Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait
Abstract
1. Introduction
1.1. Background
1.2. Step 1 to Goal: Constructing HGS Assessment Model on an IMS and Related Work
1.2.1. Research Question in Step 1
1.2.2. Ideas for Solving the Research Question in Step 1
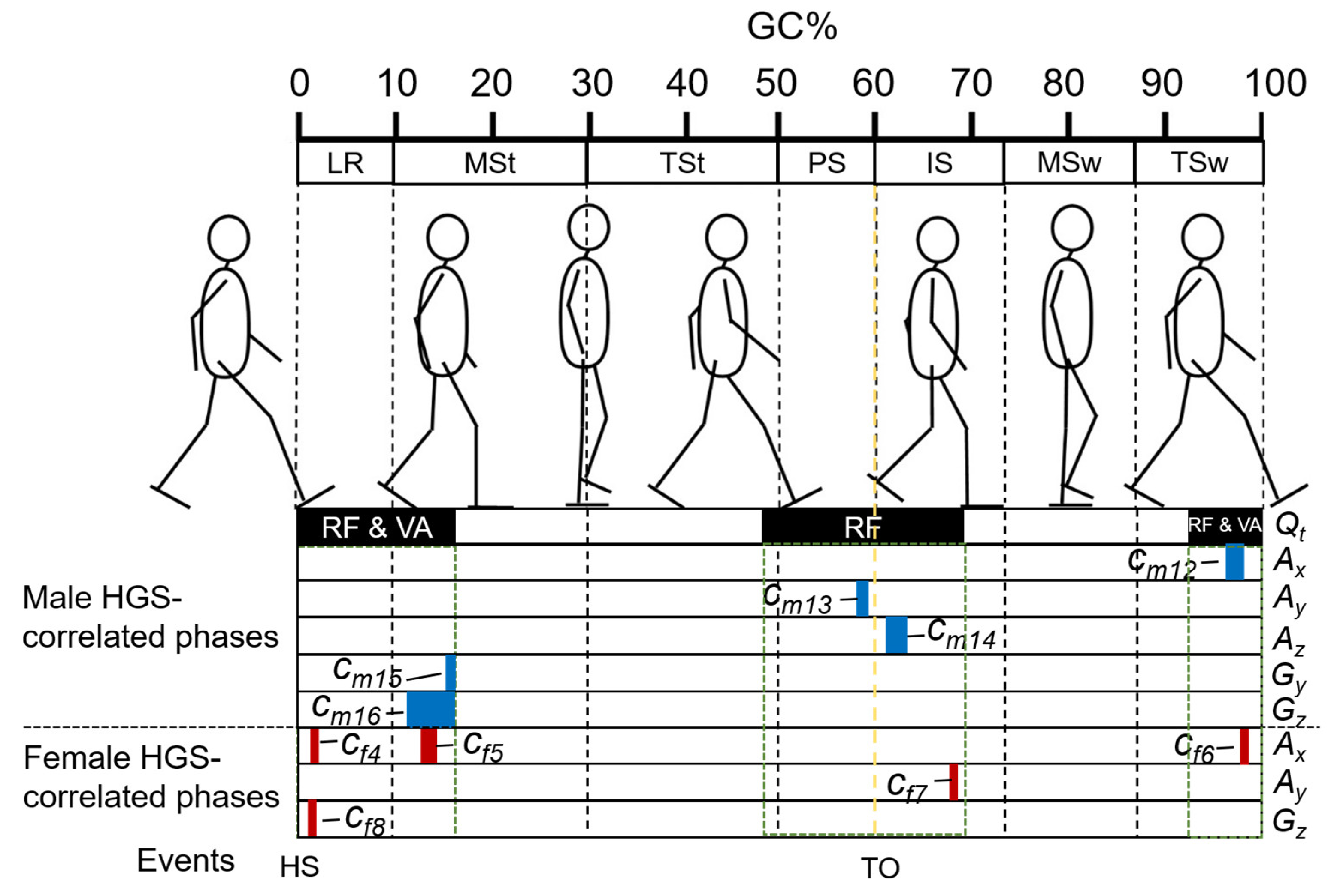
- Identifying significant gait phases with statistically significant correlation with the target variable using statistic parametric mapping (SPM) [46], which was proven effective in biomechanical studies. The significant gait phases always continuously appeared, performing as clusters on the temporal axis; thus we called them, “gait phase clusters” (GPCs).
- Conducting predictors by averaging the foot motion signals in the GPCs to obtain IMS predictors that can be implemented on the A-RROWG-type IMS. Although there are clustering algorithms, such as community detection algorithms [47], due to the temporal continuity of foot motion, using the integral average of the signals in GPC as a single predictor is sufficient and helpful for implementation on the A-RROWG-type IMS.
- Reducing redundant predictors and selecting appropriate predictors using our original algorithm, the leave-one-subject-out least absolute shrinkage and selection operator (LOSO-LASSO).
- Constructing a multivariate linear regression estimation model.
1.3. Step 2 to Goal: Related Work on Frailty Assessment and Designing a Frailty Risk Score
1.3.1. Research Question in Step 2
1.3.2. Ideas for Solving the Research Question in Step 2
1.4. Testing Constructed HGS Estimation Model and Frailty Risk Score
1.5. The Development Process and Main Contributions in this Study
- (1)
- We discovered novel predictors for HGS assessment obtained from foot motions.
- (2)
- We constructed a lightweight HGS assessment model that can be feasibly implemented in the A-RROWG-type IMS, which serves as a key module for long-term frailty assessment.
- (3)
- We tested the effectiveness and robustness of the constructed model using a group of separately recruited subjects.
- (4)
- We designed an analog frailty risk score and evaluated its effectiveness for frailty risk assessment via an IMS.

2. Materials and Methods
2.1. Subjects and Their Characteristics
2.2. Experiment
- Step 1
- At the start of the experiment, all subjects were asked to complete a questionnaire to provide basic information, including age, height, and weight, and for the calculation of BMI based on their answers.
- Step 2
- The same questionnaire included four questions based on the J-CHS criteria:
- Q1. Have you lost more than 2–3 kg in the past 6 months?
- Q2. In the past two weeks, have you felt tired for no reason?
- Q3. Do you engage in light exercise or gymnastics at least once a week?
- Q4. Do you engage in regular exercise or sports at least once a week?
- Step 3
- After answering the questionnaire, subjects were guided to measure their HGS, which served as the reference HGS value in this study.
- Step 4
- Subjects were asked to walk in a straight line. In this step, we collected foot motion data for calculating GPs and IMS predictors, as well as reference gait speed data for all subjects. Additionally, for those in Group III, video recordings were made while they walked.
- Step 5
- We sent the walking videos to clinical experts to obtain expert-rated frailty risk scores as objective frailty reference data.
2.2.1. Step 2 of the Experiment
2.2.2. Step 3 of the Experiment
2.2.3. Step 4 of the Experiment
2.2.4. Step 5 of the Experiment
2.3. Characteristics of IMS
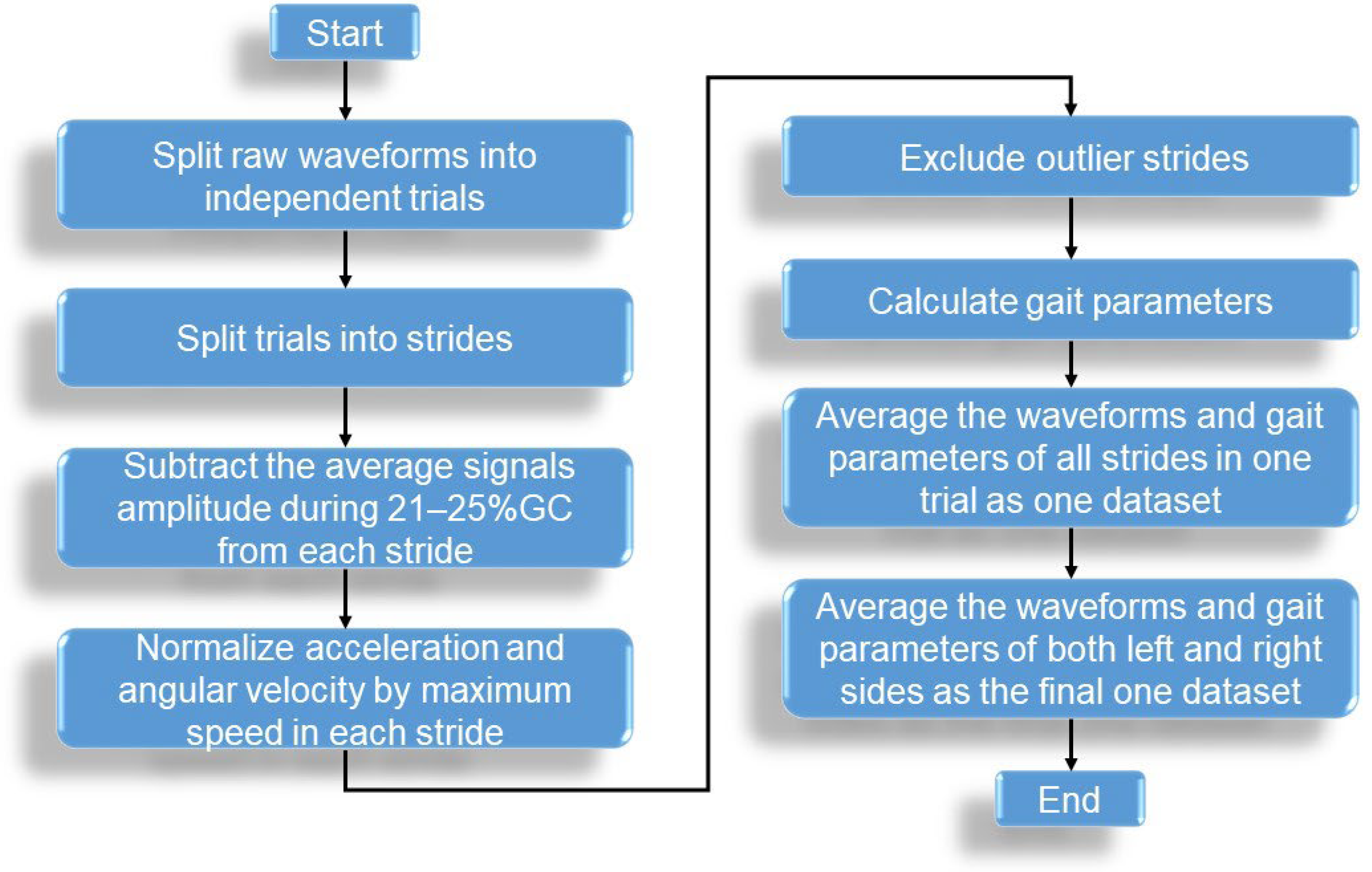
2.4. Signal Processing and GPs
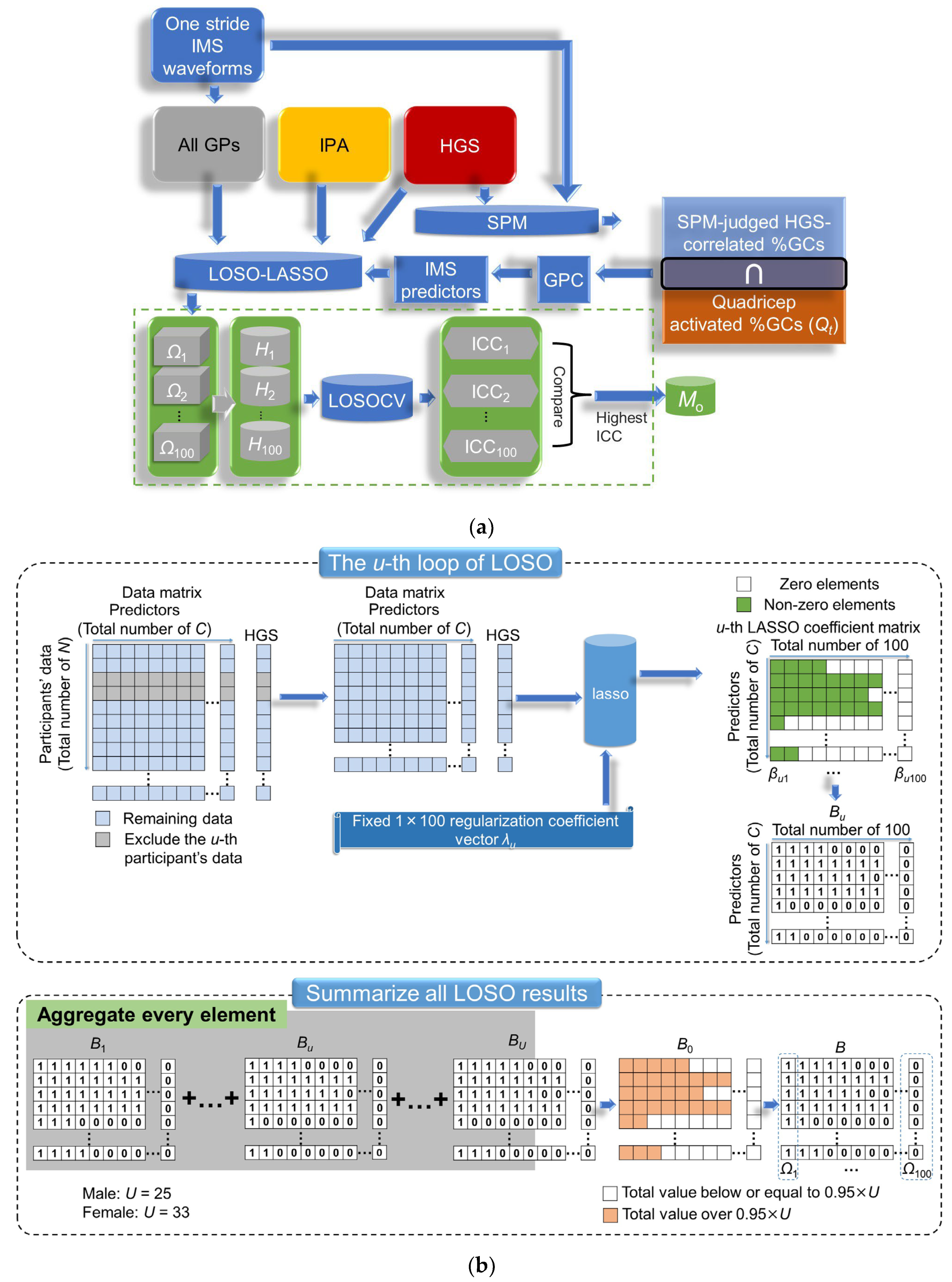
2.5. SPM-LOSO-LASSO, Model Evaluation of HGS, and Precision Evaluation of Gait Speed
2.5.1. The Details of SPM-LOSO-LASSO
2.5.2. Model Evaluation of HGS and Precision Evaluation of Gait Speed
2.6. Designing Frailty Risk Score
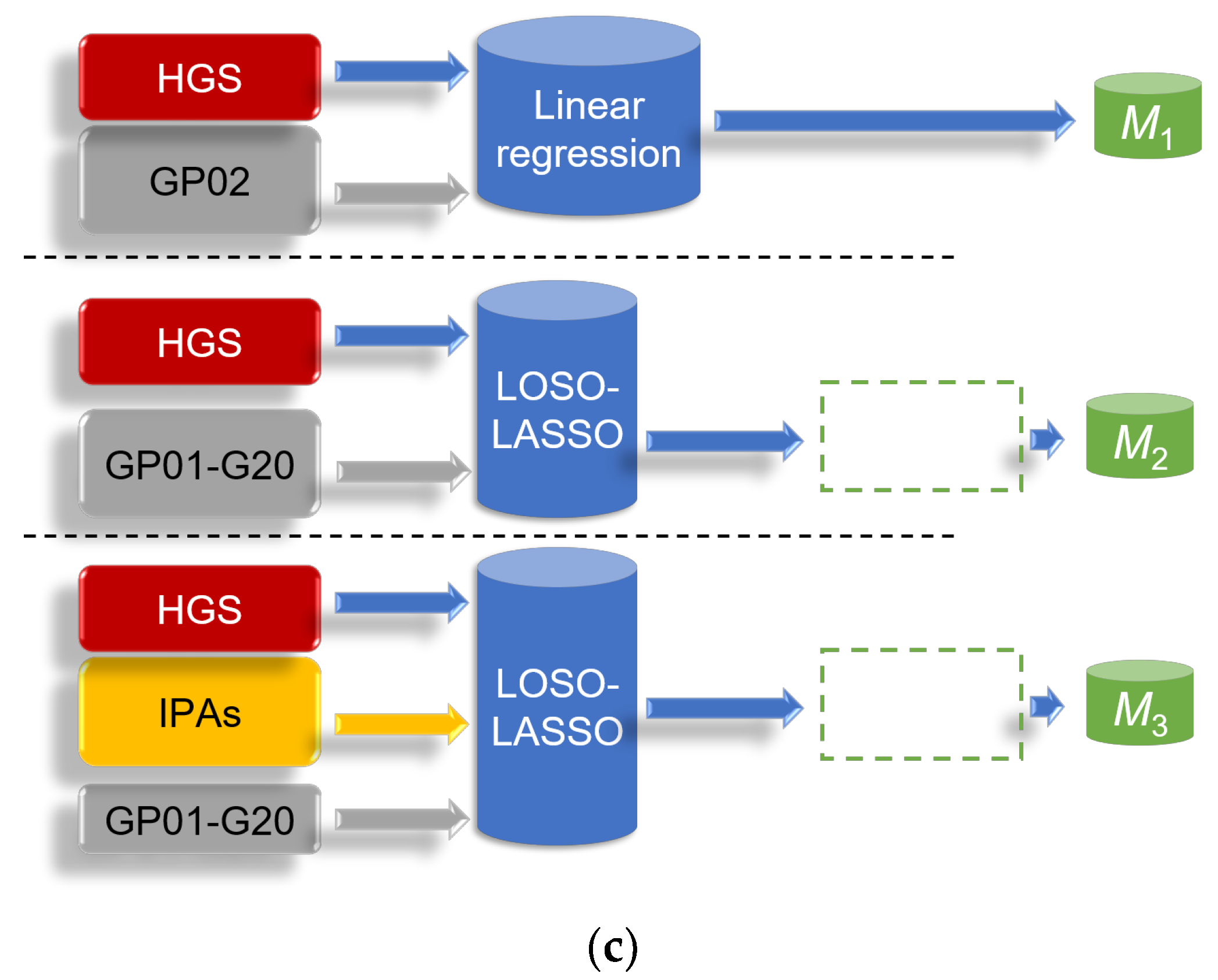
2.7. Evaluation Methods in Model Tests
2.7.1. Test 1
2.7.2. Test 2
3. Results
3.1. SPM Analysis in HGS Estimation Model Construction
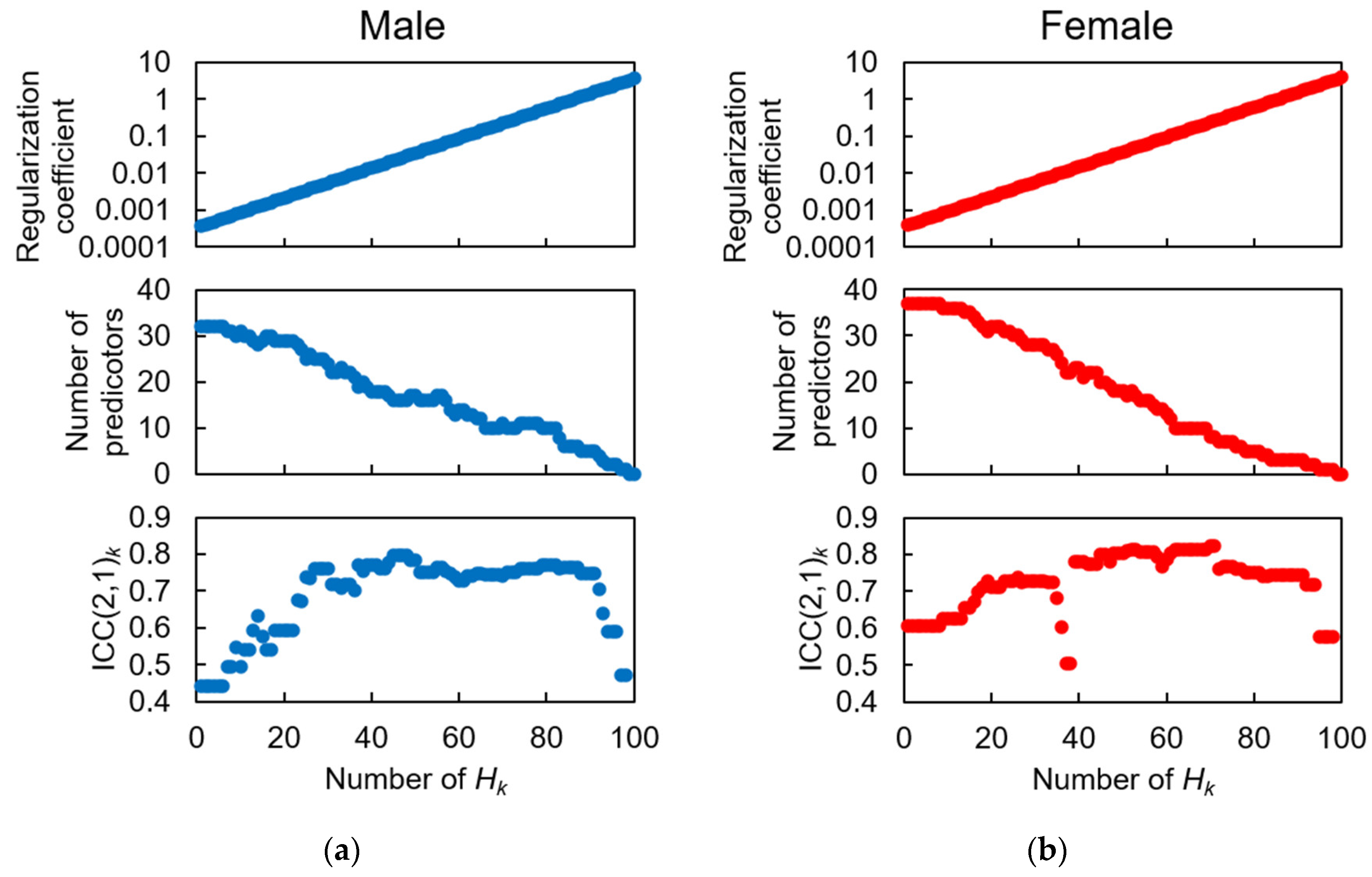
3.2. Feature Selection for HGS Estimation Model
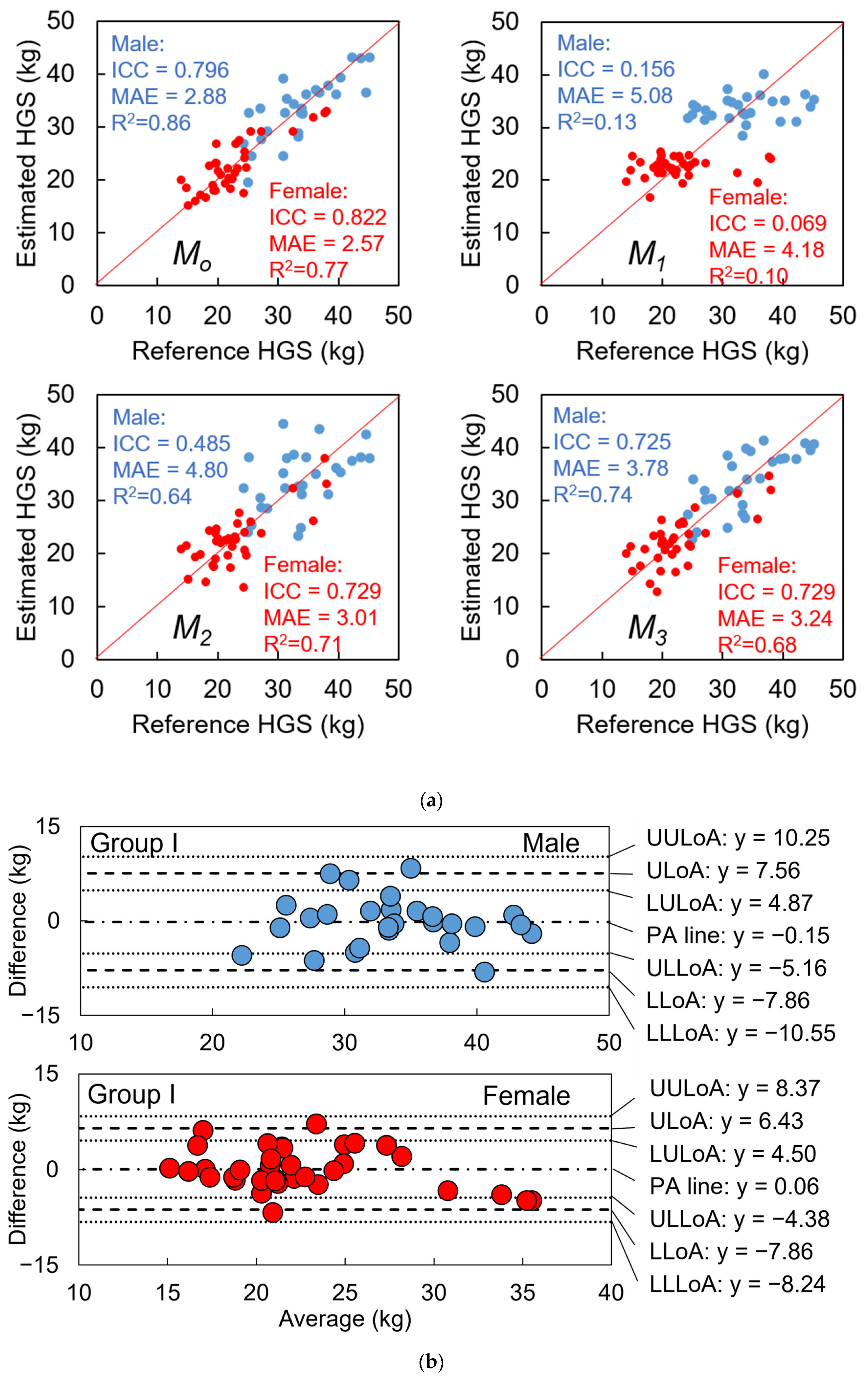
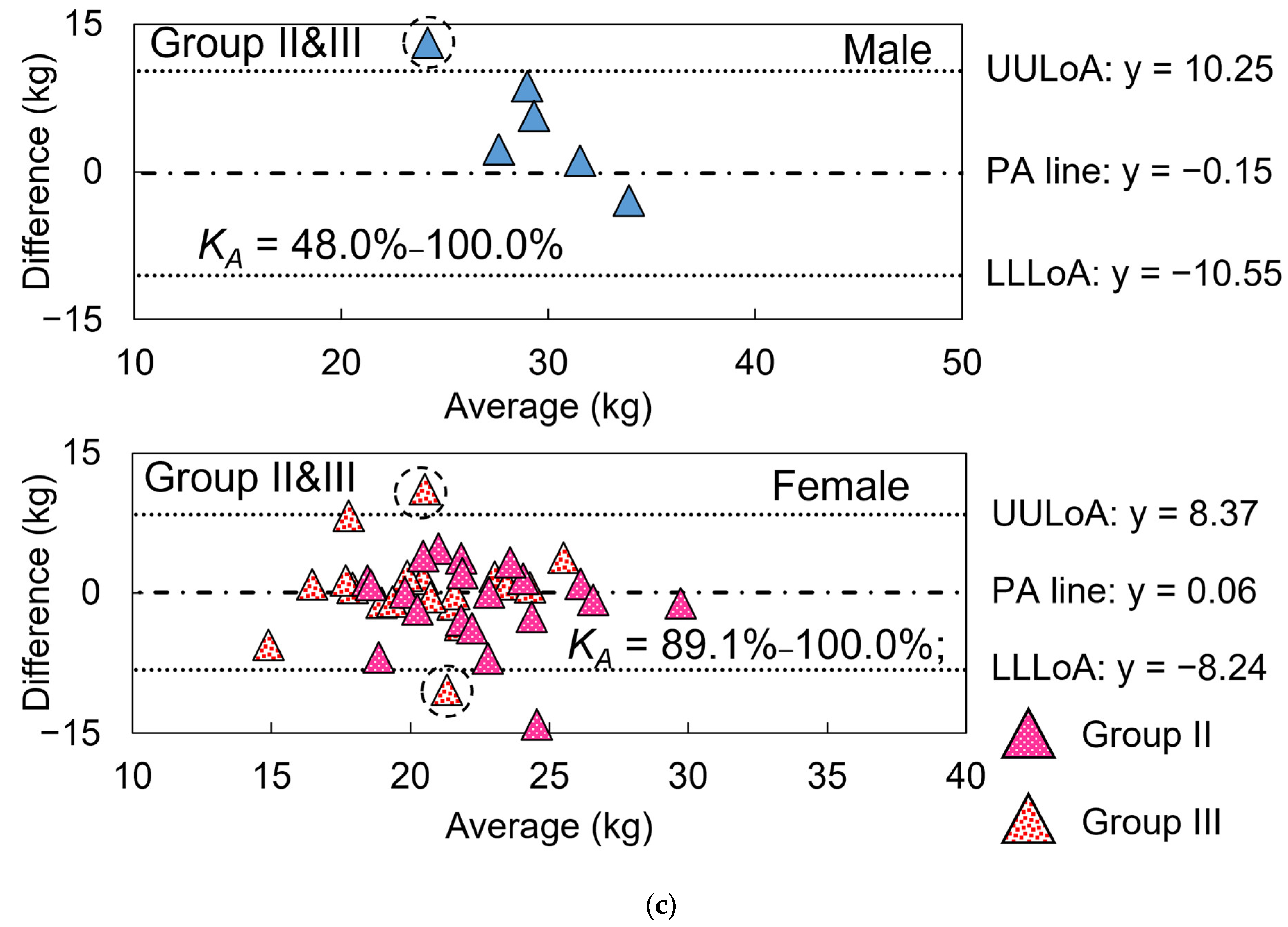
3.3. Precision Evaluation of Gait Speed, Model Evaluation of HGS Estimation, and Test 1
3.3.1. Gait Speed
3.3.2. HGS
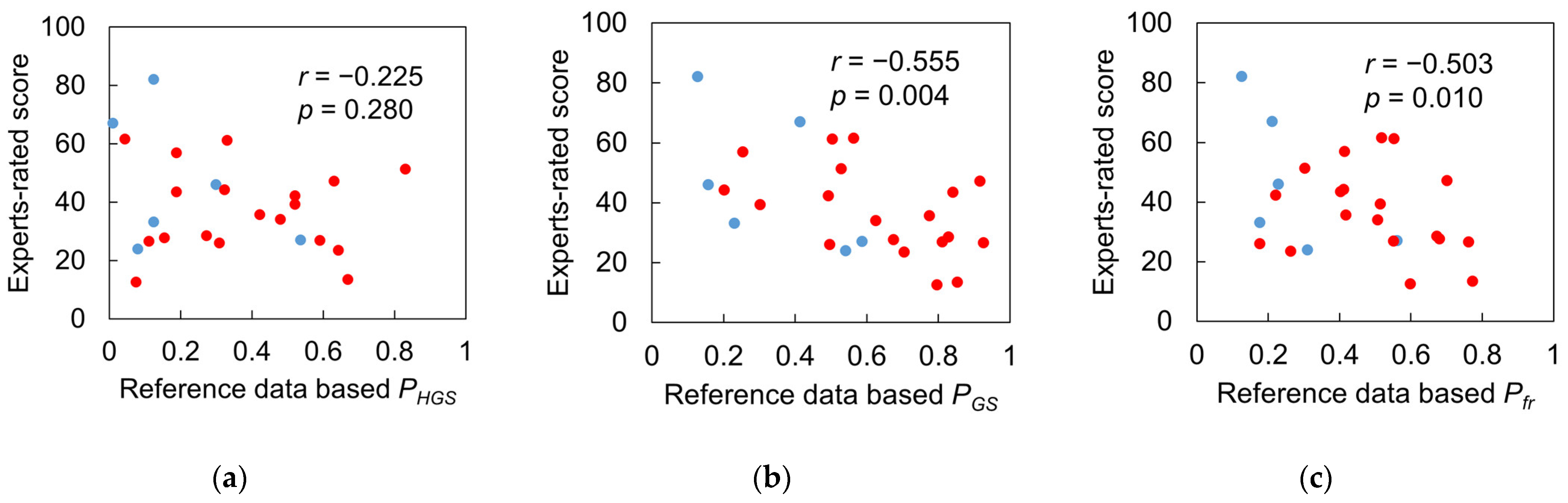
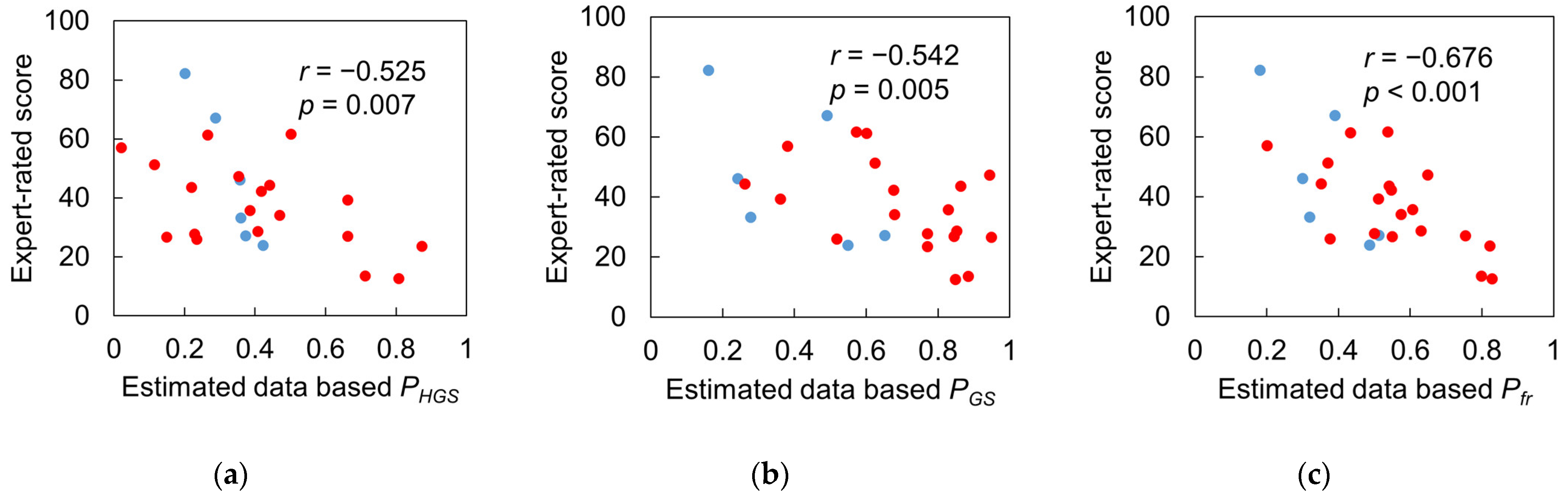
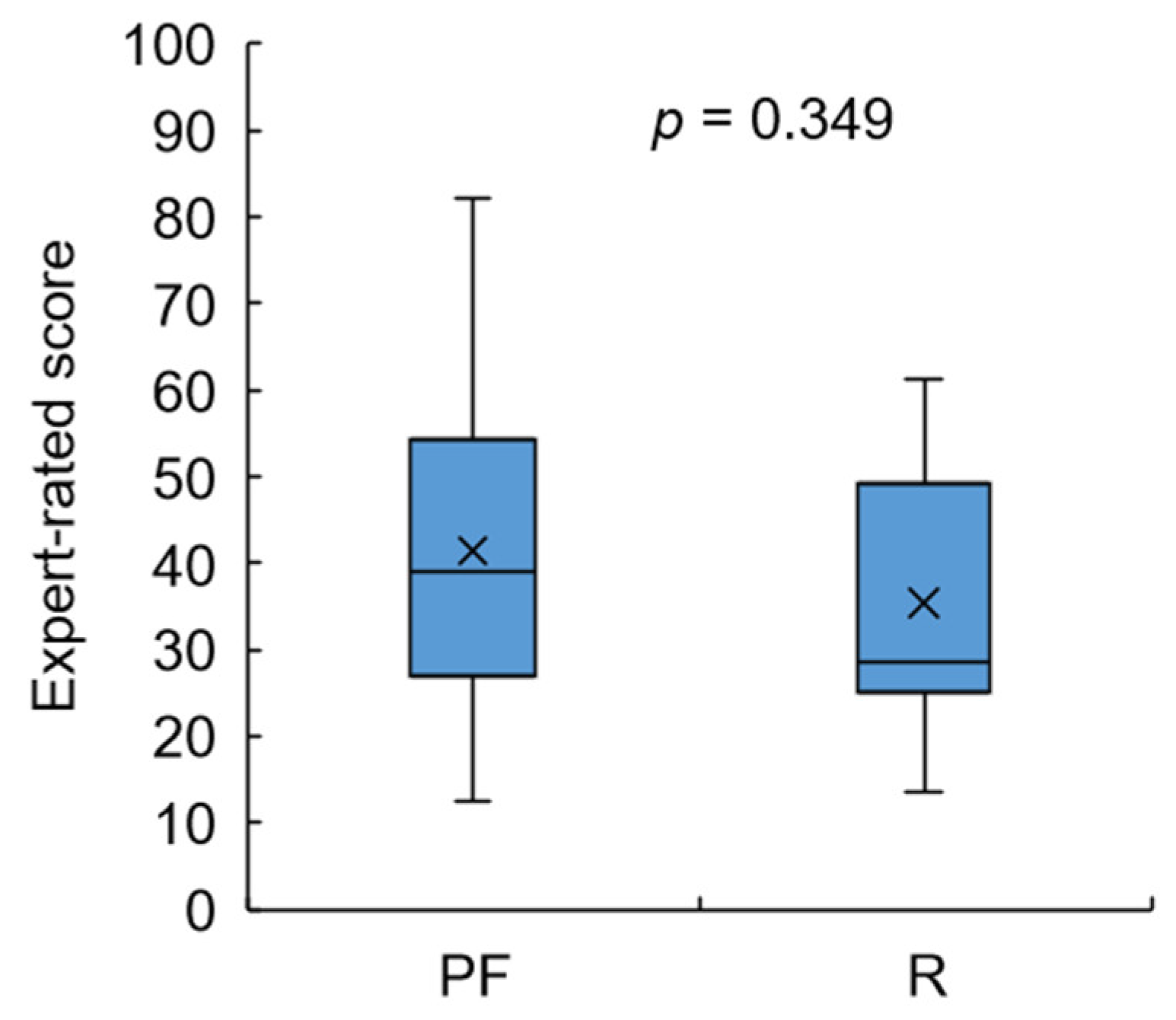
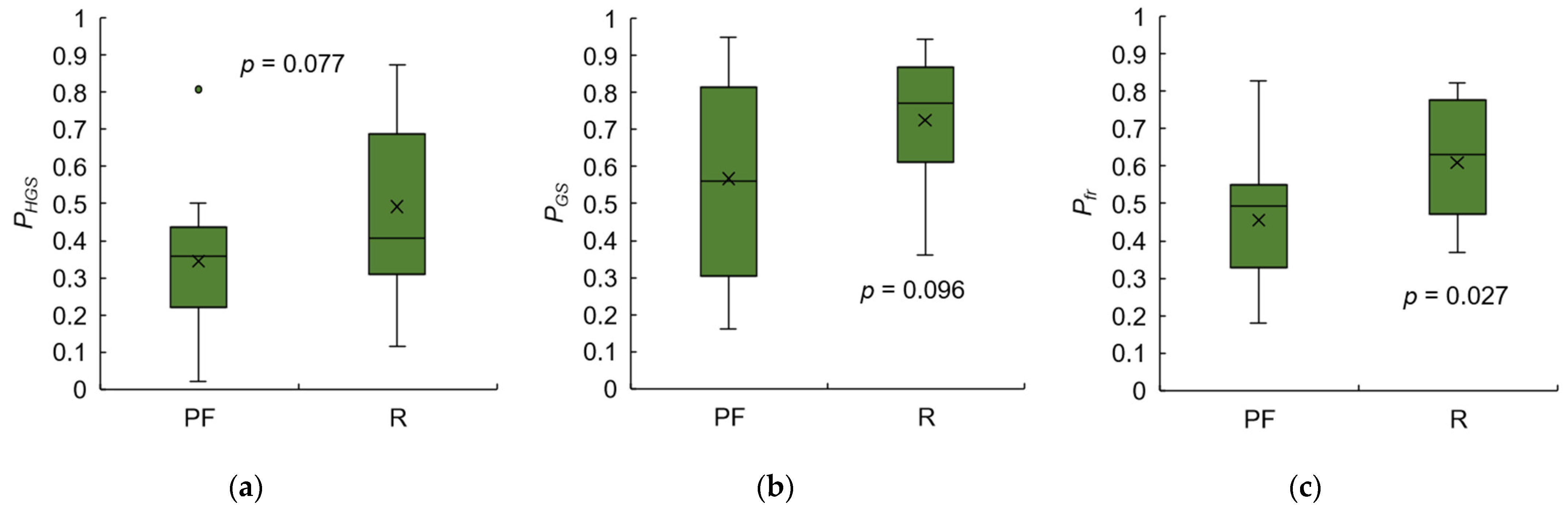
3.4. Test 2: Validity of Designed Frailty Risk Score with Estimated HGS
4. Discussion
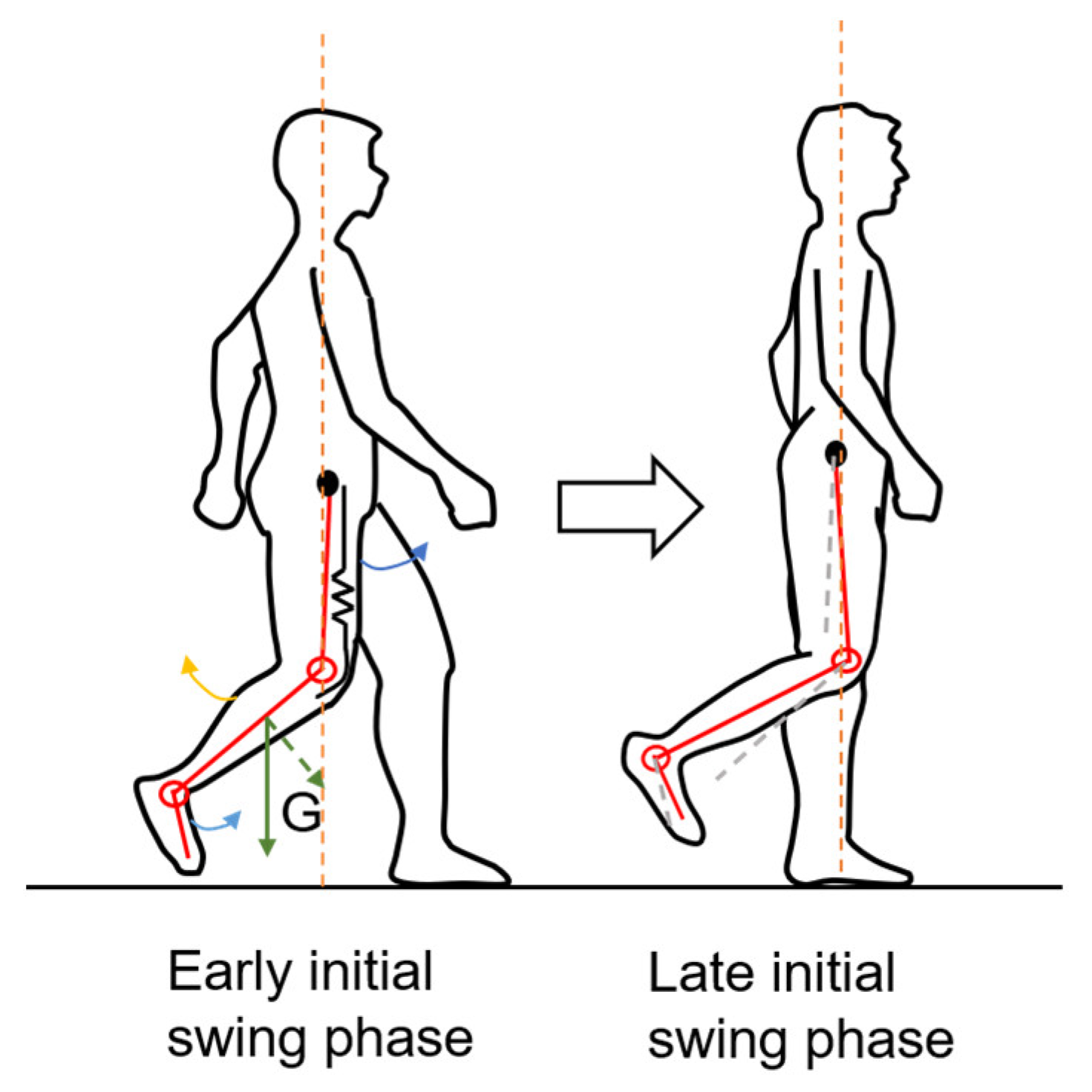
4.1. Some Significant GP Predictors for HGS Estimation
4.2. Some Significant IMS Predictors for HGS Estimation
4.3. Results Regarding Model Test and Designed Frailty Risk Score
4.4. Outlook for this Technology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Symbol | Description | Symbol | Description |
|---|---|---|---|
| %GC | Percentage gait cycle | LR | Loading response |
| AWGS | Asian Working Group on Sarcopenia | LULoA | Lower limit of ULoA |
| Ax | The acceleration signal of x-axis | M1–M3 | Three other patterns of predictor combinations |
| Ay | The acceleration signal of y-axis | MAE | Mean absolute error |
| Az | The acceleration signal of z-axis | MCU | Micro-control unit |
| BA plots | Bland–Altman plots | Mo | The optimal model |
| BMI | Body mass index | MSt | Mid-stance |
| CCA | Canonical correlation analysis | MSw | Mid-swing |
| CHS | Cardiovascular health study | PeC | Pearson’s correlation |
| Ci | The i-th predictor variable | Pfr | Frailty risk score |
| EMBC | Conference of the IEEE Engineering in Medicine and Biology Society | PGS | Designed score of the gait speed performance |
| Ex | The SGA signal of x-axis | PHGS_f | Designed score of the HGS performance of females |
| Ey | The SGA signal of y-axis | PHGS_m | Designed score of the HGS performance of males |
| Ez | The SGA signal of z-axis | PS | Pre-swing |
| FRT | Functional reach test | Qt | Quadricep-activation %GCs |
| GA | Gastrocnemius | r | Pearson’s coefficient of correlation |
| GC | Gait cycle | R2 | Adjusted coefficient of determination |
| GP | Gait parameter | RA | Residual of BA plots for training data |
| GPC | Gait phase cluster | RT | Residual of BA plots for test data |
| GS | Gait speed | RF | Rectus femoris |
| Gx | The angular velocity signal of x-axis | RFT | Random field theory |
| Gy | The angular velocity signal of y-axis | RTC | Real-time clock |
| Gz | The angular velocity signal of z-axis | SGA | Sole-to-ground angle |
| H1–H100 | 100 candidate models | SO | Soleus |
| HGS | Hand grip strength | SPM | Statistical parametric mapping |
| HGSf | HGS of the female subjects | SPM{F} | F statistic of vector field analysis by SPM-CCA |
| HGSm | HGS of the male subjects | SPM{t} | Statistic of post hoc scalar trajectory linear correlation test by SPM-PC |
| HS | Heel strike | TA | Tibialis anterior |
| ICC | Intraclass correlation coefficient | Te | The end of %GCs of GPCs |
| IMS | In-shoe motion sensor | TO | Toe-off |
| IMU | Inertial measurement unit | TSt | Terminal stance |
| IPA | Individual physical attribute | Ts | The start of %GCs of GPCs |
| ISw | Initial swing | TSw | Terminal swing |
| J-CHS | Japanese version of the Cardiovascular Health Study | ULLoA | Upper limit of LLoA |
| KA | The success rate of measurements | ULoA | Upper LoA |
| KAL | Lower limit of KA | UULoA | Upper limit of ULoA |
| KAU | Upper limit of KA | VA | Vastus muscle |
| KS test | Kolmogorov–Smirnov test | W | The waveform of the foot motion signals |
| LASSO | Least absolute shrinkage and selection operator | ZGS | Z-scores of the gait speed |
| LLLoA | Lower limit of LLoA | ZHGS_m | Z-scores of the HGS performance of males |
| LLoA | Lower LoA | ZHGS_f | Z-scores of the HGS performance of females |
| LoA | Limit of agreement | β | The set of fitted least-squares regression coefficients |
| LOSO | Leave-one-subject-out | β0 | The residual of the linear regression |
| LOSOCV | Leave-one-subject-out cross-validation | λ | Non-negative regularization parameter input to LASSO |
References
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Lusardi, M. White paper: “walking speed: The sixth vital sign”. J. Geriatr. Phys. Ther. 2009, 32, 2–5. Available online: https://digitalcommons.sacredheart.edu/cgi/viewcontent.cgi?article=1134&context=pthms_fac (accessed on 6 June 2023). [CrossRef]
- Sialino, L.D.; Schaap, L.A.; van Oostrom, S.H.; Picavet, H.S.J.; Twisk, J.W.; Verschuren, W.; Visser, M.; Wijnhoven, H.A. The sex difference in gait speed among older adults: How do sociodemographic, lifestyle, social and health determinants contribute? BMC Geriatr. 2021, 21, 340. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and physical frailty: Two sides of the same coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef]
- Wong, L.; Duque, G.; McMahon, L.P. Sarcopenia and Frailty: Challenges in Mainstream Nephrology Practice. Kidney Int. Rep. 2021, 6, 2554–2564. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Marcell, T.J. Sarcopenia: Causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M911–M916. [Google Scholar] [CrossRef]
- Hogan, D.B. Models, definitions, and criteria for frailty. In Conn’s Handbook of Models for Human Aging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–44. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Scott, D.; Hayes, A.; Sanders, K.M.; Aitken, D.; Ebeling, P.R.; Jones, G. Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos. Int. 2014, 25, 187–193. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyere, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Rejeb, A.; Rejeb, K.; Treiblmaier, H.; Appolloni, A.; Alghamdi, S.; Alhasawi, Y.; Iranmanesh, M. The Internet of Things (IoT) in healthcare: Taking stock and moving forward. Internet Things 2023, 22, 100721. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, L.; Kim, J.Y.; Li, Y.; Huang, W. Applying Probabilistic Model Checking to the Behavior Guidance and Abnormality Detection for A-MCI Patients under Wireless Sensor Network. ACM Trans. Sens. Netw. 2023, 19, 48. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Liu, J.; Zou, Y. Cost research of Internet of Things service architecture for random mobile users based on edge computing. Int. J. Web Inf. Syst. 2022, 18, 217–235. [Google Scholar] [CrossRef]
- Pradeep Kumar, D.; Toosizadeh, N.; Mohler, J.; Ehsani, H.; Mannier, C.; Laksari, K. Sensor-based characterization of daily walking: A new paradigm in pre-frailty/frailty assessment. BMC Geriatr. 2020, 20, 164. [Google Scholar] [CrossRef]
- Rast, F.M.; Labruyère, R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. Neuroeng. Rehabil. 2020, 17, 148. [Google Scholar] [CrossRef]
- Vavasour, G.; Giggins, O.M.; Doyle, J.; Kelly, D. How wearable sensors have been utilised to evaluate frailty in older adults: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Becerra, V.; Perales, F.J.; Roca, M.; Buades, J.M.; Miró-Julià, M. A Wireless Hand Grip Device for Motion and Force Analysis. Appl. Sci. 2021, 11, 6036. [Google Scholar] [CrossRef]
- Chen, X.; Gong, L.; Wei, L.; Yeh, S.-C.; Da Xu, L.; Zheng, L.; Zou, Z. A wearable hand rehabilitation system with soft gloves. IEEE Trans. Industr. Inform. 2020, 17, 943–952. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, L.; Yang, J.; Wang, S. A Grip Strength Estimation Method Using a Novel Flexible Sensor under Different Wrist Angles. Sensors 2022, 22, 2002. [Google Scholar] [CrossRef]
- Eskofier, B.M.; Lee, S.I.; Baron, M.; Simon, A.; Martindale, C.F.; Gaßner, H.; Klucken, J. An overview of smart shoes in the internet of health things: Gait and mobility assessment in health promotion and disease monitoring. Appl. Sci. 2017, 7, 986. [Google Scholar] [CrossRef]
- Gokalgandhi, D.; Kamdar, L.; Shah, N.; Mehendale, N. A Review of Smart Technologies Embedded in Shoes. J. Med. Syst. 2020, 44, 150. [Google Scholar] [CrossRef]
- Huang, C.; Fukushi, K.; Wang, Z.; Kajitani, H.; Nihey, F.; Pokka, H.; Narasaki, H.; Nakano, H.; Nakahara, K. Foot-Healthcare Application Using Inertial Sensor: Estimating First Metatarsophalangeal Angle from Foot Motion During Walking. IEEE Sens. J. 2021, 22, 2835–2844. [Google Scholar] [CrossRef]
- Fukushi, K.; Huang, C.; Wang, Z.; Kajitani, H.; Nihey, F.; Nakahara, K. On-Line Algorithms of Stride-Parameter Estimation for in-Shoe Motion-Sensor System. IEEE Sens. J. 2022, 22, 9636–9648. [Google Scholar] [CrossRef]
- Nguyen, L.V.; La, H.M.; Sanchez, J.; Vu, T. A smart shoe for building a real-time 3D map. Autom. Constr. 2016, 71, 2–12. [Google Scholar] [CrossRef]
- Bi, Z.; Yu, L.; Gao, H.; Zhou, P.; Yao, H. Improved VGG model-based efficient traffic sign recognition for safe driving in 5G scenarios. Int. J. Mach. Learn. Cybern. 2021, 12, 3069–3080. [Google Scholar] [CrossRef]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef]
- Lee, L.; Patel, T.; Costa, A.; Bryce, E.; Hillier, L.M.; Slonim, K.; Hunter, S.W.; Heckman, G.; Molnar, F. Screening for frailty in primary care: Accuracy of gait speed and hand-grip strength. Can. Fam. Physician 2017, 63, e51–e57. [Google Scholar]
- Mündermann, A.; Dyrby, C.O.; Hurwitz, D.E.; Sharma, L.; Andriacchi, T.P. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: Reduced walking speed. Arthritis Rheum. 2004, 50, 1172–1178. [Google Scholar] [CrossRef]
- Lemke, M.R.; Wendorff, T.; Mieth, B.; Buhl, K.; Linnemann, M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J. Psychiatr. Res. 2000, 34, 277–283. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Magasi, S.R.; Bubela, D.J.; Wang, Y.C.; Gershon, R.C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012, 46, 555–558. [Google Scholar] [CrossRef]
- Samuel, D.; Rowe, P. An investigation of the association between grip strength and hip and knee joint moments in older adults. Arch. Gerontol. Geriatr. 2012, 54, 357–360. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Park, T.; Casella, G. The bayesian lasso. J. Am. Stat. Assoc. 2008, 103, 681–686. [Google Scholar] [CrossRef]
- Scardapane, S.; Comminiello, D.; Hussain, A.; Uncini, A. Group sparse regularization for deep neural networks. Neurocomputing 2017, 241, 81–89. [Google Scholar] [CrossRef]
- Yoosefdoost, I.; Basirifard, M.; Álvarez-García, J. Reservoir Operation Management with New Multi-Objective (MOEPO) and Metaheuristic (EPO) Algorithms. Water 2022, 14, 2329. [Google Scholar] [CrossRef]
- Roberts, S.; Nowak, G. Stabilizing the lasso against cross-validation variability. Comput. Stat. Data Anal. 2014, 70, 198–211. [Google Scholar] [CrossRef]
- Wu, C.-F.J. Jackknife, bootstrap and other resampling methods in regression analysis. Ann. Stat. 1986, 14, 1261–1295. [Google Scholar] [CrossRef]
- Huang, C.; Nihey, F.; Fukushi, K.; Kajitani, H.; Nozaki, Y.; Ihara, K.; Nakahara, K. Feature selection, construction and validation of a lightweight model for foot function assessment during gait with in-shoe motion sensors. IEEE Sens. J. 2023, 23, 8839–8855. [Google Scholar] [CrossRef]
- Huang, C.; Nihey, F.; Fukushi, K.; Kajitani, H.; Nozaki, Y.; Wang, Z.; Ihara, K.; Nakahara, K. Assessment method of balance ability of older adults using an in-shoe motion sensor. In Proceedings of the 2022 IEEE Biomedical Circuits and Systems Conference (BioCAS), Taipei, Taiwan, 13–15 October 2022; pp. 448–452. [Google Scholar]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef]
- Rostami, M.; Oussalah, M.; Berahmand, K.; Farrahi, V. Community Detection Algorithms in Healthcare Applications: A Systematic Review. IEEE Access 2023, 11, 30247–30272. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hobara, H.; Heldoorn, T.A.; Kouchi, M.; Mochimaru, M. Age-independent and age-dependent sex differences in gait pattern determined by principal component analysis. Gait. Posture 2016, 46, 11–17. [Google Scholar] [CrossRef]
- Huang, C.; Nihey, F.; Fukushi, K.; Kajitani, H.; Nozaki, Y.; Wang, Z.; Nakahara, K. Estimation of Hand Grip Strength Using Foot motion Measured by In-shoe Motion Sensor. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 898–903. [Google Scholar]
- Segal, J.B.; Chang, H.Y.; Du, Y.; Walston, J.D.; Carlson, M.C.; Varadhan, R. Development of a Claims-based Frailty Indicator Anchored to a Well-established Frailty Phenotype. Med. Care 2017, 55, 716–722. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Schwenk, M.; Mohler, J.; Wendel, C.; D’Huyvetter, K.; Fain, M.; Taylor-Piliae, R.; Najafi, B. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: Baseline results of the Arizona frailty cohort study. Gerontology 2015, 61, 258–267. [Google Scholar] [CrossRef]
- Razjouyan, J.; Naik, A.D.; Horstman, M.J.; Kunik, M.E.; Amirmazaheri, M.; Zhou, H.; Sharafkhaneh, A.; Najafi, B. Wearable sensors and the assessment of frailty among vulnerable older adults: An observational cohort study. Sensors 2018, 18, 1336. [Google Scholar] [CrossRef]
- Greene, B.R.; Doheny, E.P.; Kenny, R.A.; Caulfield, B. Classification of frailty and falls history using a combination of sensor-based mobility assessments. Physiol. Meas. 2014, 35, 2053–2066. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D. Global incidence of frailty and prefrailty among community-dwelling older adults: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef]
- Auyeung, T.; Arai, H.; Chen, L.; Woo, J. Normative data of handgrip strength in 26344 older adults-a pooled dataset from eight cohorts in Asia. J. Nutr. Health Aging 2020, 24, 125–126. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kitamura, A.; Seino, S.; Murayama, H.; Amano, H.; Nofuji, Y.; Nishi, M.; Yokoyama, Y.; Shinozaki, T.; Yokota, I. Gait performance trajectories and incident disabling dementia among community-dwelling older Japanese. J. Am. Med. Dir. Assoc. 2017, 18, 192.e13–192.e20. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491. [Google Scholar] [CrossRef]
- Madgwick, S.O.; Harrison, A.J.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE international conference on rehabilitation robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar]
- Huang, C.; Fukushi, K.; Wang, Z.; Kajitani, H.; Nihey, F.; Nakahara, K. Initial Contact and Toe-Off Event Detection Method for In-Shoe Motion Sensor. In Activity and Behavior Computing; Smart Innovation, Systems and Technologies; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–118. [Google Scholar]
- Sangeux, M.; Polak, J. A simple method to choose the most representative stride and detect outliers. Gait. Posture 2015, 41, 726–730. [Google Scholar] [CrossRef]
- Huang, C.; Fukushi, K.; Wang, Z.; Nihey, F.; Kajitani, H.; Nakahara, K. Method for Estimating Temporal Gait Parameters Concerning Bilateral Lower Limbs of Healthy Subjects Using a Single In-Shoe Motion Sensor through a Gait Event Detection Approach. Sensors 2022, 22, 351. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Region-of-interest analyses of one-dimensional biomechanical trajectories: Bridging 0D and 1D theory, augmenting statistical power. PeerJ 2016, 4, e2652. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Šidák, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the Musculoskeletal System: Foundations of Physical Rehabilitation, 2nd ed.; Mosby: St Louis, MO, USA, 2010; pp. 627–699. [Google Scholar]
- Nene, A.; Byrne, C.; Hermens, H. Is rectus femoris really a part of quadriceps?: Assessment of rectus femoris function during gait in able-bodied adults. Gait. Posture 2004, 20, 1–13. [Google Scholar] [CrossRef]
- Di Nardo, F.; Mengarelli, A.; Maranesi, E.; Burattini, L.; Fioretti, S. Gender differences in the myoelectric activity of lower limb muscles in young healthy subjects during walking. Biomed. Signal Process. Control 2015, 19, 14–22. [Google Scholar] [CrossRef]
- Bailey, C.A.; Corona, F.; Pilloni, G.; Porta, M.; Fastame, M.C.; Hitchcott, P.K.; Penna, M.P.; Pau, M.; Côté, J.N. Sex-dependent and sex-independent muscle activation patterns in adult gait as a function of age. Exp. Gerontol. 2018, 110, 1–8. [Google Scholar] [CrossRef]
- Rowe, E.; Beauchamp, M.K.; Astephen Wilson, J. Age and sex differences in normative gait patterns. Gait Posture 2021, 88, 109–115. [Google Scholar] [CrossRef]
- Lam, N.W.; Goh, H.T.; Kamaruzzaman, S.B.; Chin, A.-V.; Poi, P.J.H.; Tan, M.P. Normative data for hand grip strength and key pinch strength, stratified by age and gender for a multiethnic Asian population. Singap. Med. J. 2016, 57, 578. [Google Scholar] [CrossRef]
- Luo, H.; Lee, P.-A.; Clay, I.; Jaggi, M.; De Luca, V. Assessment of fatigue using wearable sensors: A pilot study. Digit. Biomark. 2020, 4, 59–72. [Google Scholar] [CrossRef]
- Sehle, A.; Mündermann, A.; Starrost, K.; Sailer, S.; Becher, I.; Dettmers, C.; Vieten, M. Objective assessment of motor fatigue in multiple sclerosis using kinematic gait analysis: A pilot study. J. Neuroeng. Rehabil. 2011, 8, 59. [Google Scholar] [CrossRef]
- Li, J.-S.; Tsai, T.-Y.; Clancy, M.M.; Li, G.; Lewis, C.L.; Felson, D.T. Weight loss changed gait kinematics in individuals with obesity and knee pain. Gait. Posture 2019, 68, 461–465. [Google Scholar] [CrossRef]




| Overall Mean ± SD (Min–Max) | Male Mean ± SD (Min–Max) | Female Mean ± SD (Min–Max) | ||
|---|---|---|---|---|
| Group I | Number | 62 | 27 | 35 |
| Data size | 248 | 108 | 140 | |
| Age (years) | 70.6 ± 6.8 (60.0–84.0) | 70.3 ± 7.7 (60.0–84.0) | 70.9 ± 5.9 (60.0–82.0) | |
| Height (cm) | 160.0 ± 8.2 (140.0–176.0) | 166.7 ± 4.2 (160.0–176.0) | 154.9 ± 6.6 (140.0–171.0) | |
| Weight (kg) | 59.9 ± 11.0 (37.0–89.0) | 66.8 ± 8.8 (53.0–89.0) | 54.7 ± 9.4 (37.0–80.0) | |
| BMI | 23.3 ± 3.1 (15.2–32.9) | 24.0 ± 2.6 (19.2–29.4) | 22.8 ± 3.4 (15.2–32.9) | |
| HGS (kg) | 27.4 ± 8.1 (14.0–45.2) | 33.7 ± 6.1 (24.3–45.2) | 22.6 ± 5.8 (14.0–38.0) | |
| Gait speed (m/s) | 1.37 ± 0.18 (0.91–1.83) | 1.35 ± 0.20 (0.99–1.83) | 1.39 ± 0.17 (0.91–1.72) | |
| Group II+III | Number | 45 | 6 | 39 |
| Data size | 180 | 24 | 156 | |
| Age (years) | 71.1 ± 7.1 (50.0–86.0) | 77.7 ± 5.4 (70.0–86.0) | 70.1 ± 6.8 (50.0–83.0) | |
| Height (cm) | 155.3 ± 6.1 (146.0–172.0) | 166.5 ± 4.8 (160.0–172.0) | 153.6 ± 4.0 (146.0–164.5) | |
| Weight (kg) | 53.2 ± 10.1 (34.0–76.0) | 63.1 ± 12.2 (41.0–76.0) | 51.7 ± 8.8 (34.0–73.0) | |
| BMI | 22.0 ± 3.6 (14.5–31.1) | 22.7 ± 3.8 (15.2–26.0) | 21.9 ± 3.6 (14.5–31.1) | |
| HGS (kg) | 22.3 ± 4.5 (13.7–35.4) | 26.9 ± 5.6 (17.6–35.4) | 21.6 ± 3.9 (13.7–31.6) | |
| Gait speed (m/s) | 1.33 ± 0.19 (0.75–1.64) | 1.18 ± 0.14 (1.02–1.34) | 1.35 ± 0.19 (0.75–1.64) | |
| Group III | Number | 25 | 6 | 19 |
| Data size | 100 | 24 | 76 | |
| Age (years) | 75.1 ± 5.8 (65.0–86.0) | 77.7 ± 5.4 (70.0–86.0) | 74.2 ± 5.8 (65.0–83.0) | |
| Height (cm) | 156.4 ± 6.6 (146.0–172.0) | 166.5 ± 4.8 (160.0–172.0) | 153.4 ± 3.0 (146.0–160.0) | |
| Weight (kg) | 51.4 ± 10.9 (34.0–76.0) | 63.1 ± 12.2 (41.0–76.0) | 47.9 ± 7.5 (34.0–62.0) | |
| BMI | 20.9 ± 3.6 (14.5–27.7) | 22.7 ± 3.8 (15.2–26.0) | 20.4 ± 3.4 (14.5–27.7) | |
| HGS (kg) | 21.9 ± 4.9 (13.7–35.4) | 26.9 ± 5.6 (17.6–35.4) | 20.1 ± 3.4 (13.7–26.5) | |
| Gait speed (m/s) | 1.33 ± 0.18 (1.02–1.64) | 1.18 ± 0.14 (1.02–1.34) | 1.39 ± 0.16 (1.09–1.64) | |
| J-CHS score: 0 (Robust) | 10 | 1 | 9 | |
| J-CHS score: 1–2 (Pre-frail) | 15 | 5 | 10 | |
| J-CHS score: >2 (Frail) | 0 | 0 | 0 | |
| Average expert-rated score | 39.3 ± 17.1 (12.6–82.2) | 46.6 ± 23.5 (23.9–82.2) | 37.0 ± 14.6 (12.6–61.6) |
| No. | Description | Unit |
|---|---|---|
| GP01 | Stride length | m |
| GP02 | One-stride gait velocity | m/s |
| GP03 | Maximum Ex in dorsiflexion direction | deg |
| GP04 | Maximum Ex in plantarflexion direction | deg |
| GP05 | Maximum circumduction | m |
| GP06 | Maximum foot height | m |
| GP07 | Toe in/out angle | deg |
| GP08 | Ey at HS | deg |
| GP09 | Ey at TO | deg |
| GP10 | Cadence | step/min |
| GP11 | Stance phase time | s |
| GP12 | Swing phase time | s |
| GP13 | Double support time 1 (loading response) | s |
| GP14 | Double support time 2 (pre-swing) | s |
| GP15 | Maximum Gx in plantarflexion direction during swing phase | deg/s |
| GP16 | Maximum Gx in dorsiflexion direction during swing phase | deg/s |
| GP17 | Maximum instantaneous velocity in one stride | m/s |
| GP18 | Maximum Az in superior direction during swing phase | 9.8 m/s2 |
| GP19 | Duration of HS to foot flat | s |
| GP20 | Duration of foot flat | s |
| No. | Detail | Mean (SD) | r | Coef. | pm |
|---|---|---|---|---|---|
| Int. | Interception | 37.9 | 0.050 | ||
| Cm1 | Age | 70.3 (7.7) | −0.599 | −0.236 | 0.000 |
| Cm2 | Height | 166.7 (4.2) | 0.428 | 0.185 | 0.055 |
| Cm3 | Weight | 66.8 (8.8) | 0.209 | 0.191 | 0.000 |
| Cm4 | GP03 | 31.64 (4.83) | 0.338 | −0.525 | 0.000 |
| Cm5 | GP05 | 1.88 × 10−2 (0.75 × 10−2) | 0.204 | 132 | 0.006 |
| Cm6 | GP08 | −5.21 (3.83) | −0.005 | 0.262 | 0.009 |
| Cm7 | GP09 | 3.64 (3.88) | −0.049 | 0.246 | 0.013 |
| Cm8 | GP10 | 112.28 (9.20) | 0.052 | −0.222 | 0.000 |
| Cm9 | GP16 | −97.05 (5.46) | 0.303 | 0.314 | 0.000 |
| Cm10 | GP18 | 6.35 × 10−1 (0.61 × 10−1) | −0.190 | 15.9 | 0.017 |
| Cm11 | GP19 | 9.41 × 10−2 (2.13 × 10−2) | 0.065 | 116 | 0.000 |
| Cm12 | Ax, 97 to 98 | −2.67 × 10−1 (1.41 × 10−1) | 0.462 | 11.2 | 0.001 |
| Cm13 | Ay, 59 | −5.76 × 10−1 (1.04 × 10−1) | −0.487 | −26.0 | 0.000 |
| Cm14 | Az, 61 to 63 | −3.43 × 10−1 (0.84 × 10−1) | −0.389 | −8.11 | 0.054 |
| Cm15 | Gy, 15 to 16 | −5.26 × 10−1 (8.01 × 10−1) | −0.387 | −1.58 | 0.000 |
| Cm16 | Gz, 12 to 16 | 4.35 × 10−1 (3.75 × 10−1) | 0.582 | 4.98 | 0.000 |
| No. | Detail | Mean (SD) | r | Coef. | pm |
|---|---|---|---|---|---|
| Int. | Interception | −17.3 | 0.178 | ||
| Cf1 | Age | 70.9 (5.9) | −0.517 | −0.349 | 0.000 |
| Cf2 | Height | 154.9 (6.6) | 0.682 | 0.374 | 0.000 |
| Cf3 | GP16 | −102.37 (7.67) | 0.199 | −0.095 | 0.025 |
| Cf4 | Ax, 3 | −1.31 × 10−1 (0.76 × 10−1) | 0.419 | 28.9 | 0.000 |
| Cf5 | Ax, 13 to 14 | −2.43 × 10−3 (2.43 × 10−3) | −0.529 | −819 | 0.000 |
| Cf6 | Ax, 97 | −2.25 × 10−1 (1.30 × 10−1) | −0.362 | −7.03 | 0.002 |
| Cf7 | Ay, 69 | −2.81 × 10−1 (0.46 × 10−2) | 0.374 | 22.5 | 0.000 |
| Cf8 | Gz, 2 | 14.44 (9.76) | −0.325 | 0.244 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Nihey, F.; Ihara, K.; Fukushi, K.; Kajitani, H.; Nozaki, Y.; Nakahara, K. Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait. Sensors 2023, 23, 5446. https://doi.org/10.3390/s23125446
Huang C, Nihey F, Ihara K, Fukushi K, Kajitani H, Nozaki Y, Nakahara K. Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait. Sensors. 2023; 23(12):5446. https://doi.org/10.3390/s23125446
Chicago/Turabian StyleHuang, Chenhui, Fumiyuki Nihey, Kazuki Ihara, Kenichiro Fukushi, Hiroshi Kajitani, Yoshitaka Nozaki, and Kentaro Nakahara. 2023. "Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait" Sensors 23, no. 12: 5446. https://doi.org/10.3390/s23125446
APA StyleHuang, C., Nihey, F., Ihara, K., Fukushi, K., Kajitani, H., Nozaki, Y., & Nakahara, K. (2023). Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait. Sensors, 23(12), 5446. https://doi.org/10.3390/s23125446








