Anti-Fouling Strategies of Electrochemical Sensors for Tumor Markers
Abstract
:1. Introduction
2. Different Anti-Fouling Strategies Based on Anti-Fouling Materials
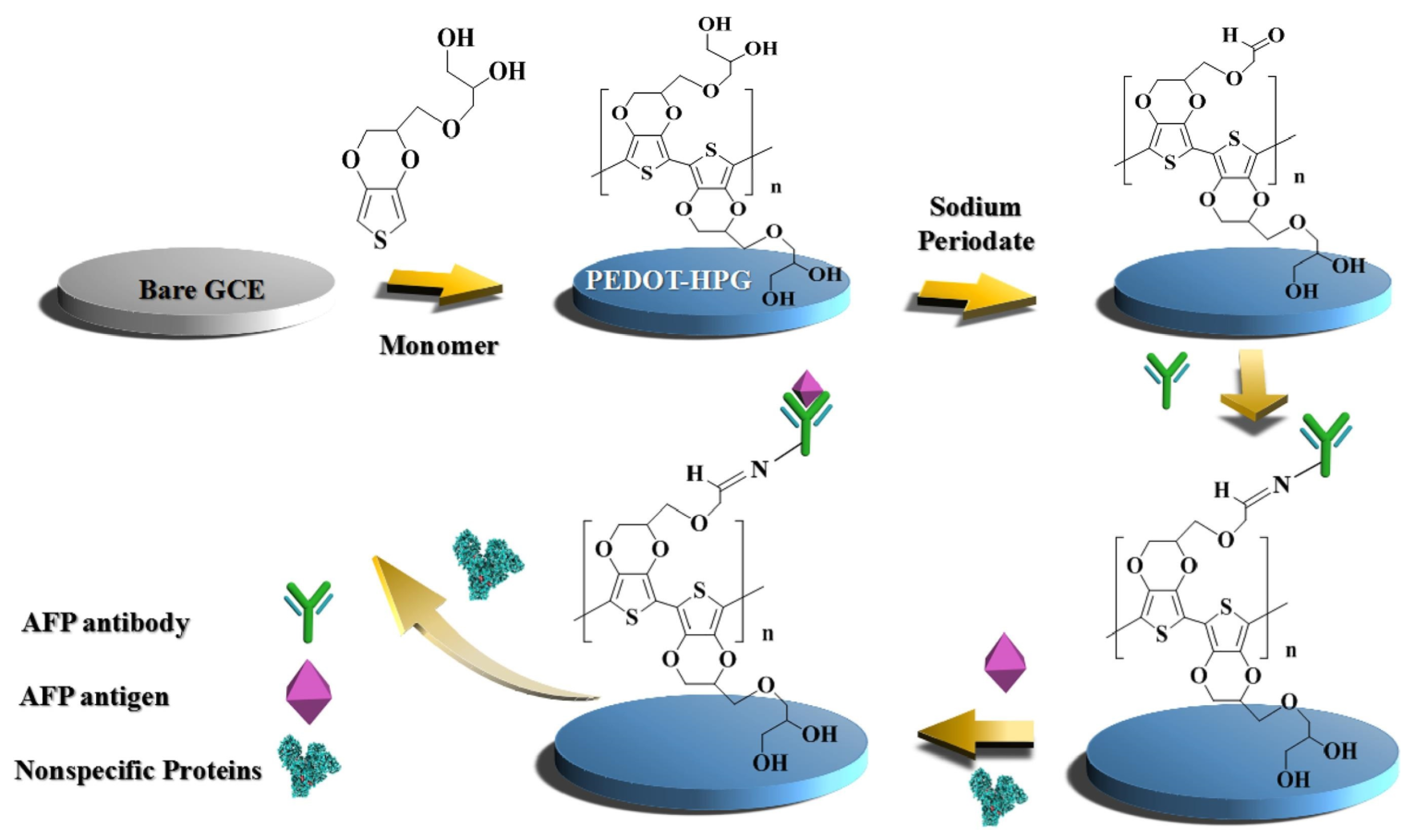
2.1. Polyethylene Glycol (PEG) and Its Derivatives
2.2. Zwitterionic Materials
2.3. Peptides
2.3.1. Straight Peptides
2.3.2. Peptides with Branches
2.4. Hydrogels
2.5. Bovine Serum Albumin (BSA)
2.6. Other Anti-Fouling Materials
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campuzano, S.; Serafín, V.; Gamella, M.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Opportunities, Challenges, and Prospects in Electrochemical Biosensing of Circulating Tumor DNA and Its Specific Features. Sensors 2019, 19, 3762. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Liu, Y.; Li, J.H. Self-phosphorylating deoxyribozyme initiated cascade enzymatic amplification for guanosine-5′-triphosphate detection. Anal. Chem. 2014, 86, 7907–7912. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; DeRosa, M.C. Recent advances in cancer early detection and diagnosis: Role of nucleic acid based aptasensors. TrAC Trends Anal. Chem. 2020, 124, 115806. [Google Scholar]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Kwong, G.A.; Ghosh, S.; Gamboa, L.; Patriotis, C.; Srivastava, S.; Bhatia, S.N. Synthetic biomarkers: A twenty-first century path to early cancer detection. Nat. Rev. Cancer 2021, 21, 655–668. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Sadighbayan, K.; Khosroushahi, A.Y.; Hasanzadeh, M. Recent advances on the DNA-based electrochemical biosensing of cancer biomarkers: Analytical approach. TrAC Trends Anal. Chem. 2019, 119, 115609. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Sen, P.; Adhikari, B.R.; Li, Y.F.; Soleymani, L. Development of nucleic-acid-based electrochemical biosensors for clinical applications. Angew. Chem. Int. Ed. 2022, 61, e202212496. [Google Scholar]
- Arya, S.K.; Estrela, P. Electrochemical ELISA protein biosensing in undiluted serum using a polypyrrole-based platform. Sensors 2020, 20, 2857. [Google Scholar] [CrossRef]
- Pashayan, N.; Pharoah, P. The challenge of early detection in cancer. Science 2020, 368, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Burns-White, K.; Chan, A.T.; Emmons, K.; Freedman, M.; Hunter, D.J.; Kraft, P.; Laden, F.; Mucci, L.; Parmigiani, G.; et al. Precision prevention and early detection of cancer: Fundamental principles. Cancer Discov. 2018, 8, 803–811. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhan, S.N.; Xu, H.Y.; Meng, X.W.; Xiong, Y.H.; Chen, X.Y. Ultrasensitive fluorescence immunoassay for detection of ochratoxin A using catalase-mediated fluorescence quenching of CdTe QDs. Nanoscale 2016, 8, 9390. [Google Scholar] [CrossRef]
- Lai, W.Q.; Wei, Q.H.; Zhuang, J.Y.; Lu, M.H.; Tang, D.P. Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens. Bioelectron. 2016, 80, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, Q.; Liu, P.K.; Zhang, G.; Song, L.; Zou, X.C.; Fu, Y.Z. Novel enhanced lanthanide electrochemiluminescence luminophores: Ce3+-doped TbPO4 facile synthesis and detection for mucin1. Anal. Chem. 2021, 93, 12289–12295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ran, P.Y.; Wu, J.L.; Chen, M.; Fu, Y.Z. An electrochemiluminesence chiral sensor for propranolol enantiomers based on functionalized graphite-like carbon nitride nanosheets. Electroanalysis 2020, 32, 185–190. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.M.; Gu, Y.X.; Yu, L.; Wang, A.J.; Yuan, P.X.; Feng, J.J. Highly Enhanced Electrochemiluminescence Luminophore Generated by Zeolitic Imidazole Framework-8-Linked Porphyrin and Its Application for Thrombin Detection. Anal. Chem. 2020, 92, 3206–3212. [Google Scholar] [CrossRef]
- Hu, L.H.; Shi, T.F.; Chen, J.Y.; Cui, Q.Q.; Yu, H.; Wu, D.; Ma, H.M.; Wei, Q.; Ju, H.X. Dual-quenching electrochemiluminescence resonance energy transfer system from CoPd nanoparticles enhanced porous g-C3N4 to FeMOFs-sCuO for neuron-specific enolase immunosensing. Biosens. Bioelectron. 2023, 226, 115132. [Google Scholar] [CrossRef]
- Tyan, Y.C.; Jong, S.B.; Yang, M.H.; Wu, T.L.; Chung, T.W.; Liao, P.C.; Lin, C.Y.; Huang, Y.F. Utilizing isotope dilution-matrix-assisted laser desorption ionization-time of flight mass spectrometry as a reference procedure for the radioimmunoassay of serum thyroxine. Clin. Chim. Acta 2013, 420, 99–103. [Google Scholar] [CrossRef]
- Bai, Y.; Leng, D.Q.; Feng, T.; Kuang, X.; Fan, D.W.; Ren, X.; Li, Y.Y.; Wei, Q.; Ju, H.X. A split-type photoelectrochemical immunosensor based on a high-performance In2O3/BiVO4 photoelectrode modulated by a ZIF-8 protective layer. Sens. Actuators B Chem. 2023, 382, 133479. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wu, T.T.; Cui, Q.Q.; Qu, Z.F.; Zhang, Y.; Ma, H.M.; Wei, Q. ReS2@Au NPs as signal labels quenching steady photocurrent generated by NiCo2O4/CdIn2S4/In2S3 heterojunction for sensitive detection of CYFRA 21-1. Biosens. Bioelectron. 2023, 222, 114992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Ren, X.; Yang, L.; Wang, W.; Fan, D.W.; Kuang, X.; Sun, X.; Wei, Q.; Ju, H.X. Ru(dcbpy)32+-functionalized γ-cyclodextrin metal-organic frameworks as efficient electrochemiluminescence tags for the detection of CYFRA21-1 in human serum. Sens. Actuators B Chem. 2023, 378, 133152. [Google Scholar] [CrossRef]
- Chen, M.; Mo, F.J.; Meng, H.; Wang, C.; Guo, J.; Fu, Y.Z. Efficient Curing Sacrificial Agent-Induced Dual-Heterojunction Photoelectrochemical System for Highly Sensitive Immunoassay. Anal. Chem. 2021, 93, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, J.; Mo, F.J.; Yu, W.Q.; Fu, Y.G. Highly Sensitive Photoelectrochemical Immunosensor Based on Organic Multielectron Donor Nanocomposite as Signal Probe. Anal. Chem. 2022, 94, 17039–17045. [Google Scholar] [CrossRef]
- Song, P.; Wang, M.L.; Duan, Y.X.; Wang, A.J.; Xue, Y.D.; Mei, L.P.; Feng, J.J. Bifunctional photoelectrochemical aptasensor based on heterostructured Ag3PO4/Ag/TiO2 nanorod array for determination of two tumor markers. Microchim. Acta 2023, 190, 85. [Google Scholar] [CrossRef]
- Tang, Z.X.; Ma, Z.F. Ratiometric ultrasensitive electrochemical immunosensor based on redox substrate and immunoprobe. Sci. Rep. 2016, 6, 35440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, N.N.; Xu, Y.; Feng, J.J.; Yao, T.; Wang, F.; Ma, Z.F.; Han, H.L. Fenton reaction-mediated dual-attenuation of signal for ultrasensitive amperometric immunoassay. Biosens. Bioelectron. 2021, 178, 113009. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Z.F. Dual-reaction triggered sensitivity amplification for ultrasensitive peptide-cleavage based electrochemical detection of matrix metalloproteinase-7. Biosens. Bioelectron. 2018, 108, 46–52. [Google Scholar] [CrossRef]
- Feng, J.J.; Liang, X.Y.; Ma, Z.F. New immunoprobe: Dual-labeling ZIF-8 embellished with multifunctional bovine serum albumin lamella for electrochemical immunoassay of tumor marker. Biosens. Bioelectron. 2021, 175, 112853. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, Y.Z.; Zhang, Y.Y.; Chen, Z.H.; Liu, Y.; Li, Z.L.; Li, J.H. Sensitive Electrochemical Aptamer Biosensor for Dynamic Cell Surface N-Glycan Evaluation Featuring Multivalent Recognition and Signal Amplification on a Dendrimer-Graphene Electrode Interface. Anal. Chem. 2014, 86, 4278–4286. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.D.; Wang, A.J.; Xue, Y.D.; Wu, L.; Feng, J.J. A new label-free electrochemical immunosensor based on dendritic core-shell AuPd@Au nanocrystals for highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 458–463. [Google Scholar] [CrossRef]
- Zhang, J.X.; Lv, C.L.; Tang, C.; Wang, A.J.; Mei, L.P.; Song, P.; Feng, J.J. Sandwich-type ultrasensitive immunosensing of breast cancer biomarker based on core-shell Au@PdAg dog-bone-like nanostructures and Au@PtRh nanorods. Sens. Actuators B Chem. 2023, 382, 133497. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.J.; Yuan, P.X.; Luo, X.L.; Xue, Y.D.; Feng, J.J. Three dimensional sea-urchin-like PdAuCu nanocrystals/ferrocene-grafted-polylysine as an efficient probe to amplify the electrochemical signals for ultrasensitive immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 132, 294–301. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.X.; Li, C.C.; Yang, J.H.; Liu, D.L.; Wang, H.B.; Xu, R.; Zhang, Y.; Wei, Q. An ultrasensitive split-type electrochemical immunosensor based on controlled-release strategy for detection of CA19-9. Biosens. Bioelectron. 2023, 227, 115180. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.J.; Zhou, Q.Y.; Gong, Y.C.; Jia, L.P.; Zhang, W.; Shang, L.; Xue, Q.W.; Wei, Q.; Wang, H.S.; Ma, R.N. A universal distance-independent ratiometric electrochemical biosensing strategy based on competitive host-guest interactions for matrix metalloproteinase-2 detection. Sens. Actuators B Chem. 2023, 378, 133144. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors 2020, 20, 5125. [Google Scholar] [CrossRef]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Anti-fouling Strategies for Electrochemical Biosensing: Mechanisms and Performance toward Point of Care Based Diagnostic Applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors-A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Feng, J.J.; Yao, T.; Ma, Z.F. Recent advances of peroxidase-active nanozymes in electrochemical immunoassay. Sens. Diagn. 2023. [Google Scholar] [CrossRef]
- Feng, T.T.; Ji, W.L.; Zhang, Y.; Wu, F.; Tang, Q.; Wei, H.; Mao, L.Q.; Zhang, M.N. Zwitterionic Polydopamine Engineered Interface for In Vivo Sensing with High Biocompatibility. Angew. Chem. Int. Ed. 2020, 59, 23445–23449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, G.X.; Hein, R.; Liu, N.Z.; Luo, X.L.; Davis, J.J. Anti-fouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, O.R.; Pelletier, J.N.; Masson, J.F. SPR Biosensing in Crude Serum Using Ultralow Fouling Binary Patterned Peptide SAM. Anal. Chem. 2010, 82, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.B.; Fan, Q.; Hu, L.L.; Cui, M.; Wang, X.Y.; Ma, H.M.; Wei, Q. Polydopamine-PEG−Folic Acid Conjugate Film Engineered TiO2 Nanotube Arrays for Photoelectrochemical Sensing of Folate Binding Protein. ACS Appl. Mater. Interfaces 2020, 12, 1877–1884. [Google Scholar] [CrossRef]
- Henrya, O.Y.F.; Sanchez, J.L.A.; O’Sullivan, C.K. Bipodal PEGylated alkanethiol for the enhanced electrochemical detection of genetic markers involved in breast cancer. Biosens. Bioelectron. 2010, 26, 1500–1506. [Google Scholar] [CrossRef]
- Ma, B.C.; Yao, T.; Meng, X.Z.; Xu, Y.; Ma, Z.F.; Han, H.L. Novel Immunoprobe Based on MOF-818 Synergizing with an Anti-fouling Sensing Interface to Improve Immunosensors. ACS Sustain. Chem. Eng. 2022, 10, 12041–12047. [Google Scholar] [CrossRef]
- Cui, M.; Song, Z.L.; Wu, Y.M.; Guo, B.; Fan, X.J.; Luo, X.L. A highly sensitive biosensor for tumor maker alpha fetoprotein based on poly(ethylene glycol) doped conducting polymer PEDOT. Biosens. Bioelectron. 2016, 79, 736–741. [Google Scholar] [CrossRef]
- Hui, N.; Sun, X.T.; Song, Z.L.; Niu, S.Y.; Luo, X.L. Gold nanoparticles and polyethylene glycols functionalized conducting polyaniline nanowires for ultrasensitive and low fouling immunosensing of alpha-fetoprotein. Biosens. Bioelectron. 2016, 86, 143–149. [Google Scholar] [CrossRef]
- Meng, X.Z.; Xu, Y.; Ma, B.C.; Ma, Z.F.; Han, H.L. Anti-fouling materials decorated immunoprobe and electrochemical sensing interface to improve immunoassay. Chem. Eng. J. 2022, 450, 137954. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Han, R.; Liu, N.Z.; Gao, F.X.; Luo, X.L. Electrochemical biosensors for the detection of carcinoembryonic antigen with low fouling and high sensitivity based on copolymerized polydopamine and zwitterionic polymer. Sens. Actuators B Chem. 2020, 319, 128253. [Google Scholar] [CrossRef]
- Leng, C.; Hung, H.; Sieggreen, O.A.; Li, Y.T.; Jiang, S.Y.; Chen, Z. Probing the Surface Hydration of Nonfouling Zwitterionic and Poly(ethylene glycol) Materials with Isotopic Dilution Spectroscopy. J. Phys. Chem. C 2015, 119, 8775–8780. [Google Scholar] [CrossRef]
- Chen, S.F.; Zheng, J.; Li, L.Y.; Jiang, S.Y. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Y.; Zhang, Y.; Ma, B.C.; Ma, Z.F.; Han, H.L. Anti-fouling and sensitive biosensor based on multifunctional peptide and urease@ZIFs for metal matrix protease-7. Sens. Actuators B Chem. 2022, 364, 131844. [Google Scholar] [CrossRef]
- Cui, M.; Ma, Y.H.; Wang, L.; Wang, Y.; Wang, S.; Luo, X.L. Anti-fouling sensors based on peptides for biomarker detection. TrAC Trends Anal. Chem. 2020, 127, 115903. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Jiao, M.X.; Jayachandran, S.; Wu, Y.M.; Fan, X.J.; Luo, X.L. Mixed Self-Assembled Aptamer and Newly Designed Zwitterionic Peptide as Anti-fouling Biosensing Interface for Electrochemical Detection of alpha-Fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Wang, H.P.; Wu, Y.M.; Luo, X.L. A label-free electrochemical DNA biosensor for breast cancer marker BRCA1 based on self-assembled anti-fouling peptide monolayer. Sens. Actuators B Chem. 2017, 244, 742–749. [Google Scholar] [CrossRef]
- Han, R.; Li, Y.; Chen, M.; Li, W.T.; Ding, C.F.; Luo, X.L. Anti-fouling Electrochemical Biosensor Based on the Designed Functional Peptide and the Electrodeposited Conducting Polymer for CTC Analysis in Human Blood. Anal. Chem. 2022, 94, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Che, Z.M.; Guo, C.E.; Liu, Y.Q.; Yang, S.H.; Zhou, M.Y.; Gong, Y.H.; Li, T.D.; Cui, M.; Luo, X.L. A durable anti-fouling protein molecularly imprinted gel interface for human serum albumin detection and antibacterial application. Chem. Eng. J. 2021, 421, 129752. [Google Scholar] [CrossRef]
- Zhao, L.H.; Yin, S.; Ma, Z.F. Ca2+-Triggered pH-Response Sodium Alginate Hydrogel Precipitation for Amplified Sandwich-Type Impedimetric Immunosensor of Tumor Marker. ACS Sens. 2019, 4, 450–455. [Google Scholar] [CrossRef]
- Li, W.X.; Shu, D.; Han, H.L.; Ma, Z.F. An amperometric immunoprobe based on multifunctional nanogel for sensitive detection of tumor marker. Sens. Actuators B Chem. 2018, 273, 1451–1455. [Google Scholar] [CrossRef]
- Cui, M.; Gong, Y.H.; Du, M.G.; Wang, K.; Li, T.D.; Zhu, X.L.; Wang, S.; Luo, X.L. An anti-fouling electrochemical biosensor based on a protein imprinted hydrogel for human immunoglobulin G recognition in complex biological media. Sens. Actuators B Chem. 2021, 337, 129820. [Google Scholar] [CrossRef]
- Ma, L.Z.; Jayachandran, S.; Li, Z.M.; Song, Z.; Wang, W.; Luo, X.L. Anti-fouling and conducting PEDOT derivative grafted with polyglycerol for highly sensitive electrochemical protein detection in complex biological media. J. Electro. Anal. Chem. 2019, 840, 272–278. [Google Scholar] [CrossRef]
- Lian, M.L.; Shi, Y.Q.; Chen, L.X.; Qin, Y.G.; Zhang, W.; Zhao, J.B.; Chen, D. Cell Membrane and V2C MXene-Based Electrochemical Immunosensor with Enhanced Anti-fouling Capability for Detection of CD44. ACS Sens. 2022, 7, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Mokni, M.; Tlili, A.; Attia, G.; Khaoulani, S.; Zerrouki, C.; Omezzine, A.; Othmane, A.; Bouslama, A.; Fourati, N. Novel sensitive immunosensor for the selective detection of Engrailed 2 urinary prostate cancer biomarker. Biosens. Bioelectron. 2022, 217, 114678. [Google Scholar] [CrossRef]
- Feng, J.J.; Yao, T.; Chu, C.S.; Ma, Z.F.; Han, H.L. Proton-responsive annunciator based on i-motif DNA structure modified metal organic frameworks for ameliorative construction of electrochemical immunosensing interface. J. Colloid Interface Sci. 2022, 608, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, Y.; Ma, Z.F. ZIF-8 silencing shell removed by complexation competition reaction for ultrasensitive electrochemical immunoassay. Sens. Actuators B Chem. 2020, 307, 127647. [Google Scholar] [CrossRef]
- Yao, T.; Feng, J.J.; Chu, C.S.; Ma, Z.F.; Han, H.L. Cascade controlled release system based on pH-responsive ZIF-8 capsule and enzyme-responsive hyaluronic acid hydrogel for tumor marker detection using electro-readout-mode. Sens. Actuators B Chem. 2021, 348, 130701. [Google Scholar] [CrossRef]
- Yin, S.; Ma, Z.F. “Smart” sensing interface for the improvement of electrochemical immunosensor based on enzyme-Fenton reaction triggered destruction of Fe3+ cross-linked alginate hydrogel. Sens. Actuators B Chem. 2019, 281, 857–863. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Ma, B.; Ma, Z.F.; Han, H.L. A novel electrochemical sensor based on process-formed laccase-like catalyst to degrade polyhydroquinone for tumor marker. Talanta 2021, 235, 122736. [Google Scholar] [CrossRef]
- Martína, C.M.; Gamellaa, M.; Pedreroa, M.; Montero-Calleb, A.; Barderasb, R.; Campuzanoa, S.; Pingarrón, J.M. Magnetic beads-based electrochemical immunosensing of HIF-1α, a biomarker of tumoral hypoxia. Sens. Actuators B Chem. 2020, 307, 127623. [Google Scholar] [CrossRef]
- Freitasa, M.; Nouwsa, H.P.A.; Keating, E.; Delerue-Matos, C. High-performance electrochemical immunomagnetic assay for breast cancer Analysis. Sens. Actuators B Chem. 2020, 308, 127667. [Google Scholar] [CrossRef]
- Koo, K.M.; Soda, N.; Shiddiky, M.J.A. Magnetic nanomaterial-based electrochemical biosensors for the detection of diverse circulating cancer biomarkers. Curr. Opin. Electrochem. 2021, 25, 100645. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure-Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Haidyrah, A.S.; Chaudhary, A.A.; Imran, M.; Khan, A. Hierarchical Molecularly Imprinted Inverse Opal-Based Platforms for Highly Selective and Sensitive Determination of Histamine. ACS Appl. Polym. Mater. 2022, 4, 2783–2793. [Google Scholar] [CrossRef]
- Imran, M.; Ahmed, S.; Abdullah, A.Z.; Hakami, J.; Chaudhary, A.A.; Rudayni, H.A.; Khan, S.-U.; Khan, A.; Basher, N.S. Nanostructured material-based optical and electrochemical detection of amoxicillin antibiotic. Luminescence 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Han, R.; Wang, G.X.; Xu, Z.Y.; Zhang, L.Y.; Li, Q.; Han, Y.F.; Luo, X.L. Designed anti-fouling peptides planted in conducting polymers through controlled partial doping for electrochemical detection of biomarkers in human serum. Biosens. Bioelectron. 2020, 164, 112317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, T.; Han, H.L.; Ma, Z.F. Universal and High-Speed Zeptomolar Protein Serum Assay with Unprecedented Sensitivity. Anal. Chem. 2022, 94, 16231–16236. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, L.; Li, J.Z.; Li, Z.; Wang, T. Enhanced Interfacial Affinity of the Supercapacitor Electrode with a Hydrogel Electrolyte by a Preadsorbed Polyzwitterion Layer. Langmuir 2022, 38, 8614–8622. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Jiang, S.Y. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Regan, E.M.; Bhalla, N.; Hopkins, N.A.E.; Goodchild, S.A.; Estrela, P. Optimisation and Characterisation of Anti-fouling Ternary SAM Layers for Impedance-Based Aptasensors. Sensors 2015, 15, 25015–25032. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Feng, J.J.; Xiong, Q.C.; Chu, C.S.; Xu, Y.; Ma, Z.F.; Han, H.L. Regenerating electrochemical detection platform by electro-oxidation me-diated host-guest dissociation between 6-mercapto-6-deoxy-β-cyclodextrin and N,N-dimethylaminomethylferrocene. Chem. Eng. J. 2022, 439, 135599. [Google Scholar] [CrossRef]
- Ederth, T.; Lerm, M.; Orihuela, B.; Rittschof, D. Resistance of Zwitterionic Peptide Monolayers to Biofouling. Langmuir 2019, 35, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Z.; Hui, N.; Davis, J.J.; Luo, X.L. Low Fouling Protein Detection in Complex Biological Media Supported by a Designed Mul-tifunctional Peptide. ACS Sens. 2018, 3, 1210–1216. [Google Scholar] [CrossRef]
- Chen, S.F.; Cao, Z.Q.; Jiang, S.Y. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Liu, N.Z.; Wang, W.Q.; Xu, Z.Y.; Wu, Y.M.; Luo, X.L. An electrochemical biosensor for alpha-fetoprotein detection in human serum based on peptides containing isomer D-Amino acids with enhanced stability and anti-fouling property. Biosens. Bioelectron. 2021, 190, 113466. [Google Scholar] [CrossRef]
- Wang, F.; Gong, Y.C.; Xu, Y.; Ma, Z.F.; Han, H.L. Electrochemical sensing interface based on the oriented self-assembly of histidine labeled peptides induced by Ni2+ for protease detection. Biosens. Bioelectron. 2023, 230, 115259. [Google Scholar] [CrossRef]
- Li, Y.X.; Song, Z.; Chen, M.; Xu, Z.Y.; Zhao, S.J.; Xu, Y.Q.; Luo, X.L. Designed multifunctional peptides with two recognizing branches specific for one target to achieve highly sensitive and low fouling electrochemical protein assay in human serum. Anal. Chim. Acta 2022, 1208, 339841. [Google Scholar] [CrossRef]
- Chen, M.; Han, R.; Li, Y.; Luo, X.L. Nonfouling and ratiometric electrochemical detection of prostate specific antigen in whole serum. Anal. Chim. Acta 2022, 1224, 340191. [Google Scholar] [CrossRef]
- Chen, M.; Song, Z.; Han, R.; Li, Y.; Luo, X. Low fouling electrochemical biosensors based on designed Y-shaped peptides with anti-fouling and recognizing branches for the detection of IgG in human serum. Biosens. Bioelectron. 2021, 178, 113016. [Google Scholar] [CrossRef]
- Zhao, W.W.; Shi, Q.H.; Sun, Y. Dual-ligand affinity systems with octapeptide ligands for affinity chromatography of hIgG and monoclonal antibody. J Chromatogr. A 2014, 1369, 64–72. [Google Scholar] [CrossRef]
- Yin, S.; Ma, Z.F. Electrochemical immunoassay for tumor markers based on hydrogels. Expert Rev. Mol. Diagn. 2018, 18, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Han, H.L.; Ma, Z.F. Conductive hydrogel composed of 1,3,5-benzenetricarboxylic acid and Fe3+ used as enhanced electrochemical immunosensing substrate for tumor biomarker. Bioelectrochemistry 2017, 114, 48–53. [Google Scholar] [CrossRef]
- Wang, H.Q.; Ma, Z.F. Ultrasensitive amperometric detection of the tumor biomarker cytokeratin antigen using a hydrogel composite consisting of phytic acid, Pb(II) ions and gold nanoparticles. Microchim. Acta 2017, 184, 1045–1050. [Google Scholar] [CrossRef]
- Zhao, L.H.; Ma, Z.F. Facile synthesis of polyaniline-polythionine redox hydrogel: Conductive, anti-fouling and enzyme-linked material for ultrasensitive label-free amperometric immunosensor toward carcinoma antigen-125. Anal. Chim. Acta 2018, 997, 60–66. [Google Scholar] [CrossRef]
- Ghaani, M.; Büyüktaş, D.; Carullo, D.; Farris, S. Development of a New Electrochemical Sensor Based on Molecularly Imprinted Biopolymer for Determination of 4,4-Methylene Diphenyl Diamine. Sensors 2023, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, R.; Chen, M.; Zhang, L.Y.; Wang, G.X.; Luo, X.L. Bovine Serum Albumin-Cross-Linked Polyaniline Nanowires for Ultralow Fouling and Highly Sensitive Electrochemical Protein Quantification in Human Serum Samples. Anal. Chem. 2021, 93, 4326–4333. [Google Scholar] [CrossRef]
- Zhang, D.S.; Li, W.X.; Ma, Z.F.; Han, H.L. Improved ELISA for tumor marker detection using electro-readout-mode based on label triggered degradation of methylene blue. Biosens. Bioelectron. 2019, 126, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Chatziharalambous, D.; Lygirou, V.; Latosinska, A.; Stravodimos, K.; Vlahou, A.; Jankowski, V.; Zoidakis, J. Analytical Performance of ELISA Assays in Urine: One More Bottleneck towards Biomarker Validation and Clinical Implementation. PLoS ONE 2016, 11, e0149471. [Google Scholar] [CrossRef]
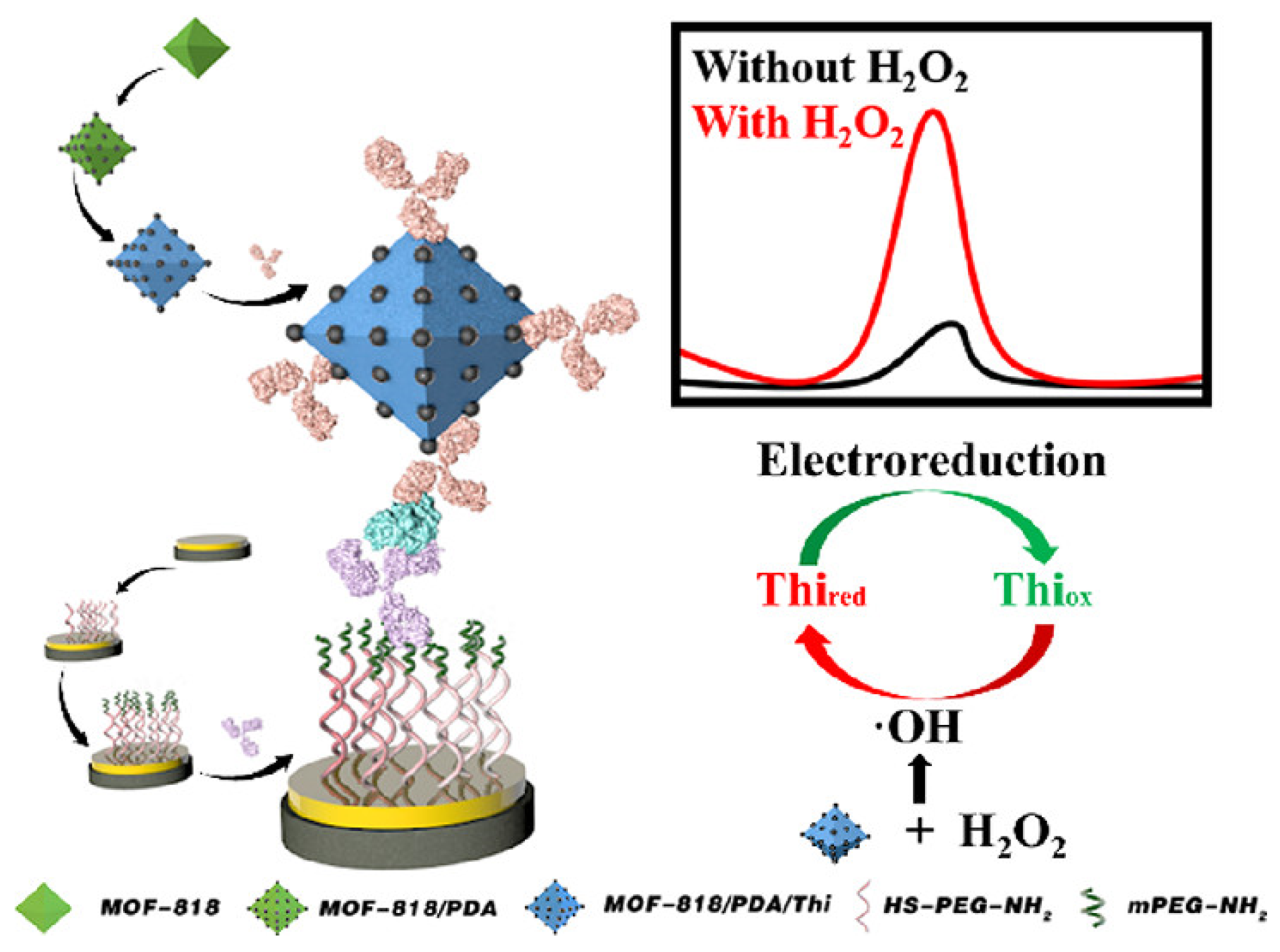

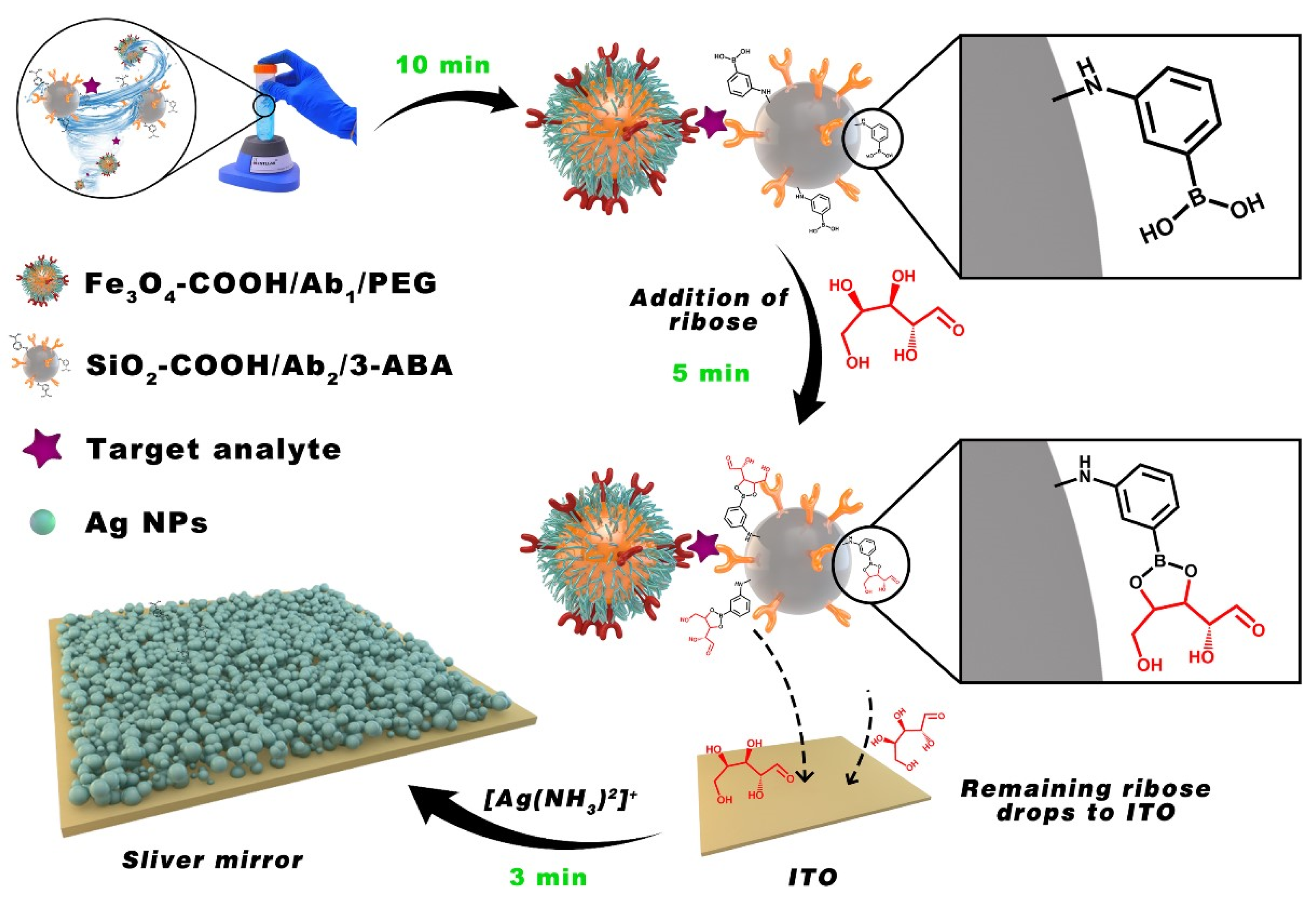
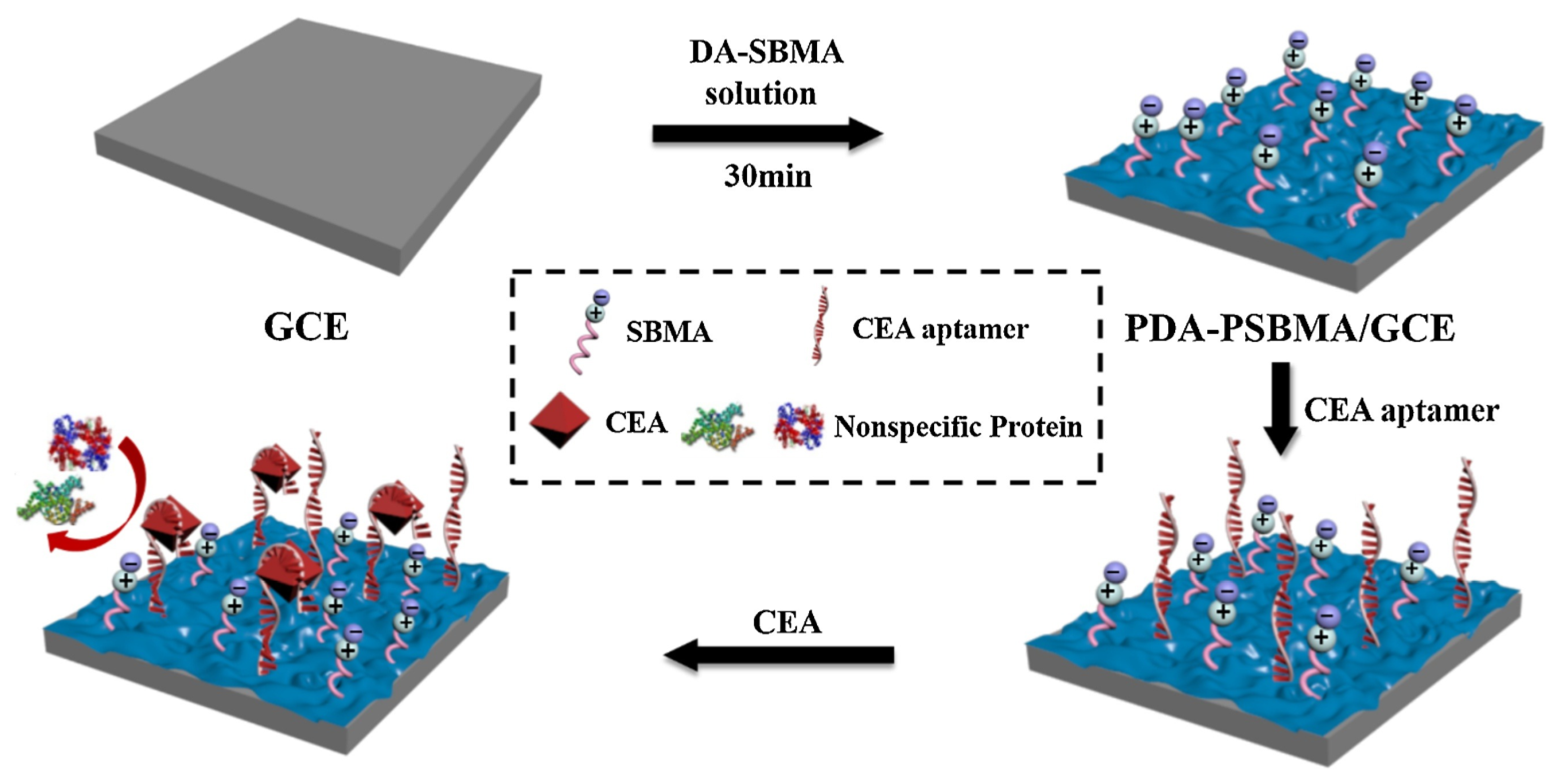
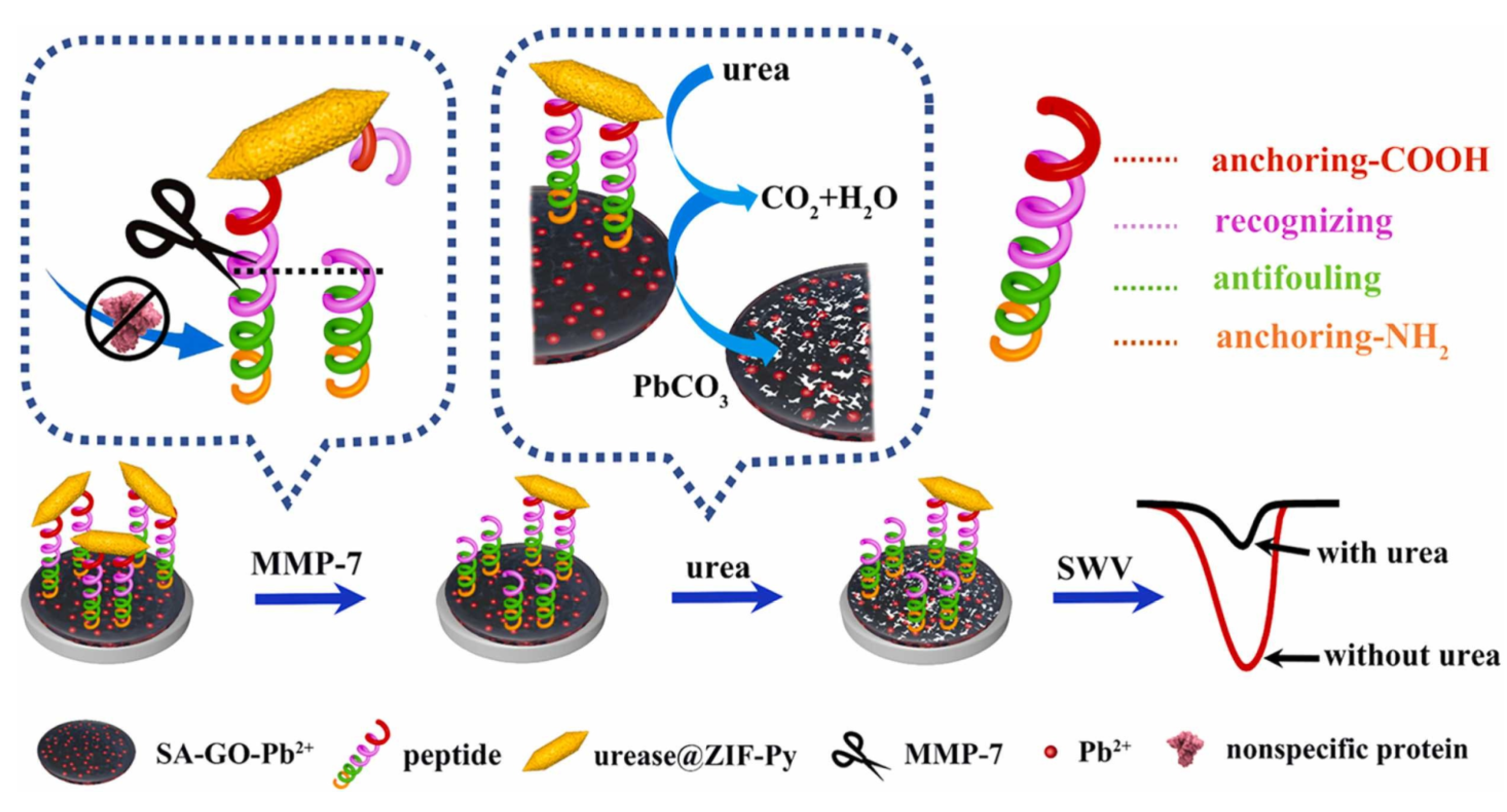
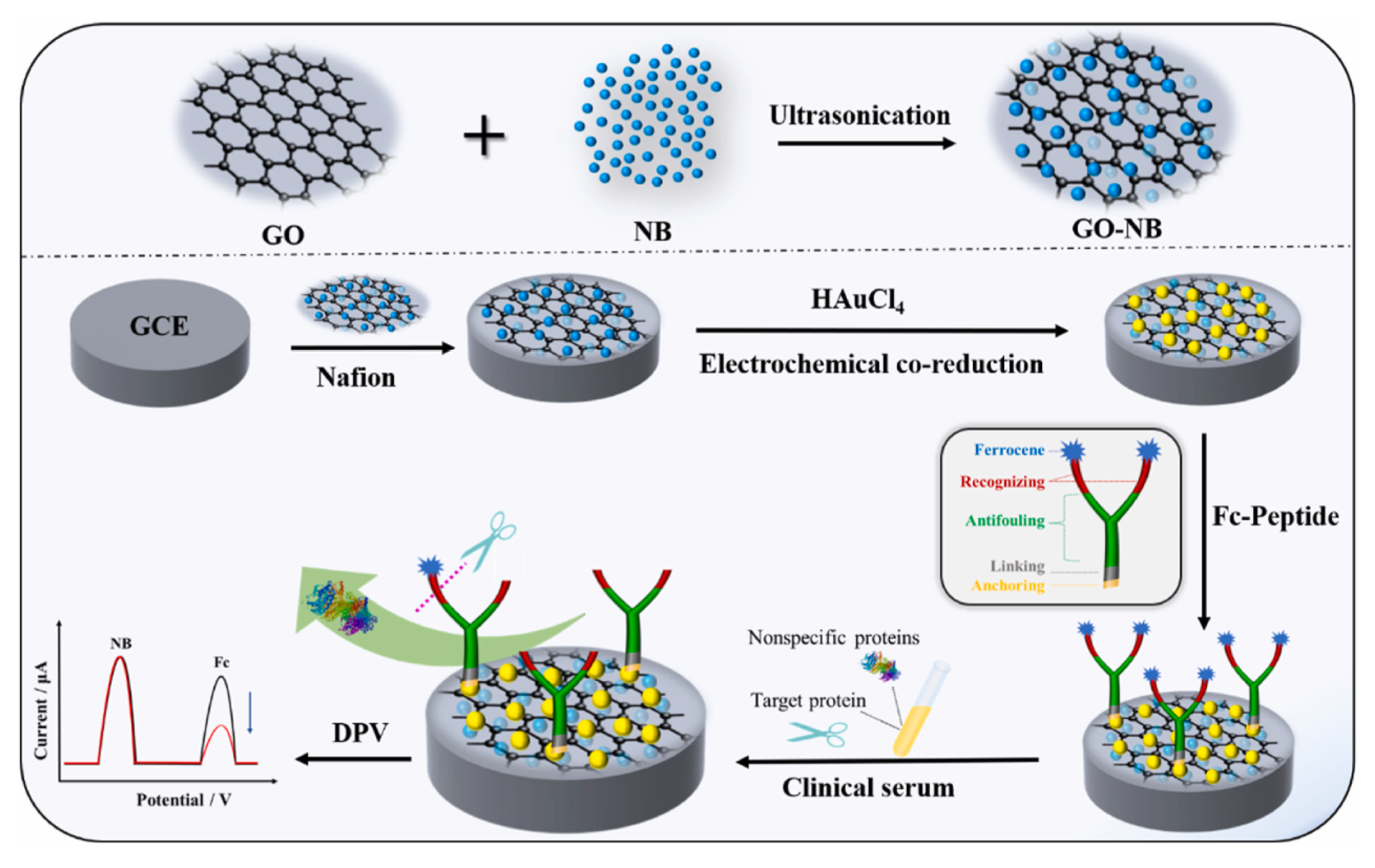
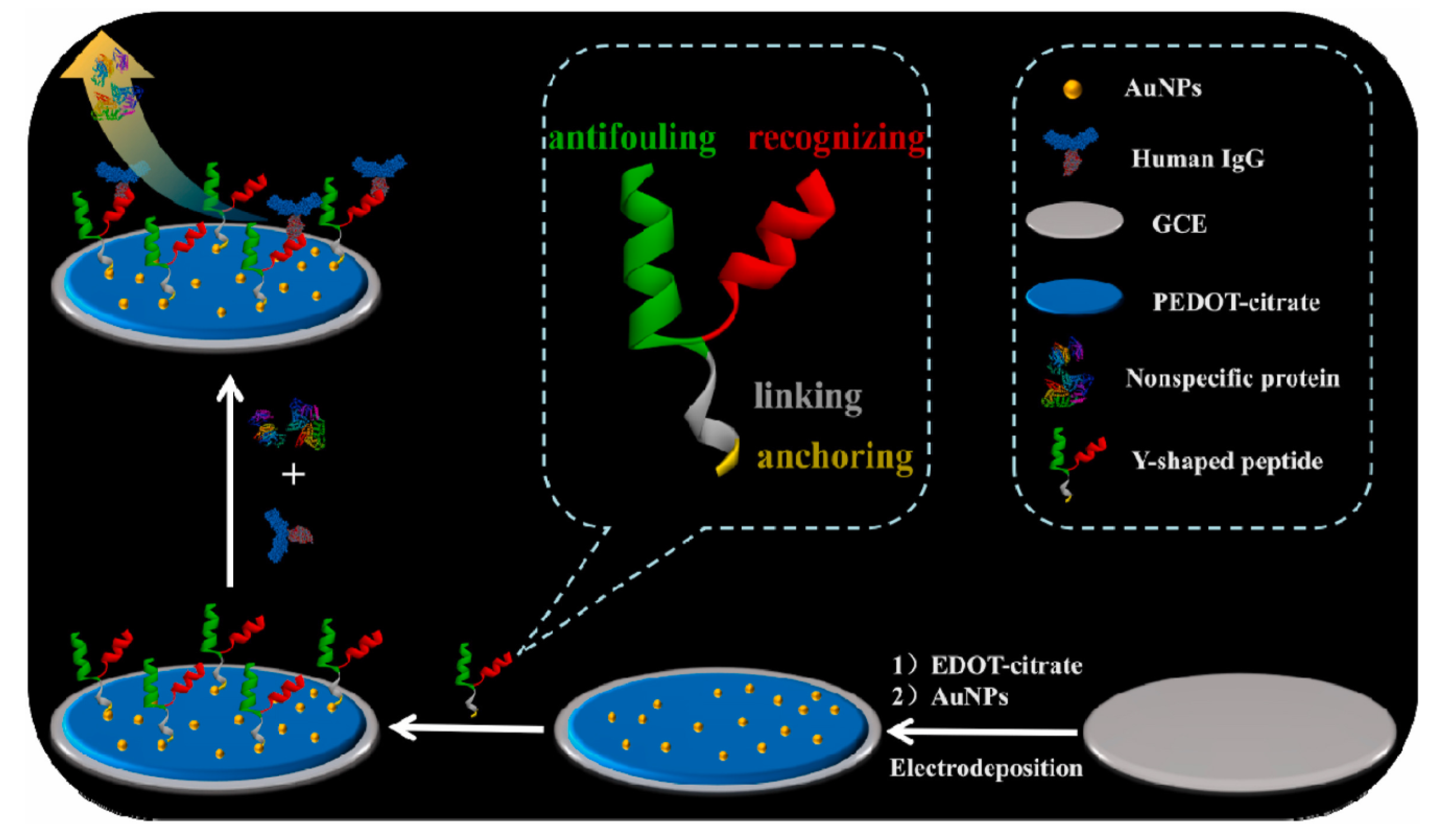
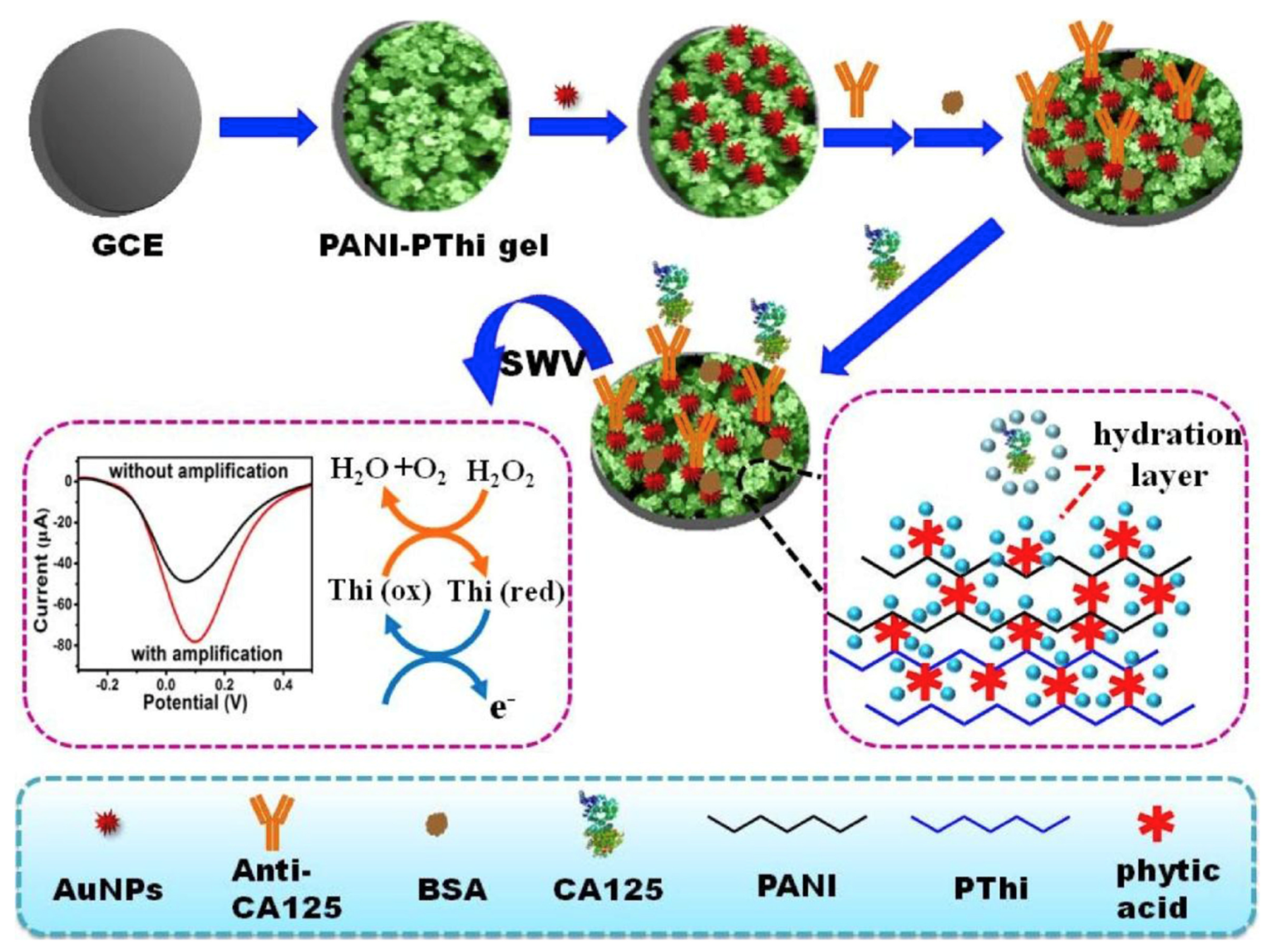

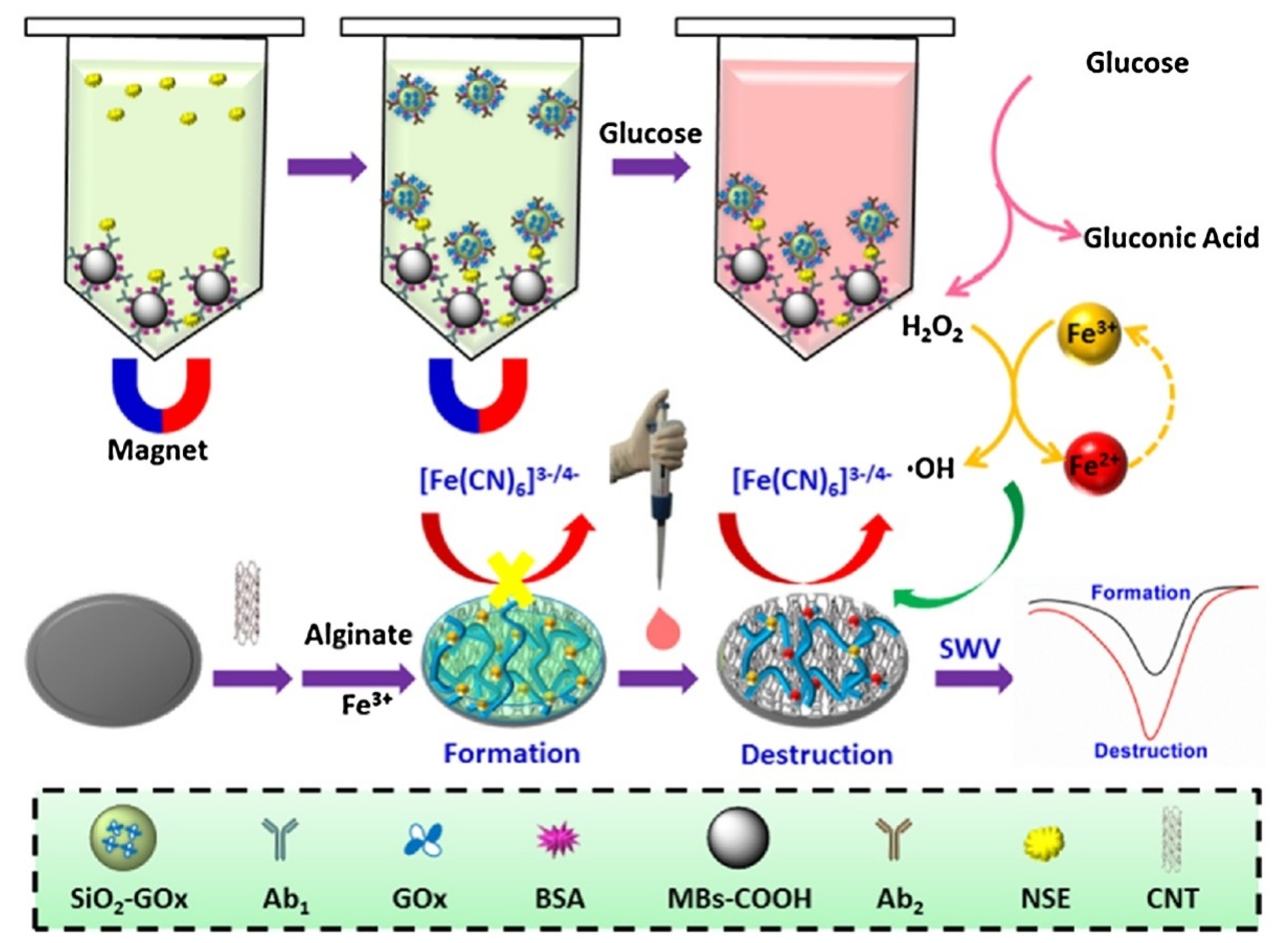

| Anti-Fouling Materials | Strong Hydrophilicity | Electrically Neutral | Limitations |
|---|---|---|---|
| PEG and its derivatives | √ | √ | Prone to oxidate into aldehydes and ethers by oxygen, resulting in polymer long-chain breakage and shedding |
| Zwitterionic materials | √ | √ | Electric field-sensitive or pH-sensitive zwitterions are used under limited conditions |
| Peptides | √ | Suffer from oxidative damage or protease degradation | |
| Hydrogels | √ | Low mechanical strength, high water content, poor freezing resistance | |
| BSA | Cannot ensure full blocking of the active sites and may detach from the surfaces gradually |
| Biomarker | Anti-Fouling Material | Detection Range | Detection Limit | Year | Ref. |
|---|---|---|---|---|---|
| FBP | PEG | 1 × 10−3 to 5 × 102 ng mL−1 | 2 × 10−4 ng mL−1 | 2019 | [44] |
| CA72-4 | PEG | 1 μU mL−1 to 10 U mL−1 | 26.48 nU mL−1 | 2022 | [46] |
| AFP | PEG | 0.001 fg mL−1 to 10 fg mL−1 | 0.0003 fg mL−1 | 2016 | [47] |
| AFP | PEG | 0.01 pg mL−1–1.0 ng mL−1 | 0.007 pg mL−1 | 2016 | [48] |
| CA19-9 | PEG | 0.00001 to 100 U mL−1 | 1.03 μU mL−1 | 2022 | [49] |
| IgG | PEG | 1 ng mL−1 to 100 ag mL−1 | 6.31 ag mL−1 (0.04 zeptomoles mL−1) | 2022 | [77] |
| CEA | Zwitterionic | 0.01–10 pg mL−1 | 3.3 fg mL−1 | 2020 | [50] |
| SCCA | Zwitterionic | 1 pg mL−1 to 1 μg mL−1 | 31.20 fg mL−1 | 2022 | [81] |
| AFP | Zwitterionic Peptide | 10.0 fg mL−1 to 100.0 pg mL−1 | 3.1 fg mL−1 | 2017 | [55] |
| CA 15-3 | Peptides | 0.01–1000 U mL−1 | 3.34 mU mL−1 | 2020 | [76] |
| AFP | Peptides | 0.1 fg mL−1 to 1.0 ng mL−1 | 0.03 fg mL−1 | 2021 | [85] |
| PSA | Peptides | 1 pg mL−1 to 100 ng mL−1 | 11.8 fg mL−1 | 2023 | [86] |
| IgG | Peptides | 0.1 pg mL−1 to 0.1 mg mL−1 | 0.031 pg mL−1 | 2022 | [87] |
| PSA | Peptides | 5.0 pg mL−1 to 100 ng mL−1 | 1.26 pg mL−1 | 2022 | [88] |
| IgG | Peptides | 100 pg mL−1 to 10 μg mL−1 | 32 pg mL−1 | 2021 | [89] |
| MMP-7 | Peptides | 0.1 pg mL−1–100 ng mL−1 | 24.34 fg mL−1 | 2022 | [53] |
| BRCA 1 | Peptides | 1.0 fM to 10.0 pM | 0.3 fM | 2017 | [56] |
| MCF-7 cancer cells | Peptides | 50–106 cells mL−1 | 17 cells mL−1 | 2022 | [57] |
| HSA | Hydrogels | 10−6–5 × 10−2 ng mL−1 | 0.03 × 10−6 ng mL−1 | 2021 | [58] |
| PSA | Hydrogels | 1.0 fg mL−1 to 100 ng mL−1 | 0.09 fg mL−1 | 2019 | [59] |
| NSE | Hydrogels | 0.01 to 1000 ng mL−1 | 4.6 pg mL−1 | 2018 | [60] |
| IgG | Hydrogels | 0.5–200.0 ng mL−1 | 0.03 ng mL−1 | 2021 | [61] |
| NSE | Hydrogels | 1 pg mL−1 to 200 ng mL−1 | 0.26 pg mL−1 | 2017 | [92] |
| CYFRA21-1 | Hydrogels | 50 fg mL−1 to 100 ng mL−1 | 38 fg mL−1 | 2017 | [93] |
| CA125 | Hydrogels | 0.0001 U mL−1 to 1 kU mL−1 | 0.00125 U mL−1 | 2018 | [94] |
| SCCA | BSA | 1 mg mL−1 to 1 pg mL−1 | 1.504 fg mL−1 | 2022 | [65] |
| CYFRA21-1 | BSA | 10 fg mL−1 to 1 μg mL−1 | 3.175 fg mL−1 | 2020 | [66] |
| PSA | BSA | 1.0 × 10−3–1.0 × 102 ng mL−1 | 37.27 fg mL−1 | 2021 | [67] |
| NSE | BSA | 1 pg mL−1 to 100 ng mL−1 | 0.447 pg mL−1 | 2019 | [68] |
| HE4 | BSA | 1 pg mL−1 to 100 ng mL−1 | 0.302 pg mL−1 | 2021 | [69] |
| HIF-1α | BSA | 0.25–10.0 ng mL−1 | 76 pg mL−1 | 2020 | [70] |
| HER 2-ECD | BSA | 5.0 and 50 ng mL−1 50 and 100 ng mL−1 | 2.8 ng mL−1 3 cells mL−1 | 2020 | [71] |
| CA 125 | BSA | 0.1 mU mL−1 to 500 U mL−1 | 0.048 mU mL−1 | 2019 | [97] |
| AFP | Polyglycerol | 0.10 pg mL−1–1.0 ng mL−1 | 0.035 pg mL−1 | 2019 | [62] |
| CD44 CD44 Cancer Cell | Cell Membrane | 0.5 ng mL−1 to 500 ng mL−1 103 to 106 cells mL−1 | 1.4 pg mL−1 140 cells mL−1 | 2022 | [63] |
| EN 2 | Histamine | 10−5 ng mL−1 to 1 μg mL−1 | 10−5 ng mL−1 | 2022 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Han, H.; Ma, Z. Anti-Fouling Strategies of Electrochemical Sensors for Tumor Markers. Sensors 2023, 23, 5202. https://doi.org/10.3390/s23115202
Song G, Han H, Ma Z. Anti-Fouling Strategies of Electrochemical Sensors for Tumor Markers. Sensors. 2023; 23(11):5202. https://doi.org/10.3390/s23115202
Chicago/Turabian StyleSong, Ge, Hongliang Han, and Zhanfang Ma. 2023. "Anti-Fouling Strategies of Electrochemical Sensors for Tumor Markers" Sensors 23, no. 11: 5202. https://doi.org/10.3390/s23115202
APA StyleSong, G., Han, H., & Ma, Z. (2023). Anti-Fouling Strategies of Electrochemical Sensors for Tumor Markers. Sensors, 23(11), 5202. https://doi.org/10.3390/s23115202






