Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS
Abstract
1. Introduction
2. Methods
2.1. Experimental Methods
2.1.1. Material Synthesis
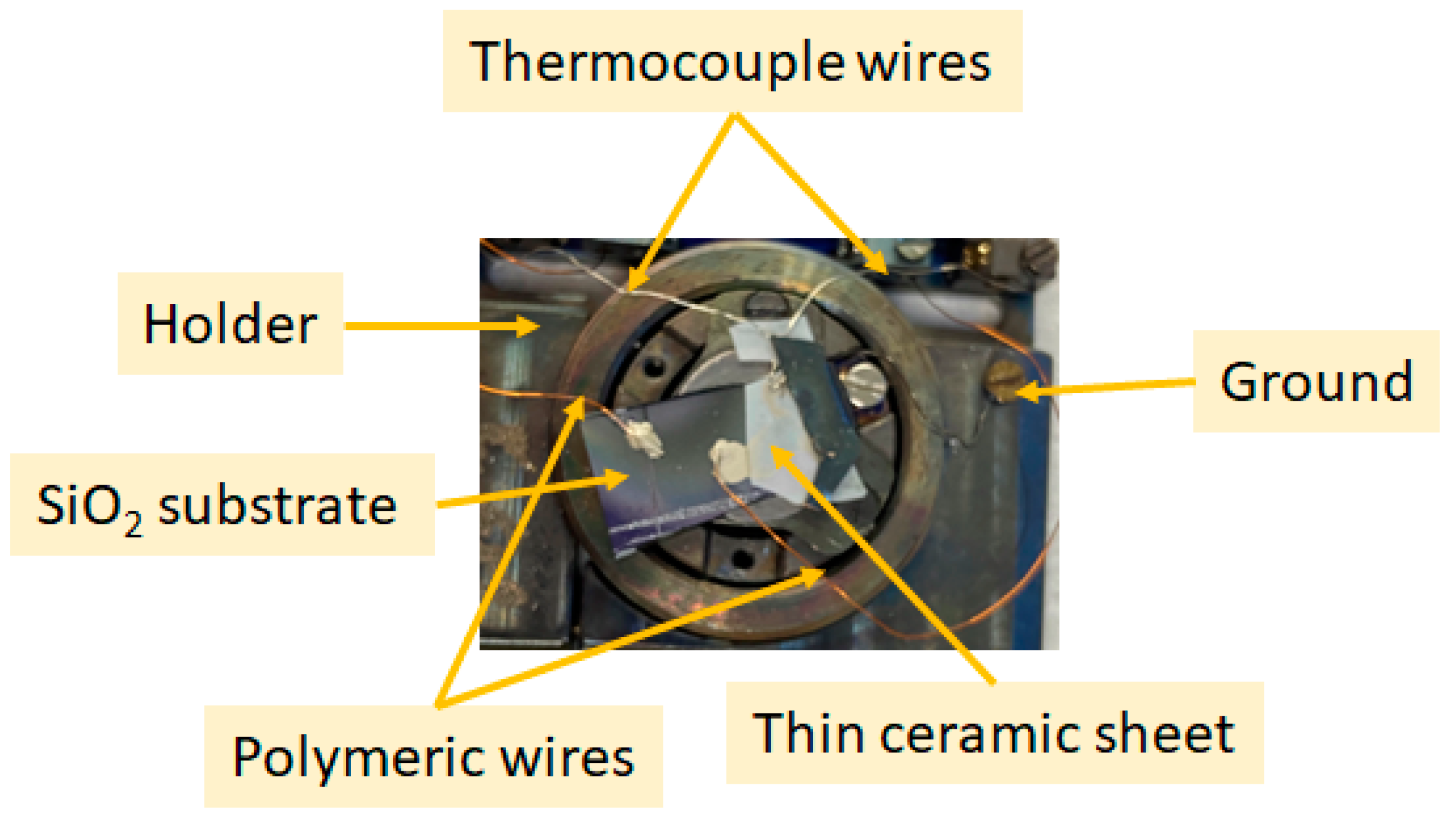
2.1.2. Sensor Connection
2.1.3. Gas Sensing Measurements
2.1.4. Material Characterization
2.2. Theoretical Methods
3. Results
3.1. Experimental Results
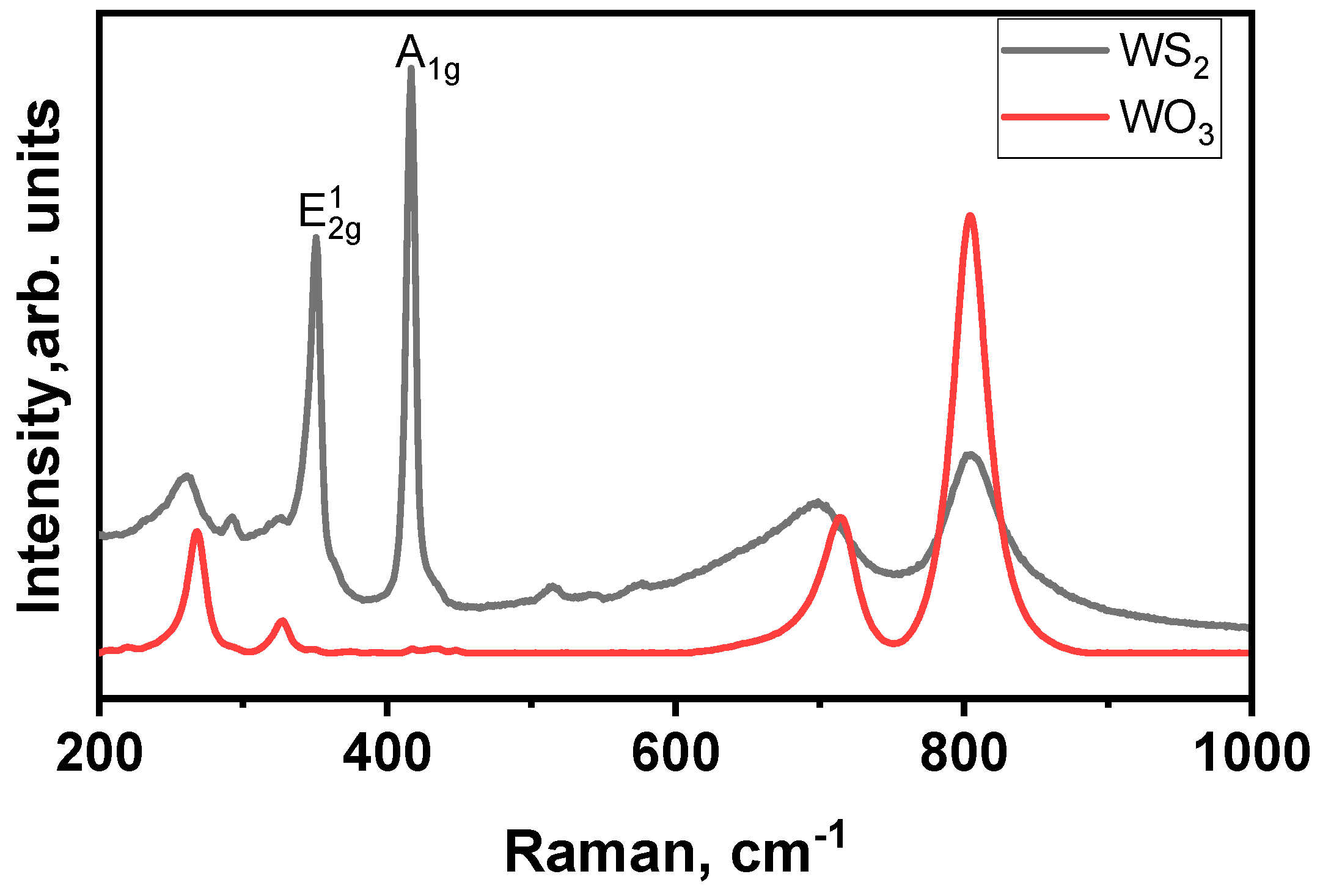
3.1.1. Characterization of the Materials
3.1.2. Characterization of the Sensors
3.1.3. NAP-XPS
3.2. Theoretical Results
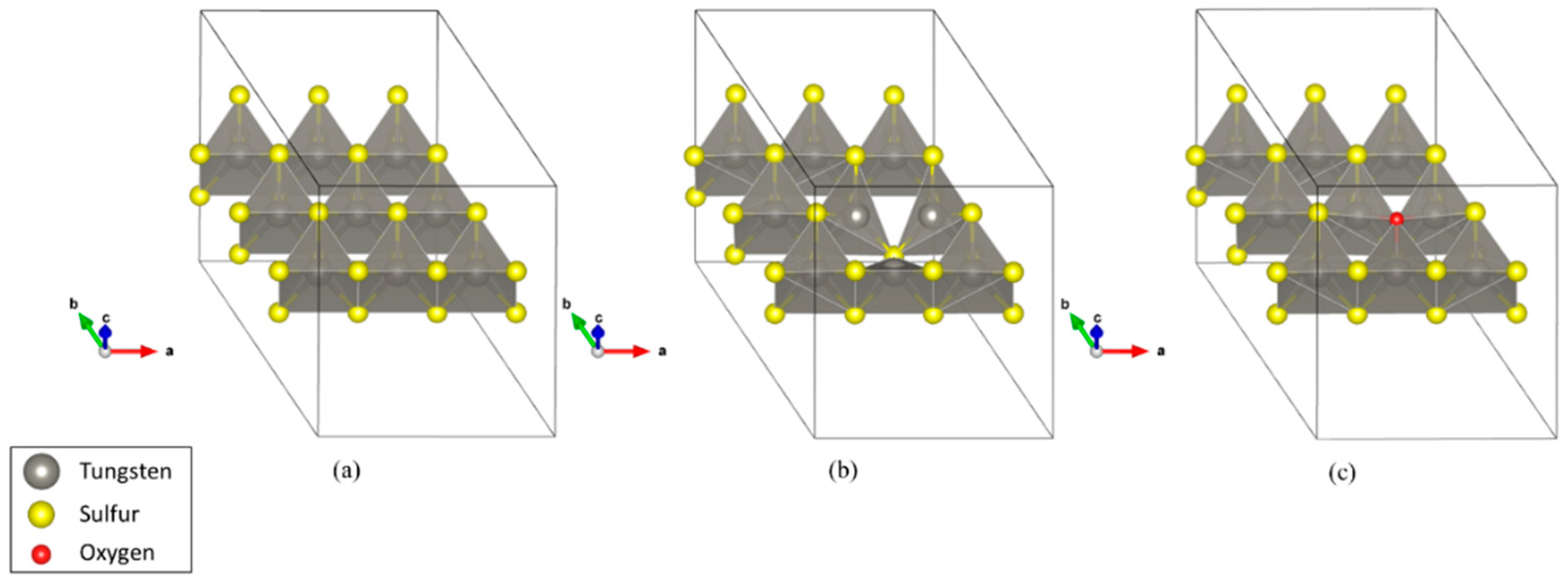
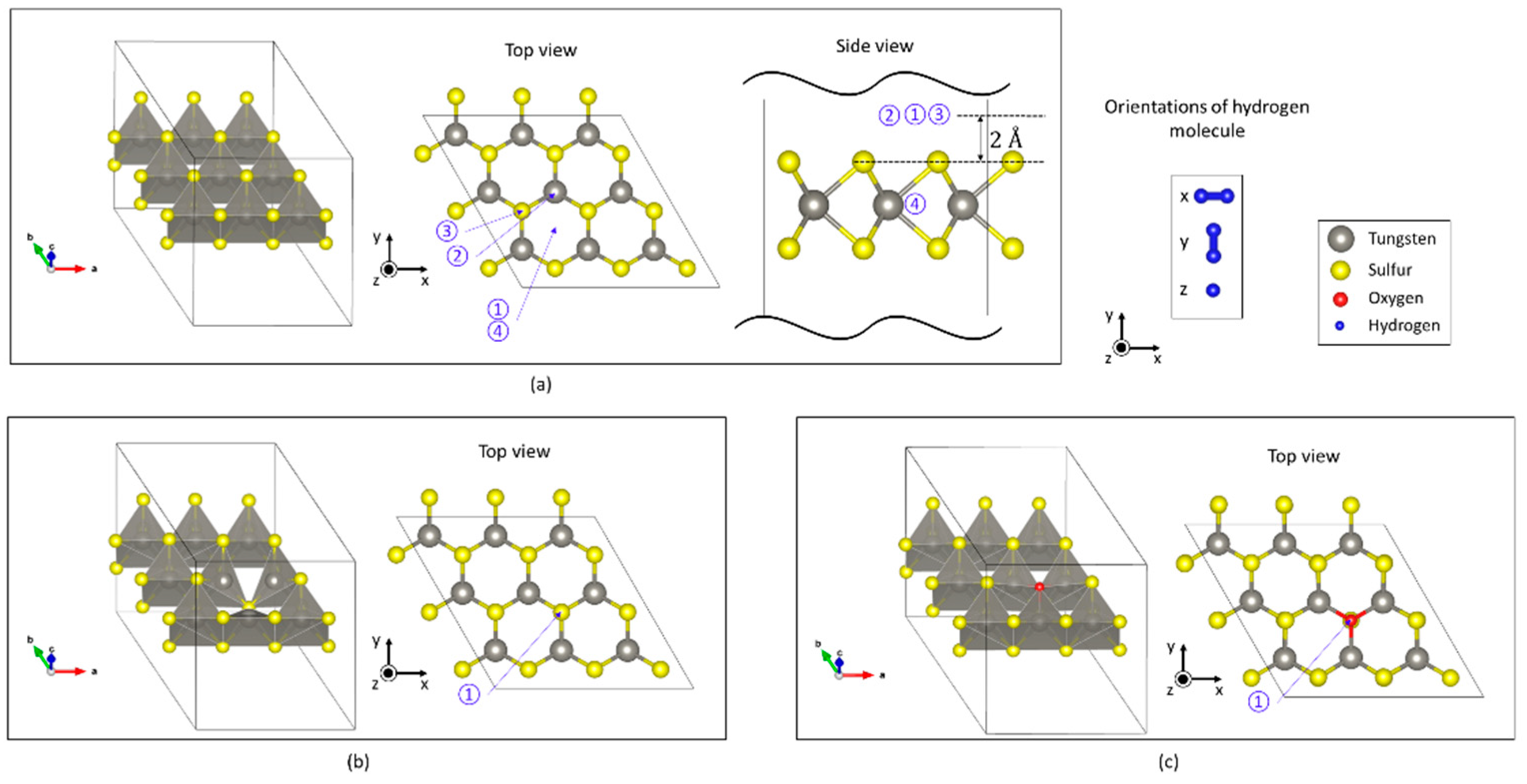
3.2.1. Stable Adsorption Sites on Each Monolayer
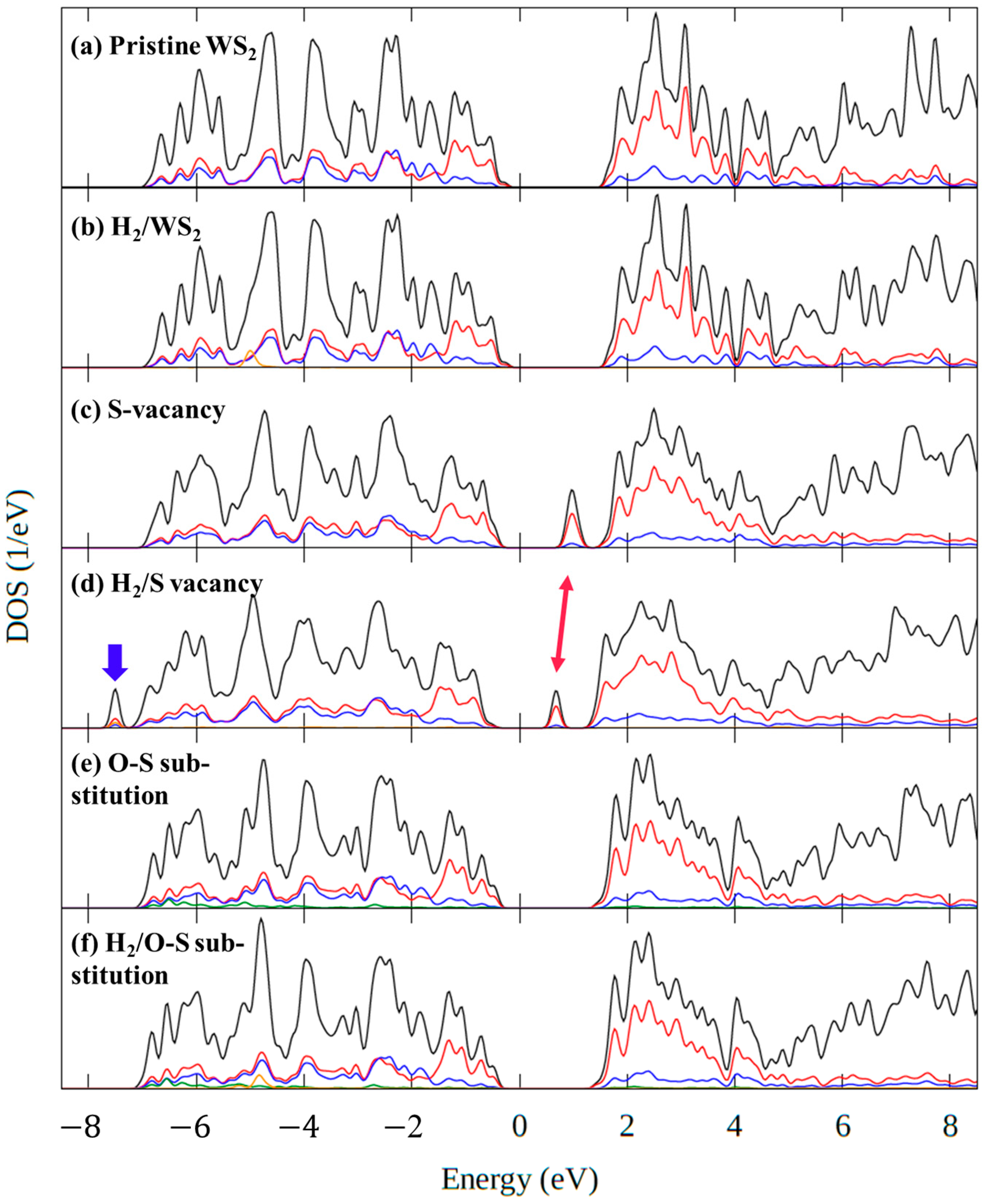
3.2.2. The Density of States
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Paris Agreement. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 6 April 2023).
- 2030 Climate Target Plan. Available online: https://climate.ec.europa.eu/eu-action/european-green-deal/2030-climate-target-plan_en (accessed on 6 April 2023).
- Greenhouse Gas Emissions by Country and Sector (Infographic). Available online: https://www.europarl.europa.eu/news/en/headlines/society/20180301STO98928/greenhouse-gas-emissions-by-country-and-sector-infographic (accessed on 6 April 2023).
- Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 6 April 2023).
- Ministry of the Environment. Available online: https://www.env.go.jp/press/110893.html (accessed on 6 April 2023).
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Cracknell, R.F.; Alcock, J.L.; Rowson, J.J.; Shirvill, L.C.; Üngüt, A. Safety Considerations in Retailing Hydrogen. SAE Trans. 2002, 111, 922–926. [Google Scholar]
- European Union EU “2050 Long-Term Strategy”. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 25 April 2023).
- International Energy Agency IEA “World Energy Outlook 2020: NZE2050”. Available online: https://www.iea.org/ (accessed on 25 April 2023).
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, C.; Wei, S.H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Jin, E.-H.; Yoo, D.-H.; Roh, J.-W.; Suh, D.; Commerell, W.; Huh, J.-S. Analysis of the Response Characteristics of Toluene Gas Sensors with a ZnO Nanorod Structure by a Heat Treatment Process. Sensors 2022, 22, 4125. [Google Scholar] [CrossRef]
- Basivi, P.K.; Pasupuleti, K.S.; Gelija, D.; Kim, M.D.; Pasupuleti, V.R.; Kim, C.W. UV-light-enhanced room temperature NO2 gas-sensing performances based on sulfur-doped graphitic carbon nitride nanoflakes. New J. Chem. 2022, 46, 19254–19262. [Google Scholar] [CrossRef]
- Subin, D.; Veeralakshmi, S.; Sandhya, J.; Nehru, S.; Kalaiselvam, S. Room temperature operatable high sensitive toluene gas sensor using chemiresistive Ag/Bi2O3 nanocomposite. Sens. Actuators B Chem. 2020, 320, 128410. [Google Scholar] [CrossRef]
- Koyama, S.; Haseda, Y.; Ishizawa, H.; Okazaki, F.; Bonefacino, J.; Tam, H.Y. Measurement of Pulsation Strain at the Fingertip Using a Plastic FBG Sensor. IEEE Sens. J. 2021, 21, 21537–21545. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, K.; Cho, B.; Kwak, Y.; Kim, J. Highly Sensitive Detection of Benzene, Toluene, and Xylene Based on CoPP-Functionalized TiO2 Nanoparticles with Low Power Consumption. ACS Sens. 2020, 5, 754–763. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Alagh, A.; Umek, P.; Casanova-Chafer, J.; Bittencourt, C.; Llobet, E. Controlled growth of 3D assemblies of edge enriched multilayer MoS2 nanosheets for dually selective NH3 and NO2 gas sensors. J. Mater. Chem. C 2022, 10, 11027–11039. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B.; Wu, X.F.; Gu, D.; Li, X. Enhanced ammonia sensing properties of rGO/WS2 heterojunction based hemiresistive sensor by marginal sulfonate decoration. Sens. Actuators B Chem. 2021, 337, 129776. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Sierra-Castillo, A.; Haye, E.; Colomer, J.F.; Llobet, E. CVD growth of self-assembled 2D and 1D WS2 nanomaterials for the ultrasensitive detection of NO2. Sens. Actuators B Chem. 2021, 326, 128813. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Youssef, K.A.; Bittencourt, C.; Güell, F.; Martínez-Alanis, P.R.; Reguant, M.; Llobet, E. PdO and PtO loaded WS2 boosts NO2 gas sensing characteristics at room temperature. Sens. Actuators B Chem. 2022, 364, 131905. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.H.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Colomer, J.F.; Llobet, E. An Ultrasensitive Room-Temperature H2S Gas Sensor Based on 3D Assembly of Cu2O Decorated WS2 Nanomaterial. IEEE Sens. J. 2021, 21, 21212–21220. [Google Scholar] [CrossRef]
- Asres, G.A.; Baldoví, J.J.; Dombovari, A.; Järvinen, T.; Lorite, G.S.; Mohl, M.; Shchukarev, A.; Paz, A.P.; Xian, L.; Mikkola, J.P.; et al. Ultrasensitive H2S gas sensors based on p-type WS2 hybrid materials. Nano Res. 2018, 11, 4215–4224. [Google Scholar] [CrossRef]
- Lanzhong, H.; Liu, H.; Xu, H.; Dong, S.; Du, Y.; Wu, Y.; Zeng, H.; Zhu, J.; Liu, Y. Flexible Pd-WS2/Si heterojunction sensors for highly sensitive detection of hydrogen at room temperature. Sens. Actuators B Chem. 2019, 283, 740–748. [Google Scholar] [CrossRef]
- Deepa, K.; Huang, B.R.; Saravanan, A.; Tzeng, Y. Role of Nanodiamond Grains in the Exfoliation of WS2 Nanosheets and Their Enhanced Hydrogen-Sensing Properties. ACS Appl. Mater. Interfaces 2021, 13, 48260–48269. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.J. Two-dimensional transition metal dichalcogenides and metal oxide hybrids for gas sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, M.; Moradinasab, M.; Fathipour, M. The transport and optical sensing properties of MoS2, MoSe2, WS2 and WSe2 semiconducting transition metal dichalcogenides. Semicond. Sci. Technol. 2018, 33, 025002. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W.; Zhu, H. Mechanism of charge transfer and its impacts on Fermi-level pinning for gas molecules adsorbed on monolayer WS2. J. Chem. Phys. 2015, 142, 214704. [Google Scholar] [CrossRef]
- Vallejos, S.; Umek, P.; Stoycheva, T.; Annanouch, F.; Llobet, E.; Correig, X.; De Marco, P.; Bittencourt, C.; Blackman, C. Single-Step Deposition of Au- and Pt-Nanoparticle-Functionalized Tungsten Oxide Nanoneedles Synthesized via Aerosol-Assisted CVD, and Used for Fabrication of Selective Gas Microsensor Arrays. Adv. Funct. Mater. 2013, 23, 1313–1322. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef]
- Pérez-Dieste, V.; Aballe, L.; Ferrer, S.; Nicolàs, J.; Escudero, C.; Milán, A.; Pellegrin, E. Near Ambient Pressure XPS at ALBA. J. Phys. Conf. Ser. 2013, 425, 072023. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to prolem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Tungsten X-ray Photoelectron Spectra, Tungsten Electron Configuration, and Other Elemental Information. Available online: https://www.thermofisher.com/be/en/home/materials-science/learning-center/periodic-table/transition-metal/tungsten.html (accessed on 6 April 2023).
- Nandasiri, M.I.; Camacho-Forero, L.E.; Schwarz, A.M.; Shutthanandan, V.; Thevuthasan, S.B.; Perla, B.; Mueller, K.T.; Murugesan, V. In Situ Chemical Imaging of Solid-Electrolyte Interphase Layer Evolution in Li–S Batteries. Chem. Mater. 2017, 29, 4728–4737. [Google Scholar] [CrossRef]
- Su, L.; Liu, C.; Liang, K.; Chen, Y.; Zhang, L.; Li, X.; Han, Z.; Zhen, G.; Chai, X.; Sun, X. Performance evaluation of zero-valent iron nanoparticles (NZVI) for high-concentration H2S removal from biogas at different temperatures. RSC Adv. 2018, 8, 13798–13805. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Wang, F.; Ma, Z.; Li, X.; Xu, K.; Wang, C. Reversible S0/MgSx Redox Chemistry in a MgTFSI2/MgCl2/DME Electrolyte for Rechargeable Mg/S Batteries. Angew. Chem. Int. Ed. 2017, 56, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Schoutte, W.J.; De Boer, J.L.; Jellinek, F. Cristal structures of tungsten disulfide and diselenide. J. Solid State Chem. 1987, 70, 207. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; Di Maggio, F.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-Assisted CVD-Grown PdO Nanoparticle-Decorated Tungsten Oxide Nanoneedles Extremely Sensitive and Selective to Hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Colomer, J.F.; Llobet, E. 3D Assembly of WS2 Nanomaterial for H2S Gas Sensing Application. In Proceedings of the IEEE SENSORS 2020, Rotterdam, The Netherlands, 25–28 October 2020. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992; pp. 41–172. ISBN 0-9627026-2-5. [Google Scholar]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.Y.; Wallace, R.M. Interface Chemistry and Band Alignment Study of Ni and Ag Contacts on MoS2. ACS Appl. Mater. Interfaces 2021, 13, 15802–15810. [Google Scholar] [CrossRef]
- Wang, X.; Cormier, C.R.; Khosravi, A.S.; Christopher, M.; Shallenberger, J.R.; Addou, R.; Wallace, R.M. In situ exfoliated 2D molybdenum disulfide analyzed by XPS. Surf. Sci. Spectra 2020, 27, 1055–5269. [Google Scholar] [CrossRef]
- Gong, C.; Colombo, L.; Wallace, R.M.; Cho, K. The Unusual Mechanism of Partial Fermi Level Pinning at Metal–MoS2 Interfaces. Nano Lett. 2014, 14, 1714–1720. [Google Scholar] [CrossRef]


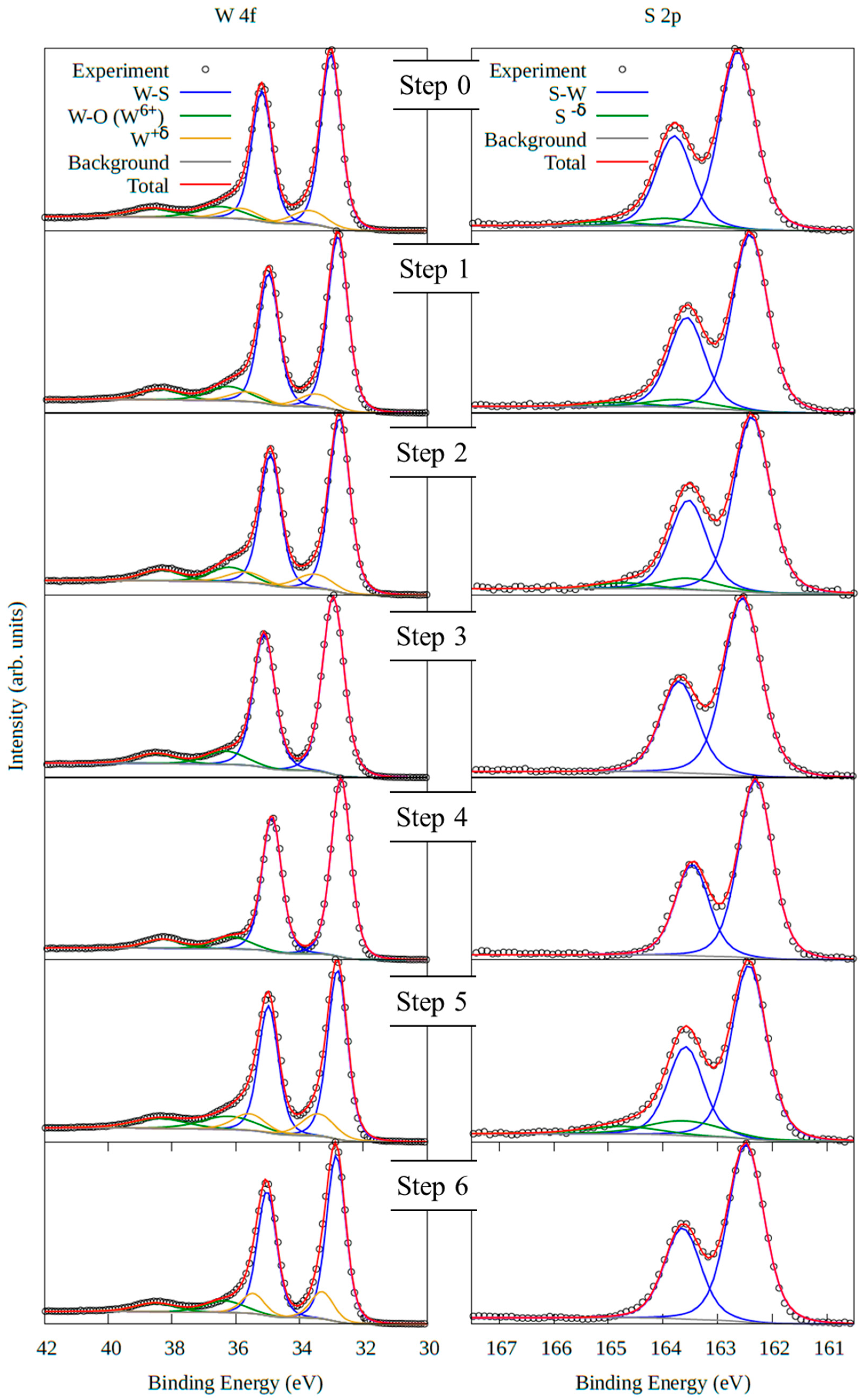

| Steps | NAP-XPS Chamber Environment | R (kΩ) | W (%) | S (%) | O (%) | S/W |
|---|---|---|---|---|---|---|
| 0 | UHV at RT | 110 | 34.8 | 52.1 | 13.1 | 1.5 |
| 1 | 1000 ppm H2 at RT | 120 | 33.6 | 48.8 | 17.6 | 1.5 |
| 2 | 5000 ppm H2 at RT | 145 | 32.6 | 49.6 | 17.8 | 1.5 |
| 3 | UHV at RT | 135 | 32.0 | 50.0 | 18.0 | 1.6 |
| 4 | UHV with the device heating at 150 °C | 80 | 33.5 | 48.8 | 17.7 | 1.5 |
| 5 | 1000 ppm H2 with the device heating at 150 °C | 110 | 31.3 | 49.8 | 18.9 | 1.6 |
| 6 | UHV with the device heating at 150 °C | 75 | 27.1 | 42.7 | 30.2 | 1.6 |
| Adsorption Site | Adsorption Energy (meV) | Charge Transfer (e) |
|---|---|---|
| Above a hexagon in defect-free WS2 | −70 | −0.005 |
| On a sulfur defect | −299 | −0.56 |
| On an oxygen impurity | −116 | −0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minezaki, T.; Krüger, P.; Annanouch, F.E.; Casanova-Cháfer, J.; Alagh, A.; Villar-Garcia, I.J.; Pérez-Dieste, V.; Llobet, E.; Bittencourt, C. Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS. Sensors 2023, 23, 4623. https://doi.org/10.3390/s23104623
Minezaki T, Krüger P, Annanouch FE, Casanova-Cháfer J, Alagh A, Villar-Garcia IJ, Pérez-Dieste V, Llobet E, Bittencourt C. Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS. Sensors. 2023; 23(10):4623. https://doi.org/10.3390/s23104623
Chicago/Turabian StyleMinezaki, Tomoya, Peter Krüger, Fatima Ezahra Annanouch, Juan Casanova-Cháfer, Aanchal Alagh, Ignacio J. Villar-Garcia, Virginia Pérez-Dieste, Eduard Llobet, and Carla Bittencourt. 2023. "Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS" Sensors 23, no. 10: 4623. https://doi.org/10.3390/s23104623
APA StyleMinezaki, T., Krüger, P., Annanouch, F. E., Casanova-Cháfer, J., Alagh, A., Villar-Garcia, I. J., Pérez-Dieste, V., Llobet, E., & Bittencourt, C. (2023). Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS. Sensors, 23(10), 4623. https://doi.org/10.3390/s23104623







