---|
| Radical Cystectomy | Mesenteric angiography [97,99] | Can confirm adequate vascularization of a bowel segment May reduce bowel ischemic complications | Unclear whether it can effectively prevent anastomotic complications | Intravenous injection before bowel resection and after the completion of the anastomosis |
| Ureteral vascularization assessment [98,99] | Confirm adequate vascularization May reduce post operative stricture rate | Unclear utility in centers with already low incidence rates of strictures | Intravenous injection prior to anastomosis | |
| Lymphangiography and sentinel node biopsy [94,96] | May improve staging by targeted removal of LNs outside the standard ePLND template | Only exploratory reports Low specificity It cannot be used as an alternative to ePLND or in association with radiotracers High variable drainage related to tumor localization, size, type and extent of disease | Cystoscopic mucosal injection around the tumor | |
| Ureteral reconstruction, Pyeloplasty | Ureteral vascularization assessment [87,88,89,90] | May predict and prevent postoperative obstruction and pyeloplasty failure Reduce unnecessary tissue disruption | Only reports with low sample size | Intravenous injection |
| Identification of ureteral strictures, ureter and renal pelvis [87,88,89,90,100,101] | Simpler identification of anatomical structures in complex anatomy Facilitate better localization and delineation of stenosis’s characteristics Ensure quality control after anastomosis or reimplantation | Only reports with low sample size | Trans-nephrostomic injection Retrograde injection through ureteral catheter |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Birch, D.J.S. Fluorescence Guided Surgery. Methods Appl. Fluoresc. 2021, 9, 042002. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-J.; Lee, N.R.; Son, S.K.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Robot-Assisted Radical Prostatectomy and Open Radical Prostatectomy Outcomes: A Systematic Review and Meta-Analysis. Yonsei Med. J. 2016, 57, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic Review and Meta-analysis of Studies Reporting Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, T.L.; Corleta, O.C. 30 Years of Robotic Surgery. World J. Surg. 2016, 40, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.S.; Patel, V.R. Applications of indocyanine green in robotic urology. J. Robot. Surg. 2016, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2015, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kochubey, V.I.; Kulyabina, T.V.; Tuchin, V.V.; Altshuler, G.B. Spectral Characteristics of Indocyanine Green upon Its Interaction with Biological Tissues. Opt. Spectrosc. 2005, 99, 560–566. [Google Scholar] [CrossRef]
- Speich, R.; Saesseli, B.; Hoffmann, U.; Neftel, K.A.; Reichen, J. Anaphylactoid Reactions after Indocyanine-Green Administration. Ann. Intern. Med. 1988, 109, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, M.H.G.M.; van Dongen, G.A.M.S.; Cailler, F.; Pèlegrin, A.; Meijerink, W.J.H.J. Sentinel node procedure of the sigmoid using indocyanine green: Feasibility study in a goat model. Surg. Endosc. 2010, 24, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Calabrese, A.; Santucci, D.; Corti, R.; Cionfoli, N.; Pusceddu, C.; de Felice, C.; Bozzini, G.; Mazzoleni, F.; Muraca, R.M.; et al. Green Tattoo Pre-Operative Renal Embolization for Robotic-Assisted and Laparoscopic Partial Nephrectomy: A Practical Proof of a New Technique. J. Clin. Med. 2022, 11, 6816. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.; Löwik, C.W.; Frangioni, J.V.; Van De Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Golijanin, D.J.; Marshall, J.; Cardin, A.; A Singer, E.; Wood, R.W.; E Reeder, J.; Wu, G.; Yao, J.L.; Passamonti, S.; Messing, E.M. Bilitranslocase (BTL) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumors accurately corresponding to intraoperative near infrared fluorescence (NIRF) expression of renal cortical tumors using intravenous indocyanine green (ICG). J. Urol. 2008, 179, 137. [Google Scholar] [CrossRef]
- Tobis, S.; Knopf, J.; Silvers, C.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Near Infrared Fluorescence Imaging with Robotic Assisted Laparoscopic Partial Nephrectomy: Initial Clinical Experience for Renal Cortical Tumors. J. Urol. 2011, 186, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tobis, S.; Knopf, J.K.; Silvers, C.; Messing, E.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Robot-Assisted and Laparoscopic Partial Nephrectomy with Near Infrared Fluorescence Imaging. J. Endourol. 2012, 26, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Krane, L.S.; Manny, T.B.; Hemal, A.K. Is Near Infrared Fluorescence Imaging Using Indocyanine Green Dye Useful in Robotic Partial Nephrectomy: A Prospective Comparative Study of 94 Patients. Urology 2012, 80, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Manny, T.B.; Krane, L.S.; Hemal, A.K. Indocyanine Green Cannot Predict Malignancy in Partial Nephrectomy: Histopathologic Correlation with Fluorescence Pattern in 100 Patients. J. Endourol. 2013, 27, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Sentell, K.T.; Ferroni, M.C.; Abaza, R. Near-infrared fluorescence imaging for intraoperative margin assessment during robot-assisted partial nephrectomy. BJU Int. 2020, 126, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Angell, J.E.; Khemees, T.A.; Abaza, R. Optimization of Near Infrared Fluorescence Tumor Localization during Robotic Partial Nephrectomy. J. Urol. 2013, 190, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Eisenberg, M.S.; Aron, M.; Berger, A.; Ukimura, O.; Patil, M.B.; Campese, V.; Thangathurai, D.; Desai, M.M. “Zero Ischemia” Partial Nephrectomy: Novel Laparoscopic and Robotic Technique. Eur. Urol. 2011, 59, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Borofsky, M.S.; Gill, I.S.; Hemal, A.K.; Marien, T.P.; Jayaratna, I.; Krane, L.S.; Stifelman, M.D. Near-infrared fluorescence imaging to facilitate super-selective arterial clamping during zero-ischaemia robotic partial nephrectomy. BJU Int. 2013, 111, 604–610. [Google Scholar] [CrossRef] [PubMed]
- McClintock, T.R.; Bjurlin, M.A.; Wysock, J.S.; Borofsky, M.S.; Marien, T.P.; Okoro, C.; Stifelman, M.D. Can Selective Arterial Clamping with Fluorescence Imaging Preserve Kidney Function During Robotic Partial Nephrectomy? Urology 2014, 84, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, D.; Luciani, L.G.; Mantovani, W.; Cai, T.; Chiodini, S.; Vattovani, V.; Puglisi, M.; Malossini, G. Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J. Robot. Surg. 2019, 13, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gadus, L.; Kocarek, J.; Chmelik, F.; Matejkova, M.; Heracek, J. Robotic Partial Nephrectomy with Indocyanine Green Fluorescence Navigation. Contrast Media Mol. Imaging 2020, 2020, 1287530. [Google Scholar] [CrossRef] [PubMed]
- Lanchon, C.; Arnoux, V.; Fiard, G.; Descotes, J.-L.; Rambeaud, J.-J.; Lefrancq, J.-B.; Poncet, D.; Terrier, N.; Overs, C.; Franquet, Q.; et al. Super-selective robot-assisted partial nephrectomy using near-infrared flurorescence versus early-unclamping of the renal artery: Results of a prospective matched-pair analysis. Int. Braz. J. Urol. 2018, 44, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Veccia, A.; Antonelli, A.; Hampton, L.J.; Greco, F.; Perdonà, S.; Lima, E.; Hemal, A.K.; Derweesh, I.; Porpiglia, F.; Autorino, R. Near-infrared Fluorescence Imaging with Indocyanine Green in Robot-assisted Partial Nephrectomy: Pooled Analysis of Comparative Studies. Eur. Urol. Focus 2020, 6, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Buffi, N.M.; Lughezzani, G.; Dell’oglio, P.; Mazzone, E.; Porter, J.; Mottrie, A. The Role of Intraoperative Indocyanine Green in Robot-assisted Partial Nephrectomy: Results from a Large, Multi-institutional Series. Eur. Urol. 2020, 78, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Hsieh, M.-L.; Chen, S.-Y.; Liu, C.-Y.; Lin, P.-H.; Kan, H.-C.; Pang, S.-T.; Yu, K.-J. Clinical Benefits of Indocyanine Green Fluorescence in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 3032. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-A.; Fiard, G.; Giai, J.; Teyssier, Y.; Fontanell, A.; Overs, C.; Poncet, D.; Descotes, J.-L.; Rambeaud, J.-J.; Moreau-Gaudry, A.; et al. Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur. Urol. Focus 2022, 8, 769–776. [Google Scholar] [CrossRef] [PubMed]
- De Backer, P.; Vermijs, S.; Van Praet, C.; De Visschere, P.; Vandenbulcke, S.; Mottaran, A.; Bravi, C.A.; Berquin, C.; Lambert, E.; Dautricourt, S.; et al. A Novel Three-dimensional Planning Tool for Selective Clamping During Partial Nephrectomy: Validation of a Perfusion Zone Algorithm. Eur. Urol. 2023, 83, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Novara, G.; Borghesi, M.; Ficarra, V.; Ahlawat, R.; Moon, D.A.; Porpiglia, F.; Challacombe, B.J.; Dasgupta, P.; Brunocilla, E.; et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: Analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017, 119, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Khene, Z.-E.; Peyronnet, B.; Gasmi, A.; Verhoest, G.; Mathieu, R.; Bensalah, K. Endophytic Renal Cell Carcinoma Treated with Robot-Assisted Surgery: Functional Outcomes—A Comprehensive Review of the Current Literature. Urol. Int. 2020, 104, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Tuderti, G.; Anceschi, U.; Ferriero, M.; Costantini, M.; Minisola, F.; Vallati, G.; Pizzi, G.; Guaglianone, S.; Misuraca, L.; et al. “Ride the Green Light”: Indocyanine Green–marked Off-clamp Robotic Partial Nephrectomy for Totally Endophytic Renal Masses. Eur. Urol. 2019, 75, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Tuderti, G.; Brassetti, A.; Mastroianni, R.; Misuraca, L.; Bove, A.; Anceschi, U.; Ferriero, M.; Guaglianone, S.; Gallucci, M.; Simone, G. Expanding the limits of nephron-sparing surgery: Surgical technique and mid-term outcomes of purely off-clamp robotic partial nephrectomy for totally endophytic renal tumors. Int. J. Urol. 2022, 29, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Nardis, P.G.; Cipollari, S.; Lucatelli, P.; Basilico, F.; Rocco, B.; Corona, M.; Cannavale, A.; Leonardo, C.; Flammia, R.S.; Proietti, F.; et al. Cone-Beam CT-Guided Transarterial Tagging of Endophytic Renal Tumors with Indocyanine Green for Robot-Assisted Partial Nephrectomy. J. Vasc. Interv. Radiol. 2022, 33, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Amparore, D.; Checcucci, E.; Piazzolla, P.; Piramide, F.; De Cillis, S.; Piana, A.; Verri, P.; Manfredi, M.; Fiori, C.; Vezzetti, E.; et al. Indocyanine Green Drives Computer Vision Based 3D Augmented Reality Robot Assisted Partial Nephrectomy: The Beginning of “Automatic” Overlapping Era. Urology 2022, 164, e312–e316. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.; Salvador, J.T.; Urdaneta, G.; Patiño, D.; Montlleó, M.; Esquena, S.; Caffaratti, J.; de León, J.P.; Guirado, L.; Villavicencio, H. Laparoscopic Kidney Transplantation. Eur. Urol. 2010, 57, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Sood, A.; Bhandari, M.; Kher, V.; Ghosh, P.; Abaza, R.; Jeong, W.; Ghani, K.R.; Kumar, R.K.; Modi, P.; et al. Robotic Kidney Transplantation with Regional Hypothermia: A Step-by-step Description of the Vattikuti Urology Institute–Medanta Technique (IDEAL Phase 2a). Eur. Urol. 2013, 65, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J. No surgical innovation without evaluation: The IDEAL recommendations. Lancet 2009, 374, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Breda, A.; Territo, A.; Gausa, L.; Tuğcu, V.; Alcaraz, A.; Musquera, M.; Decaestecker, K.; Desender, L.; Stockle, M.; Janssen, M.; et al. Robot-assisted Kidney Transplantation: The European Experience. Eur. Urol. 2018, 73, 273–281. [Google Scholar] [CrossRef]
- Hoffmann, C.; Compton, F.; Schäfer, J.; Steiner, U.; Fuller, T.; Schostak, M.; Zidek, W.; van der Giet, M.; Westhoff, T. Intraoperative Assessment of Kidney Allograft Perfusion by Laser-Assisted Indocyanine Green Fluorescence Videography. Transplant. Proc. 2010, 42, 1526–1530. [Google Scholar] [CrossRef]
- Vignolini, G.; Sessa, F.; Greco, I.; Cito, G.; Vanacore, D.; Cocci, A.; Sessa, M.; Grandi, V.; Pili, A.; Giancane, S.; et al. Intraoperative assessment of ureteral and graft reperfusion during robotic kidney transplantation with indocyanine green fluorescence videography. Minerva Urol. Nefrol. 2019, 71, 79–84. [Google Scholar] [CrossRef]
- Ietto, G.; Iori, V.; Inversini, D.; Parise, C.; Zani, E.; Iovino, D.; Tozzi, M.; Gasperina, D.D.; Carcano, G.; Workgroup, I. Indocyanine Green Angiography for Quality Assessment of Renal Graft Before Transplantation: A Pilot Study. Exp. Clin. Transplant. 2023, 21, 110–115. [Google Scholar] [CrossRef]
- Arora, E.; Bhandarwar, A.; Wagh, A.; Gandhi, S.; Patel, C.; Gupta, S.; Talwar, G.; Agarwal, J.; Rathore, J.; Chatnalkar, S. Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: A retrospective review of 55 Cases. Surg. Endosc. 2018, 32, 4649–4657. [Google Scholar] [CrossRef]
- Manny, T.B.; Pompeo, A.S.; Hemal, A.K. Robotic Partial Adrenalectomy Using Indocyanine Green Dye with Near-infrared Imaging: The Initial Clinical Experience. Urology 2013, 82, 738–742. [Google Scholar] [CrossRef]
- Sound, S.; Okoh, A.K.; Bucak, E.; Yigitbas, H.; Dural, A.C.; Berber, E. Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: A feasibility study. Surg. Endosc. 2016, 30, 657–662. [Google Scholar] [CrossRef]
- Gokceimam, M.; Kahramangil, B.; Akbulut, S.; Erten, O.; Berber, E. Robotic Posterior Retroperitoneal Adrenalectomy: Patient Selection and Long-Term Outcomes. Ann. Surg. Oncol. 2021, 28, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.; Zaidi, N.; Berber, E. The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J. Surg. Oncol. 2016, 114, 153–156. [Google Scholar] [CrossRef]
- Kahramangil, B.; Kose, E.; Berber, E. Characterization of fluorescence patterns exhibited by different adrenal tumors: Determining the indications for indocyanine green use in adrenalectomy. Surgery 2018, 164, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Donmez, M.; Kahramangil, B.; Kose, E.; Erten, O.; Akbulut, S.; Gokceimam, M.; Berber, E. A visual quantification of tissue distinction in robotic transabdominal lateral adrenalectomy: Comparison of indocyanine green and conventional views. Surg. Endosc. 2022, 36, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Suardi, N.; Moschini, M.; Gallina, A.; Gandaglia, G.; Abdollah, F.; Capitanio, U.; Bianchi, M.; Tutolo, M.; Passoni, N.; Salonia, A.; et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2012, 111, 717–722. [Google Scholar] [CrossRef]
- Patel, V.R.; Schatloff, O.; Chauhan, S.; Sivaraman, A.; Valero, R.; Coelho, R.F.; Rocco, B.; Palmer, K.J.; Kameh, D. The Role of the Prostatic Vasculature as a Landmark for Nerve Sparing During Robot-Assisted Radical Prostatectomy. Eur. Urol. 2012, 61, 571–576. [Google Scholar] [CrossRef]
- Kumar, A.; Samavedi, S.; Bates, A.; Coelho, R.; Rocco, B.; Marquinez, J.; Palmer, K.J.; Patel, V.R. Using Indocyanine Green and Near-Infrared Fluorescence Technology to Identify the “Landmark Artery” During Robot-Assisted Radical Prostatectomy. Videourology 2015, 29. [Google Scholar] [CrossRef]
- Mangano, M.S.; De Gobbi, A.; Beniamin, F.; Lamon, C.; Ciaccia, M.; Maccatrozzo, L. Robot-assisted nerve-sparing radical prostatectomy using near-infrared fluorescence technology and indocyanine green: Initial experience. Urol. J. 2018, 85, 29–31. [Google Scholar] [CrossRef]
- Amara, N.; Al Youssef, T.; Massa, J.; Fidjel, A.; El Khoury, E.; Patel, B.; Flais, M.; Deswarte, C. Intraoperative angiography of the neurovascular bundle using indocyanine green and near-infrared fluorescence improves anatomical dissection during robot-assisted radical prostatectomy: Initial clinical experience. J. Robot. Surg. 2023, 17, 687–694. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.-J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic Efficacy of 68 Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Manny, T.B.; Patel, M.; Hemal, A.K. Fluorescence-enhanced Robotic Radical Prostatectomy Using Real-time Lymphangiography and Tissue Marking with Percutaneous Injection of Unconjugated Indocyanine Green: The Initial Clinical Experience in 50 Patients. Eur. Urol. 2014, 65, 1162–1168. [Google Scholar] [CrossRef]
- Harke, N.N.; Godes, M.; Wagner, C.; Addali, M.; Fangmeyer, B.; Urbanova, K.; Hadaschik, B.; Witt, J.H. Fluorescence-supported lymphography and extended pelvic lymph node dissection in robot-assisted radical prostatectomy: A prospective, randomized trial. World J. Urol. 2018, 36, 1817–1823. [Google Scholar] [CrossRef]
- Shimbo, M.; Endo, F.; Matsushita, K.; Hattori, K. Impact of indocyanine green-guided extended pelvic lymph node dissection during robot-assisted radical prostatectomy. Int. J. Urol. 2020, 27, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Köseoğlu, E.; Canda, A.E.; Çil, B.E.; Aykanat, I.C.; Sarıkaya, A.F.; Tarım, K.; Armutlu, A.; Kulaç, I.; Barçın, E.; et al. Fluorescence-guided extended pelvic lymphadenectomy during robotic radical prostatectomy. J. Robot. Surg. 2022, 17, 885–890. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, H.G.; Grivas, N.; van Leeuwen, F. Comprehensive Assessment of Indocyanine Green Usage: One Tracer, Multiple Urological Applications. Eur. Urol. Focus 2018, 4, 665–668. [Google Scholar] [CrossRef]
- Mazzone, E.; Dell’oglio, P.; Grivas, N.; Wit, E.; Donswijk, M.; Briganti, A.; Van Leeuwen, F.; van der Poel, H. Diagnostic Value, Oncologic Outcomes, and Safety Profile of Image-Guided Surgery Technologies During Robot-Assisted Lymph Node Dissection with Sentinel Node Biopsy for Prostate Cancer. J. Nucl. Med. 2021, 62, 1363–1371. [Google Scholar] [CrossRef]
- de Korne, C.M.; Wit, E.M.; de Jong, J.; Olmos, R.A.V.; Buckle, T.; van Leeuwen, F.W.B.; van der Poel, H.G. Anatomical localization of radiocolloid tracer deposition affects outcome of sentinel node procedures in prostate cancer. Eur. J. Nucl. Med. 2019, 46, 2558–2568. [Google Scholar] [CrossRef]
- Buckle, T.; Brouwer, O.R.; Olmos, R.A.V.; van der Poel, H.G.; van Leeuwen, F.W. Relationship Between Intraprostatic Tracer Deposits and Sentinel Lymph Node Mapping in Prostate Cancer Patients. J. Nucl. Med. 2012, 53, 1026–1033. [Google Scholar] [CrossRef]
- Wit, E.M.K.; van Beurden, F.; Kleinjan, G.H.; Grivas, N.; de Korne, C.M.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. The impact of drainage pathways on the detection of nodal metastases in prostate cancer: A phase II randomized comparison of intratumoral vs intraprostatic tracer injection for sentinel node detection. Eur. J. Nucl. Med. 2022, 49, 1743–1753. [Google Scholar] [CrossRef]
- Wit, E.M.K.; KleinJan, G.H.; Berrens, A.-C.; van Vliet, R.; van Leeuwen, P.J.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur. J. Nucl. Med. 2023, 1–11. [Google Scholar] [CrossRef]
- Dixon, A.R.; Lord, P.H.; Madigan, M.R. The Madigan Prostatectomy. J. Urol. 1990, 144, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, D.; Ye, S.; Kong, D.; Qin, J.; Jing, T.; Mao, Y.; Meng, H.; Wang, S. Robotic-assisted Urethra-sparing Simple Prostatectomy Via an Extraperitoneal Approach. Urology 2018, 119, 85–90. [Google Scholar] [CrossRef]
- Simone, G.; Misuraca, L.; Anceschi, U.; Minisola, F.; Ferriero, M.; Guaglianone, S.; Tuderti, G.; Gallucci, M. Urethra and Ejaculation Preserving Robot-assisted Simple Prostatectomy: Near-infrared Fluorescence Imaging-guided Madigan Technique. Eur. Urol. 2019, 75, 492–497. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Albersen, M.; Parnham, A.; Protzel, C.; Pettaway, C.A.; Ayres, B.; Antunes-Lopes, T.; Barreto, L.; Campi, R.; Crook, J.; et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 2023, 83, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Kroon, B.; Lont, A.; Olmos, R.V.; Nieweg, O.; Horenblas, S. Morbidity of dynamic sentinel node biopsy in penile carcinoma. J. Urol. 2005, 173, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Horenblas, S.; Jansen, L.; Meinhardt, W.; Hoefnagel, C.A.; De Jong, D.; Nieweg, O.E. Detection of occult Metastasis in Squamous Cell Carcinoma of the Penis Using a Dynamic Sentinel Node Procedure. J. Urol. 2000, 163, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhu, T.; Gao, J.; Li, H.; Liu, X.; Zhang, X. Impact of Examined Lymph Node Count and Lymph Node Density on Overall Survival of Penile Cancer. Front. Oncol. 2021, 11, 706531. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Berg, N.S.V.D.; Mathéron, H.M.; van der Poel, H.G.; van Rhijn, B.W.; Bex, A.; van Tinteren, H.; Olmos, R.A.V.; van Leeuwen, F.W.; Horenblas, S. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur. Urol. 2014, 65, 600–609. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van Werkhoven, E.; Berg, N.S.V.D.; Karakullukcu, M.B.; Zijlmans, H.J.M.A.A.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. 2018, 45, 1915–1925. [Google Scholar] [CrossRef]
- Dell’oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.; Brouwer, O.R. Hybrid Indocyanine Green–99mTc-nanocolloid for Single-photon Emission Computed Tomography and Combined Radio- and Fluorescence-guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, O.R.; Buckle, T.; Vermeeren, L.; Klop, W.M.C.; Balm, A.J.; van der Poel, H.G.; van Rhijn, B.W.; Horenblas, S.; Nieweg, O.E.; van Leeuwen, F.W.; et al. Comparing the Hybrid Fluorescent–Radioactive Tracer Indocyanine Green–99mTc-Nanocolloid with 99mTc-Nanocolloid for Sentinel Node Identification: A Validation Study Using Lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 2012, 53, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Torbrand, C.; Warnolf, Å.; Glombik, D.; Davidsson, S.; Carlsson, J.; Baseckas, G.; Håkansson, U.; Trägårdh, E.; Geijer, H.; Liedberg, F.; et al. Sentinel Node Identification with Hybrid Tracer-guided and Conventional Dynamic Sentinel Node Biopsy in Penile Cancer: A Prospective Study in 130 Patients from the Two National Referral Centres in Sweden. Eur. Urol. Oncol. 2022, 5, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Sávio, L.F.; Barboza, M.P.; Alameddine, M.; Ahdoot, M.; Alonzo, D.; Ritch, C.R. Combined Partial Penectomy with Bilateral Robotic Inguinal Lymphadenectomy Using Near-infrared Fluorescence Guidance. Urology 2018, 113, 251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yao, K.; Zhou, Z.; Liu, J.; Li, C.; Hou, W.; Tang, Y.; Hu, S.; Wang, L. “Light green up”: Indocyanine Green Fluorescence Imaging–guided Robotic Bilateral Inguinal Lymphadenectomy by the Hypogastric Subcutaneous Approach for Penile Cancer. Eur. Urol. Open Sci. 2022, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Zhao, L.C.; Kenigsberg, A.P.; Mass, A.Y.; Taneja, S.S.; Huang, W.C. Novel Use of Fluorescence Lymphangiography During Robotic Groin Dissection for Penile Cancer. Urology 2017, 107, 267. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Simhan, J.; Parker, D.C.; Reilly, C.; Llukani, E.; Lee, D.I.; Mydlo, J.H.; Eun, D.D. Novel Use of Indocyanine Green for Intraoperative, Real-time Localization of Ureteral Stenosis During Robot-assisted Ureteroureterostomy. Urology 2013, 82, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Gan, M.; McClintock, T.R.; Volpe, A.; Borofsky, M.S.; Mottrie, A.; Stifelman, M.D. Near-infrared Fluorescence Imaging: Emerging Applications in Robotic Upper Urinary Tract Surgery. Eur. Urol. 2014, 65, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Moore, B.; Giusto, L.; Eun, D.D. Use of Indocyanine Green During Robot-assisted Ureteral Reconstructions. Eur. Urol. 2015, 67, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Xing, S.; Xing, W.; Bai, Z.; Zhang, J.; Li, Y.; Wang, H.; Liu, Q. Application of Indocyanine Green in Combination with Da Vinci Xi Robot in Surgeries on the Upper Urinary Tract: A Case Series Study. J. Clin. Med. 2023, 12, 1980. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; West, A.; Hayes, J.; Teoh, J.; Decaestecker, K.; Vasdev, N. Methods of Sentinel Lymph Node Detection and Management in Urinary Bladder Cancer—A Narrative Review. Curr. Oncol. 2022, 29, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Shiina, H.; Mitsui, Y.; Yasumoto, H.; Matsubara, A.; Igawa, M. Identification of lymphatic pathway involved in the spread of bladder cancer: Evidence obtained from fluorescence navigation with intraoperatively injected indocyanine green. Can. Urol. Assoc. J. 2012, 7, E322–E328. [Google Scholar] [CrossRef]
- Schaafsma, B.; Verbeek, F.; Elzevier, H.; Tummers, Q.; van der Vorst, J.; Frangioni, J.; van de Velde, C.; Pelger, R.; Vahrmeijer, A. Optimization of sentinel lymph node mapping in bladder cancer using near-infrared fluorescence imaging. J. Surg. Oncol. 2014, 110, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Manny, T.B.; Hemal, A.K. Fluorescence-enhanced Robotic Radical Cystectomy Using Unconjugated Indocyanine Green for Pelvic Lymphangiography, Tumor Marking, and Mesenteric Angiography: The Initial Clinical Experience. Urology 2014, 83, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Polom, W.; Markuszewski, M.; Cytawa, W.; Czapiewski, P.; Lass, P. Fluorescent Versus Radioguided Lymph Node Mapping in Bladder Cancer. Clin. Genitourin. Cancer 2017, 15, e405–e409. [Google Scholar] [CrossRef] [PubMed]
- Rietbergen, D.D.; van Gennep, E.J.; KleinJan, G.H.; Donswijk, M.; Olmos, R.A.M.V.; van Rhijn, B.W.M.; van der Poel, H.G.M.; van Leeuwen, F.W. Evaluation of the Hybrid Tracer Indocyanine Green–99mTc-Nanocolloid for Sentinel Node Biopsy in Bladder Cancer—A Prospective Pilot Study. Clin. Nucl. Med. 2022, 47, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Abreu, A.L.D.C.; Berger, A.K.; Sehgal, S.; Gill, I.; Aron, M.; Desai, M.M. Evolution of robot-assisted orthotopic ileal neobladder formation: A step-by-step update to the University of Southern California (USC) technique. BJU Int. 2017, 119, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Doshi, C.P.; Wozniak, A.; Quek, M.L. Near-infrared Fluorescence Imaging of Ureters with Intravenous Indocyanine Green During Radical Cystectomy to Prevent Ureteroenteric Anastomotic Strictures. Urology 2020, 144, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Ashrafi, A.N.; Hartman, N.; Shakir, A.; Cacciamani, G.E.; Freitas, D.; Rajarubendra, N.; Fay, C.; Berger, A.; Desai, M.M.; et al. Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int. 2019, 124, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Sterling, M.E.; Keehn, A.Y.; Lee, M.; Metro, M.J.; Eun, D.D. The use of indocyanine green during robotic ureteroenteric reimplantation for the management of benign anastomotic strictures. World J. Urol. 2018, 37, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Tuderti, G.; Brassetti, A.; Minisola, F.; Anceschi, U.; Ferriero, M.; Leonardo, C.; Misuraca, L.; Vallati, G.; Guaglianone, S.; Gallucci, M.; et al. Transnephrostomic Indocyanine Green-Guided Robotic Ureteral Reimplantation for Benign Ureteroileal Strictures After Robotic Cystectomy and Intracorporeal Neobladder: Step-By-Step Surgical Technique, Perioperative and Functional Outcomes. J. Endourol. 2019, 33, 823–828. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licari, L.C.; Bologna, E.; Proietti, F.; Flammia, R.S.; Bove, A.M.; D’annunzio, S.; Tuderti, G.; Leonardo, C. Exploring the Applications of Indocyanine Green in Robot-Assisted Urological Surgery: A Comprehensive Review of Fluorescence-Guided Techniques. Sensors 2023, 23, 5497. https://doi.org/10.3390/s23125497
Licari LC, Bologna E, Proietti F, Flammia RS, Bove AM, D’annunzio S, Tuderti G, Leonardo C. Exploring the Applications of Indocyanine Green in Robot-Assisted Urological Surgery: A Comprehensive Review of Fluorescence-Guided Techniques. Sensors. 2023; 23(12):5497. https://doi.org/10.3390/s23125497
Chicago/Turabian StyleLicari, Leslie Claire, Eugenio Bologna, Flavia Proietti, Rocco Simone Flammia, Alfredo Maria Bove, Simone D’annunzio, Gabriele Tuderti, and Costantino Leonardo. 2023. "Exploring the Applications of Indocyanine Green in Robot-Assisted Urological Surgery: A Comprehensive Review of Fluorescence-Guided Techniques" Sensors 23, no. 12: 5497. https://doi.org/10.3390/s23125497
APA StyleLicari, L. C., Bologna, E., Proietti, F., Flammia, R. S., Bove, A. M., D’annunzio, S., Tuderti, G., & Leonardo, C. (2023). Exploring the Applications of Indocyanine Green in Robot-Assisted Urological Surgery: A Comprehensive Review of Fluorescence-Guided Techniques. Sensors, 23(12), 5497. https://doi.org/10.3390/s23125497






