Comparison of Characteristics of a ZnO Gas Sensor Using a Low-Dimensional Carbon Allotrope
Abstract
1. Introduction
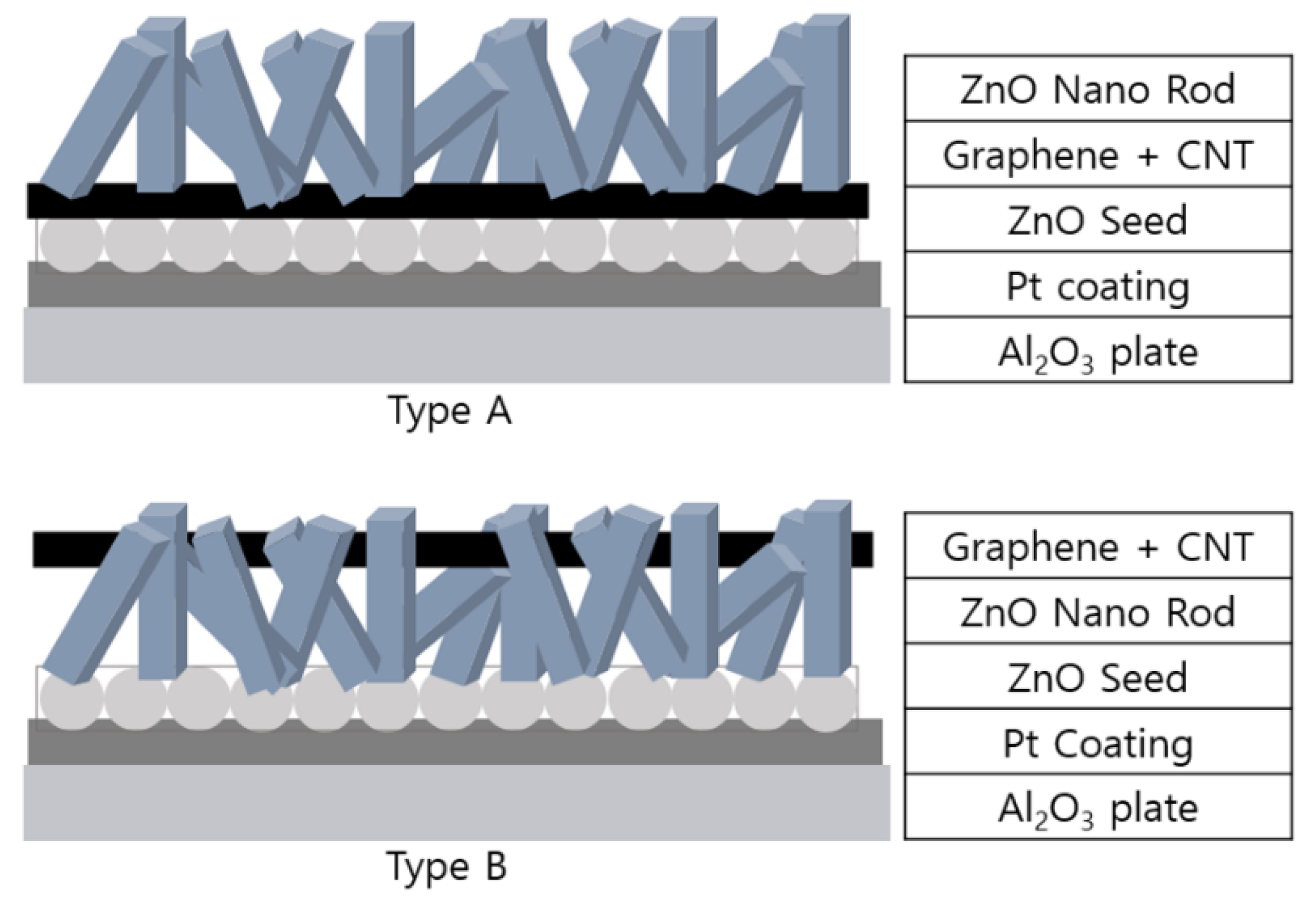
2. Materials and Methods
3. Results and Discussion
3.1. FE-SEM (Field Emission Scanning Electron Microscope)
3.2. XRD (X-ray Diffraction) Analysis
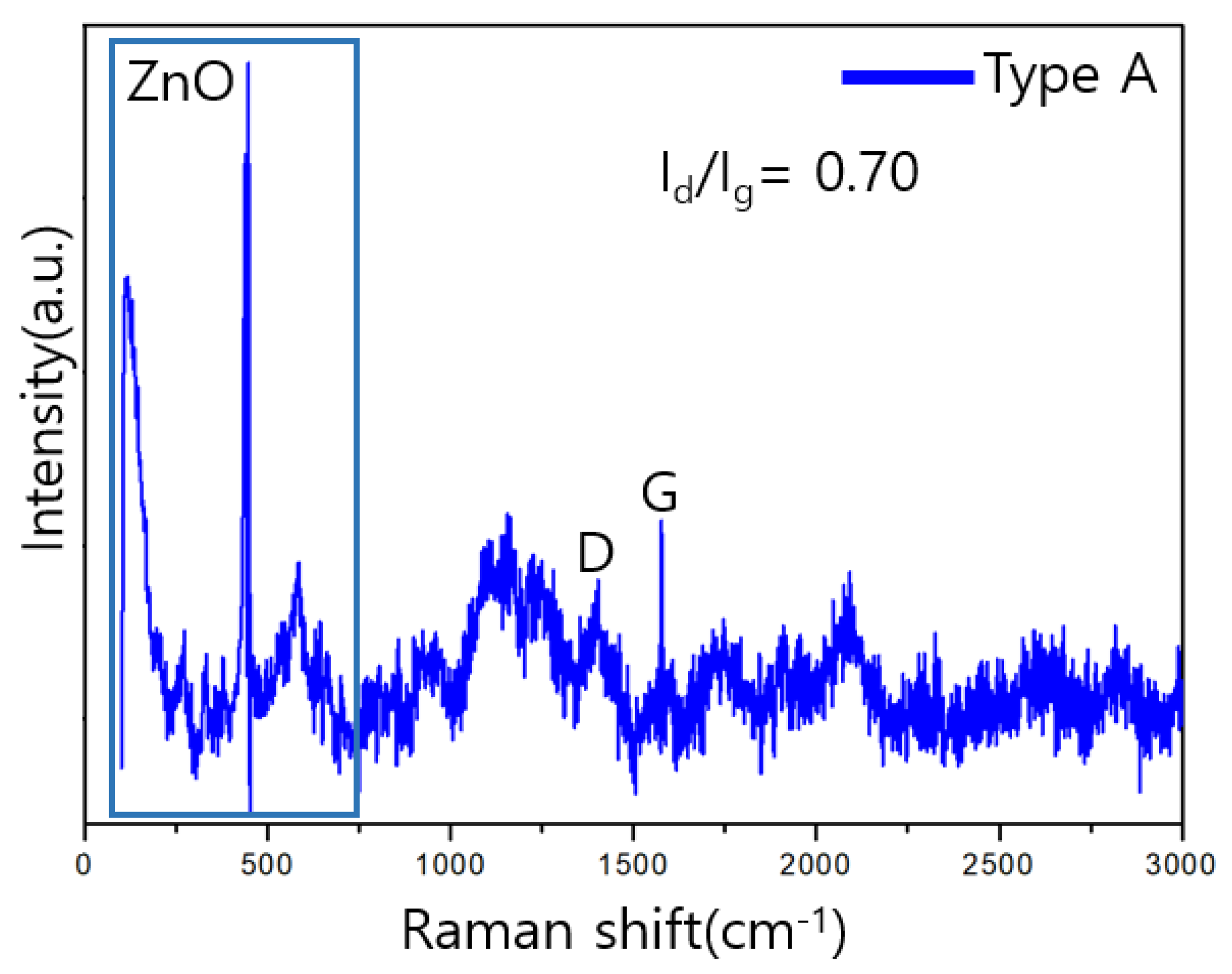
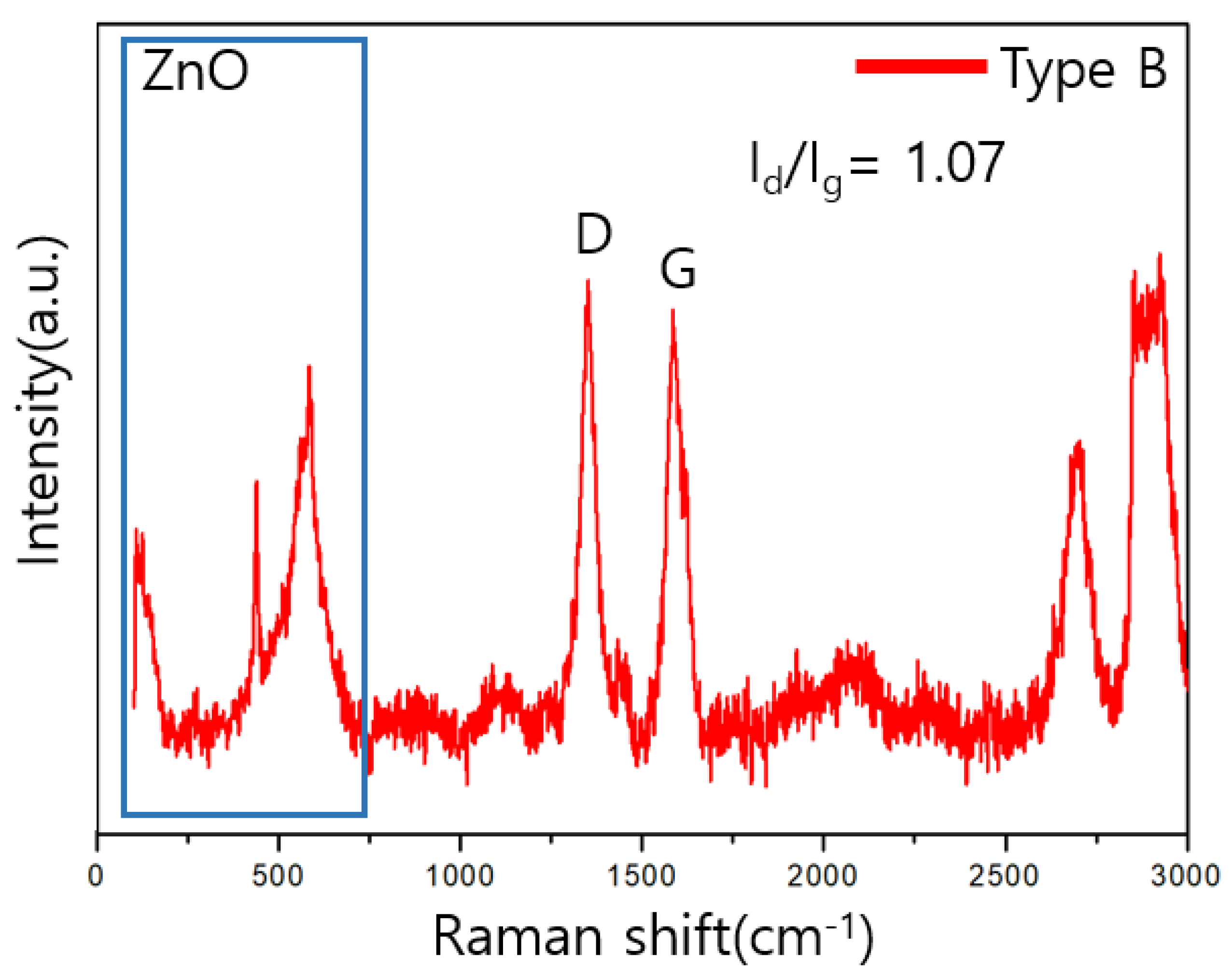
3.3. Raman Analysis
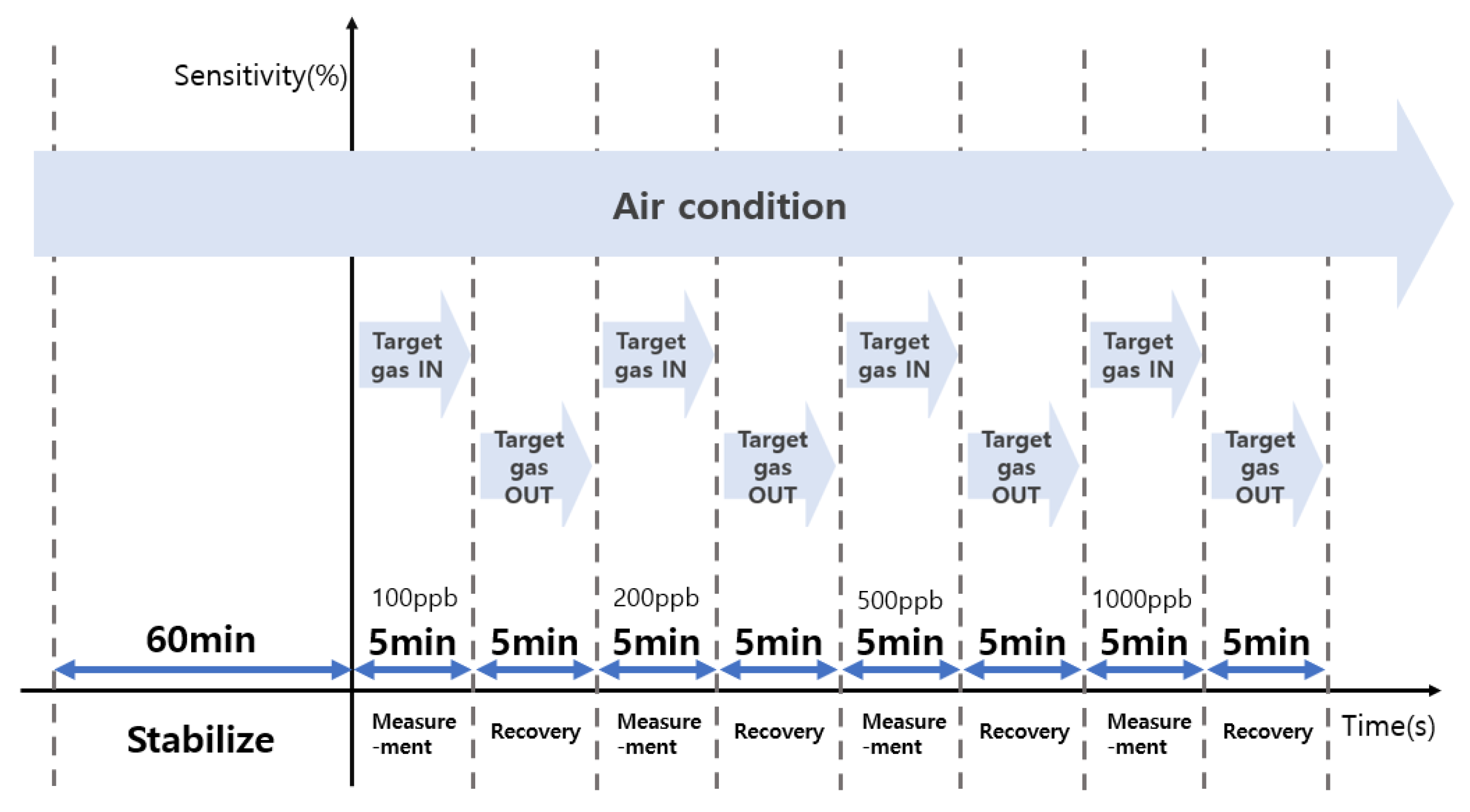
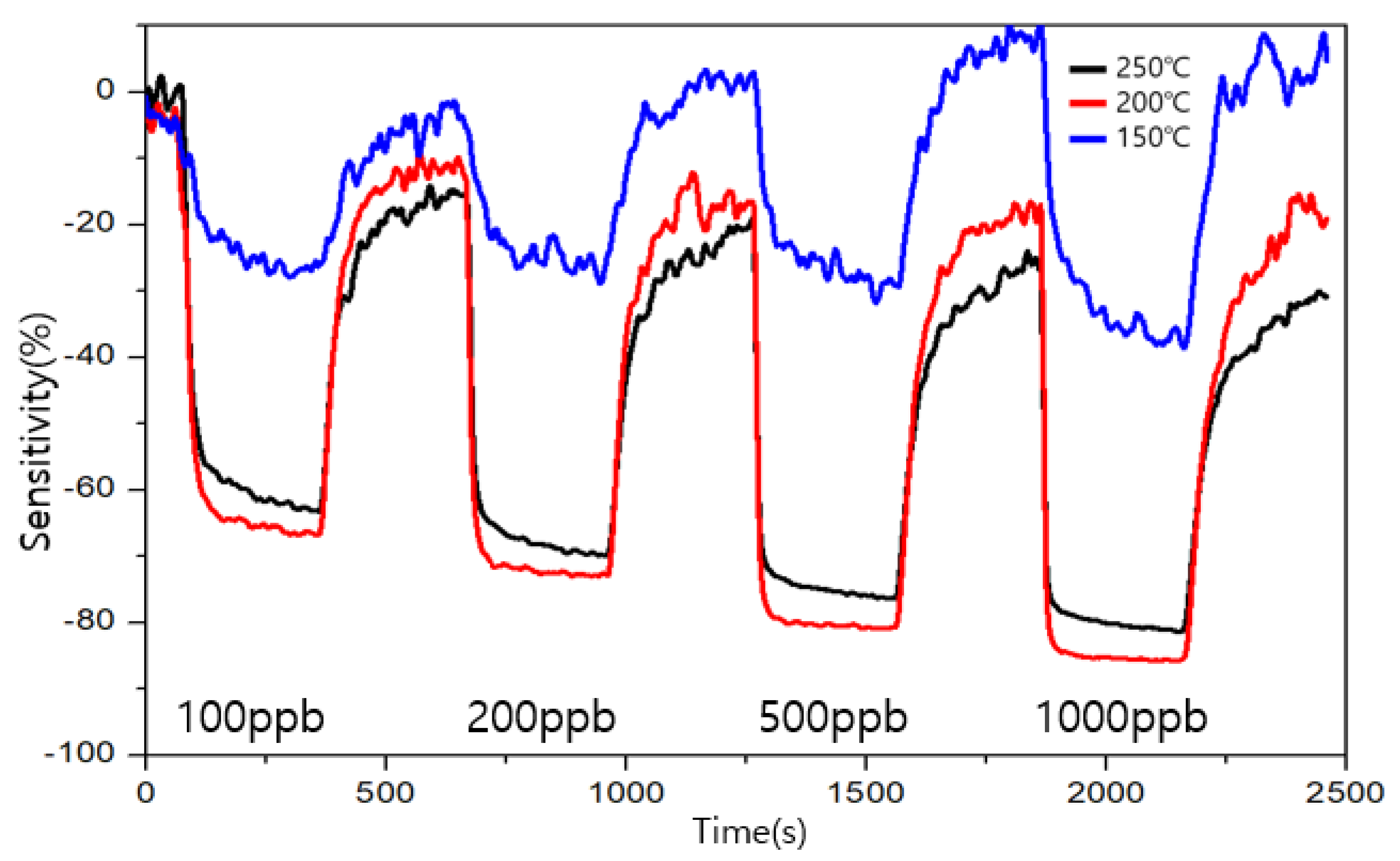
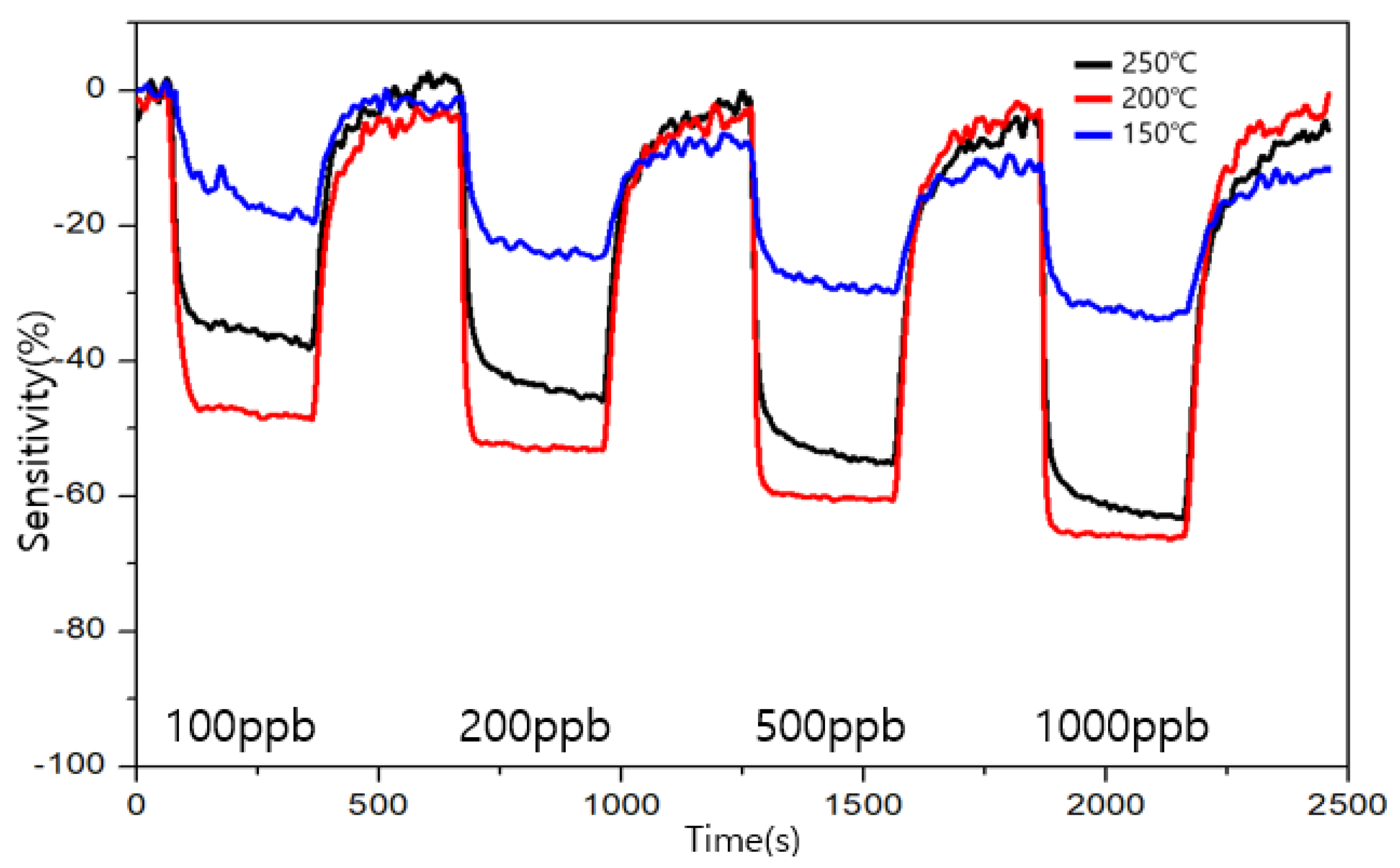
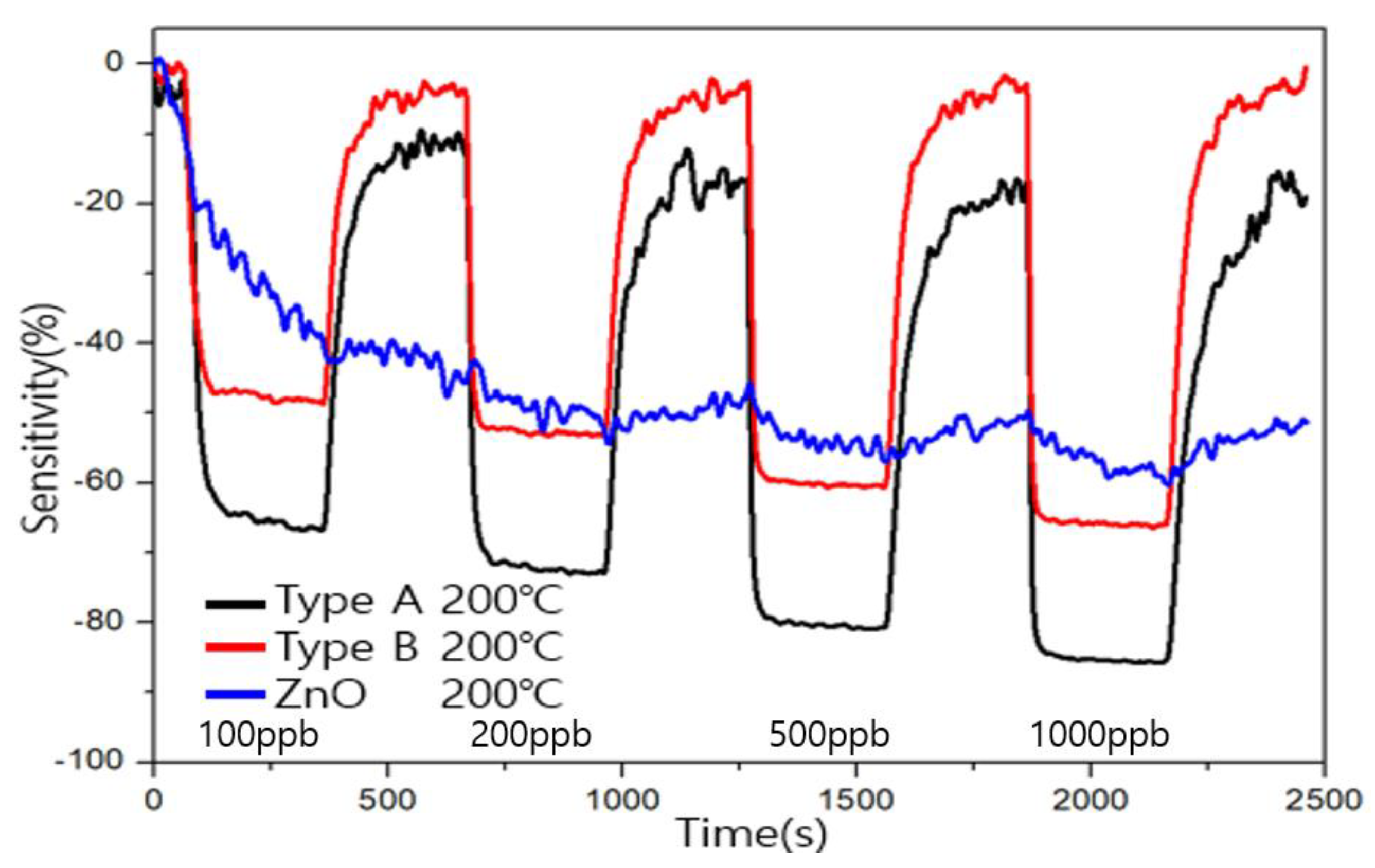
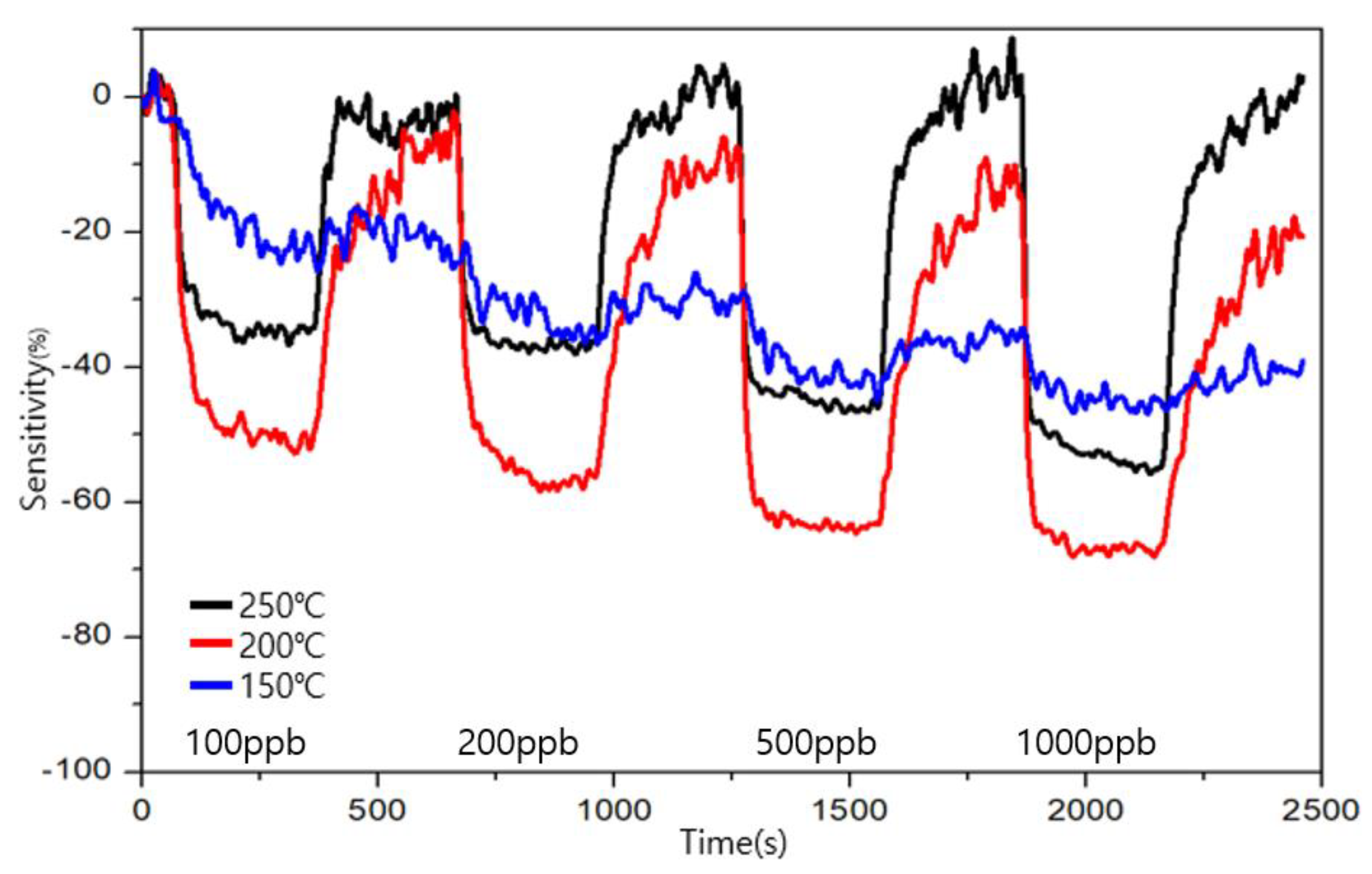
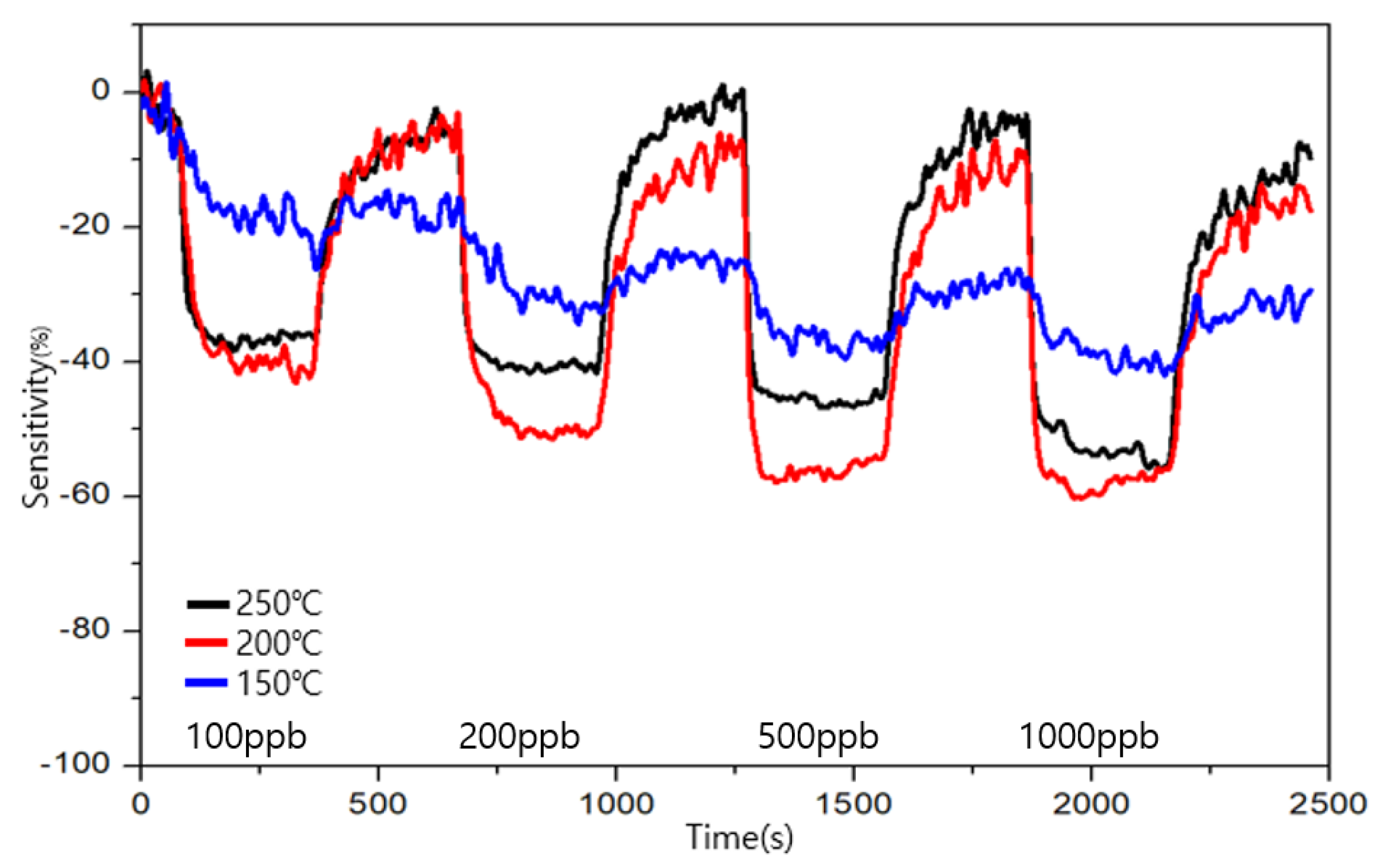
3.4. Comparison of Sensitivity and Recovery for Each Sensor for 1 ppb Level of Formaldehyde
3.5. Comparison of Sensitivity and Recovery for Each Sensor for 1 ppb Level of Toluene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandari, M.; Wang, J.; Jang, D.; Nam, I.; Huang, B. A comparative study on the electrical and piezoresistive sensing characteristics of GFRP and CFRP composites with hybridized incorporation of carbon nanotubes, graphenes, carbon nanofibers, and graphite nanoplatelets. Sensors 2021, 21, 7291. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Chen, Y.; Jiang, H.L.; Qiu, X.B.; Yu, D.L. Smartphone-based microfluidic colorimetric sensor for gaseous formaldehyde determination with high sensitivity and selectivity. Sensors 2018, 18, 3141. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Inorganic-diverse nanostructured materials for volatile organic compound sensing. Sensors 2021, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, S.; Weschler, C.; Zhao, B.; Zhang, Y. Analysis of the dynamic interaction between SVOCs and airborne particles. Aerosol Sci. Technol. 2013, 47, 125–136. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor metal oxides as chemoresistive sensors for detecting volatile organic compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef]
- Taurino, A.M.; Epifani, M.; Toccoli, T.; Iannotta, S.; Siciliano, P. Innovative aspects in thin film technologies for nanostructured materials in gas sensor devices. Thin Solid Film. 2003, 436, 52–63. [Google Scholar] [CrossRef]
- Saukko, S.; Lantto, V. Influence of electrode material on properties of SnO2-based gas sensor. Thin Solid Film. 2003, 436, 137–140. [Google Scholar] [CrossRef]
- Bo, Z.; Yuan, M.; Mao, S.; Chen, X.; Yan, J.; Cen, K. Decoration of vertical graphene with tin dioxide nanoparticles for highly sensitive room temperature formaldehyde sensing. Sens. Actuators B Chem. 2018, 256, 1011–1020. [Google Scholar] [CrossRef]
- Behi, S.; Bohli, N.; Casanova-Cháfer, J.; Llobet, E.; Abdelghani, A. Metal oxide nanoparticle-decorated few layer graphene nanoflake chemoresistors for the detection of aromatic volatile organic compounds. Sensors 2020, 20, 3413. [Google Scholar] [CrossRef]
- Fang, F.; Bai, L.; Song, D.; Yang, H.; Sun, X.; Sun, H.; Zhu, J. Ag-modified In2O3/ZnO nanobundles with high formaldehyde gas-sensing performance. Sensors 2015, 15, 20086–20096. [Google Scholar] [CrossRef]
- Shen, T.; Dai, X.; Zhang, D.; Wang, W.; Feng, Y. ZnO composite graphene coating micro-fiber interferometer for ultraviolet detection. Sensors 2020, 20, 1478. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Hammed, N.A.; Aziz, A.A.; Usman, A.I.; Qaeed, M.A. The sonochemical synthesis of vertically aligned ZnO nanorods and their UV photodetection properties: Effect of ZnO buffer layer. Ultrason. Sonochemistry 2019, 50, 172–181. [Google Scholar] [CrossRef]
- Kwon, D.H.; Jin, E.H.; Yoo, D.H.; Roh, J.W.; Suh, D.; Commerell, W.; Huh, J.S. Analysis of the Response Characteristics of Toluene Gas Sensors with a ZnO Nanorod Structure by a Heat Treatment Process. Sensors 2022, 22, 4125. [Google Scholar] [CrossRef]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Horrillo, M.C.; Getino, J.; Gutiérrez, J.; Arés, L.; Robla, J.I.; Garcia, C.; Sayago, I. Measurements of VOCs in soils through a tin oxide multisensor system. Sens. Actuators B Chem. 1997, 43, 193–199. [Google Scholar] [CrossRef]
- Patil, L.A.; Bari, A.R.; Deo, V. Ultrasonically synthesized nanocrystalline ZnO powder-based thick film sensor for ammonia sensing. Sens. Rev. 2010, 30, 290–296. [Google Scholar] [CrossRef]
- Savage, N.; Chwieroth, B.; Ginwalla, A.; Patton, B.R.; Akbar, S.A.; Dutta, P.K. Composite n–p semiconducting titanium oxides as gas sensors. Sens. Actuators B Chem. 2001, 79, 17–27. [Google Scholar] [CrossRef]
- Yamaura, H.; Jinkawa, T.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Indium oxide-based gas sensor for selective detection of CO. Sens. Actuators B Chem. 1996, 36, 325–332. [Google Scholar] [CrossRef]
- Festinger, N.; Morawska, K.; Ivanovski, V.; Ziąbka, M.; Jedlińska, K.; Ciesielski, W.; Smarzewska, S. Comparative electroanalytical studies of graphite flake and multilayer graphene paste electrodes. Sensors 2020, 20, 1684. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Aksay, I.A. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Ribeiro, J.; DaBoit, K.; Flores, D.; Kronbauer, M.A.; Silva, L.F. Extensive FE-SEM/EDS, HR-TEM/EDS and ToF-SIMS studies of micron-to nano-particles in anthracite fly ash. Sci. Total Environ. 2013, 452, 98–107. [Google Scholar] [CrossRef]
- Van Heerden, J.L.; Swanepoel, R. XRD analysis of ZnO thin films prepared by spray pyrolysis. Thin Solid Film. 1997, 299, 72–77. [Google Scholar] [CrossRef]
- Emani, P.S.; Maddah, H.A.; Rangoonwala, A.; Che, S.; Prajapati, A.; Singh, M.R.; Behura, S.K. Organophilicity of graphene oxide for enhanced wettability of ZnO nanorods. ACS Appl. Mater. Interfaces 2020, 12, 39772–39780. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J. Mechanism for toluene detection of flower-like ZnO sensors prepared by hydrothermal approach: Charge transfer. Sens. Actuators B Chem. 2015, 207, 66–73. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, J.; Huh, J.-S. Comparison of Characteristics of a ZnO Gas Sensor Using a Low-Dimensional Carbon Allotrope. Sensors 2023, 23, 52. https://doi.org/10.3390/s23010052
Lee J, Park J, Huh J-S. Comparison of Characteristics of a ZnO Gas Sensor Using a Low-Dimensional Carbon Allotrope. Sensors. 2023; 23(1):52. https://doi.org/10.3390/s23010052
Chicago/Turabian StyleLee, Jihoon, Jaebum Park, and Jeung-Soo Huh. 2023. "Comparison of Characteristics of a ZnO Gas Sensor Using a Low-Dimensional Carbon Allotrope" Sensors 23, no. 1: 52. https://doi.org/10.3390/s23010052
APA StyleLee, J., Park, J., & Huh, J.-S. (2023). Comparison of Characteristics of a ZnO Gas Sensor Using a Low-Dimensional Carbon Allotrope. Sensors, 23(1), 52. https://doi.org/10.3390/s23010052







