Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage–Turner Syndrome—A Case Report
Abstract
1. Introduction
2. Case Description
| May | Symptoms: severe, throbbing, and diffuse pain in the biceps muscle of his right arm (pain 8/10 NPRS). Assessment: medical history (family doctor). Diagnosis: exercise-related overload at the gym. Treatment: pharmacological analgesics. |
| June | Symptom: increasing muscle weakness (pain 6/10 NPRS). Assessment: physical examination (physiotherapist). Diagnosis: overload of the biceps muscle. Treatment: cold compresses. Effects: no positive therapeutic effect. |
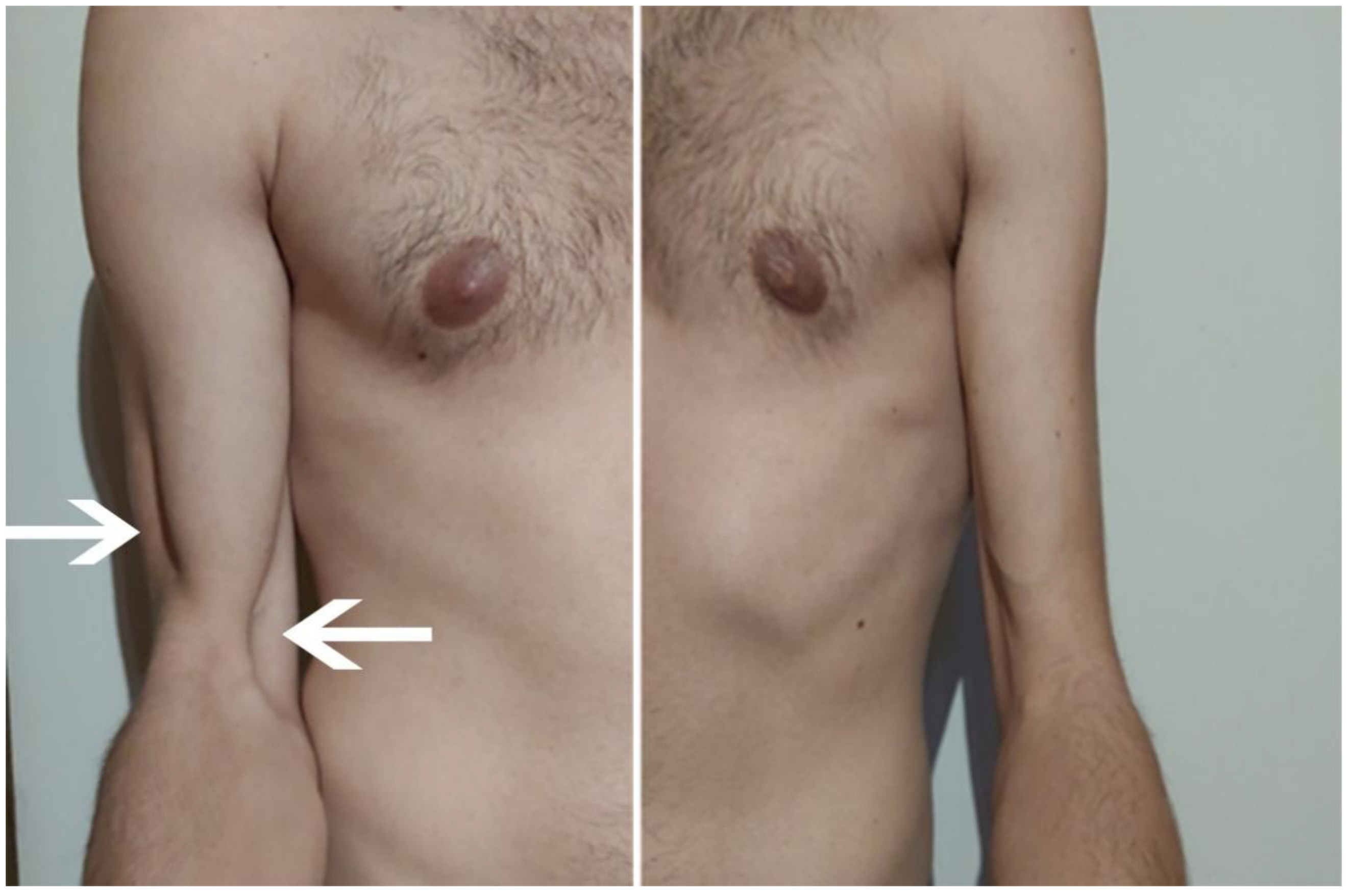
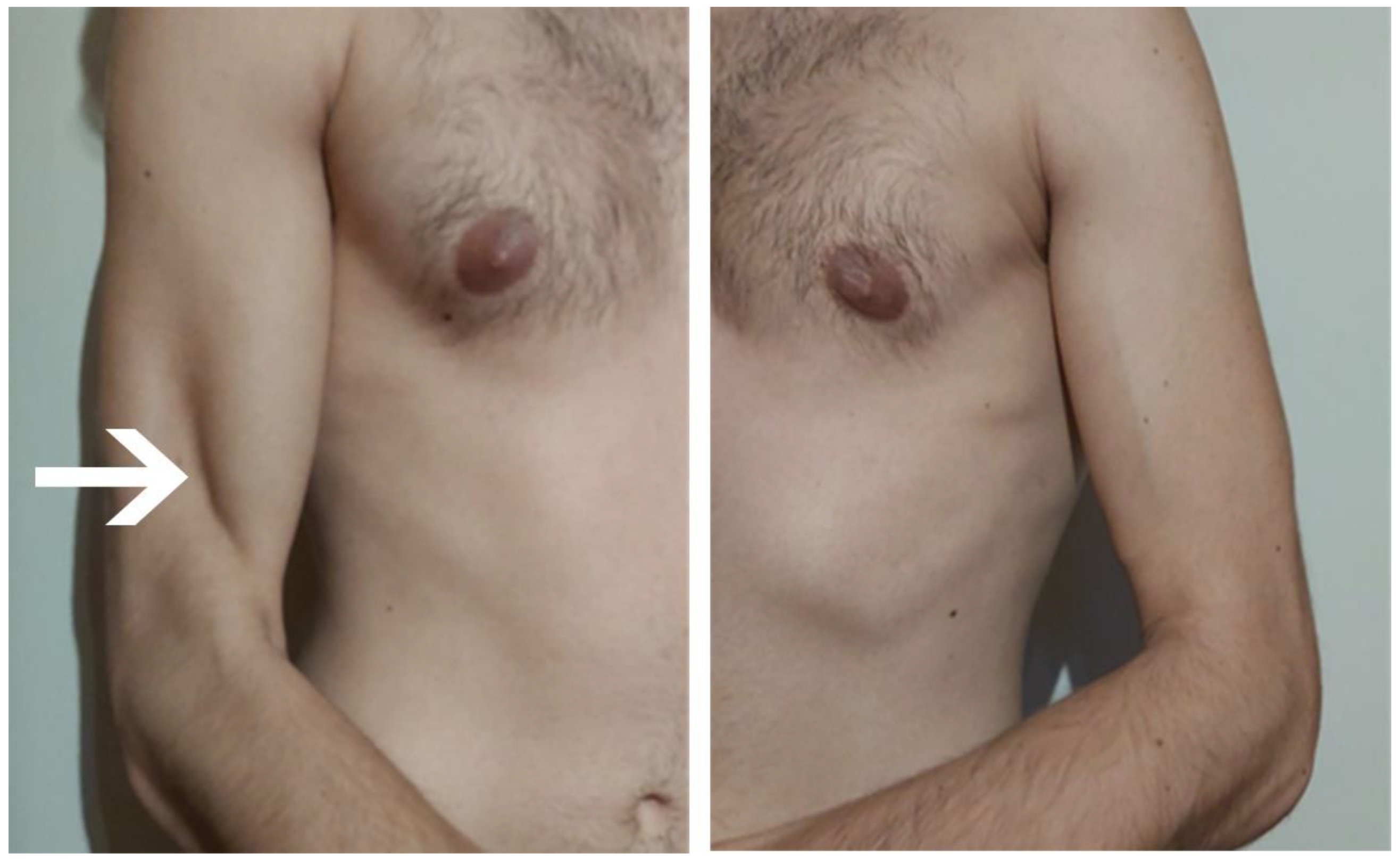
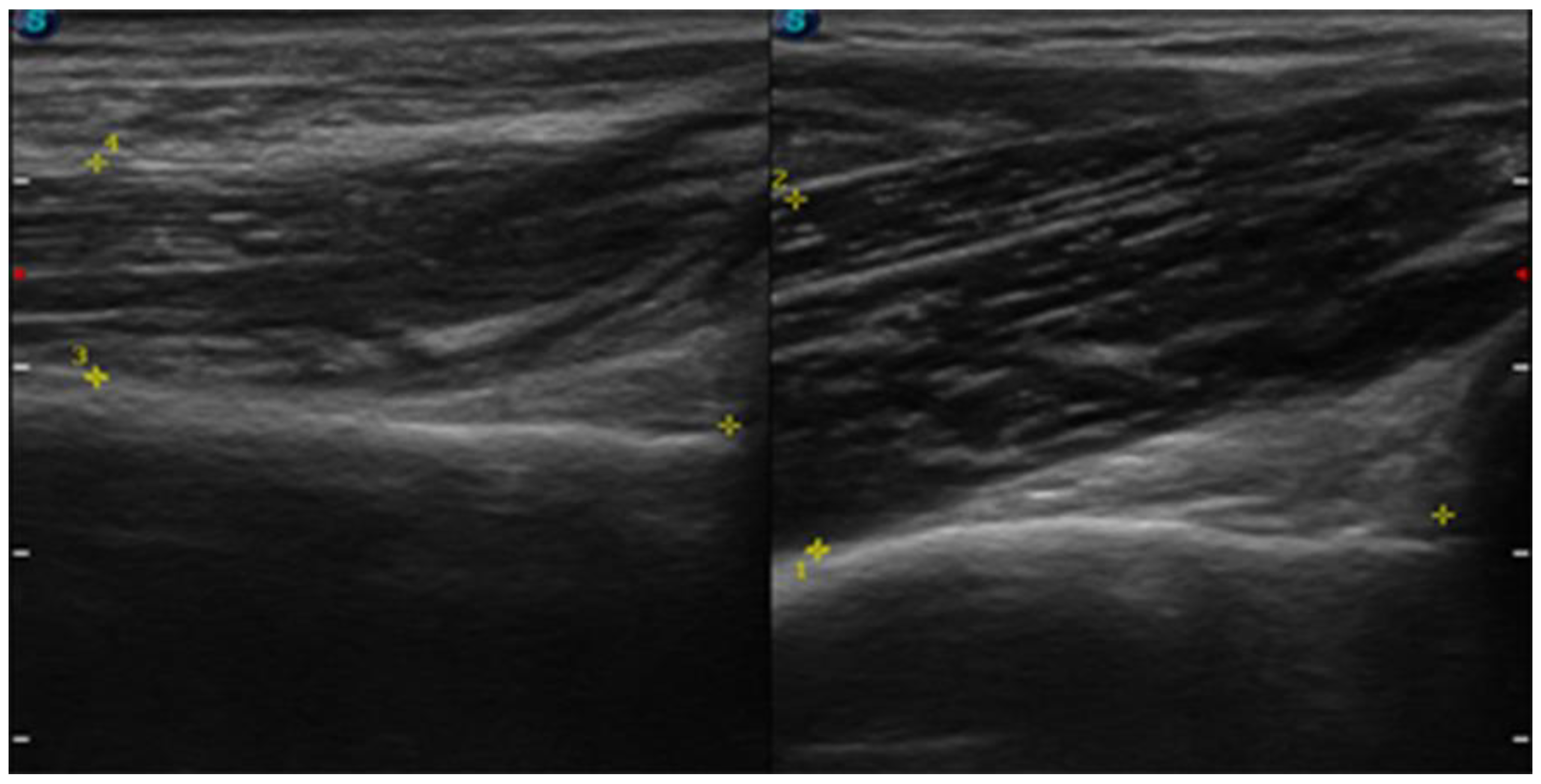
| July | Symptoms: brachialis muscle atrophy (Figure 1 and Figure 2) (pain 4/10 NPRS). Assessment: medical history, physical examination, ultrasound (orthopaedic surgeon). Diagnosis: unspecified soft tissue diseases associated with their use overload and overexertion (M70.9 ICD). Treatment: pharmacological treatment, avoid full weight-bearing. Effects: no positive therapeutic effect. |
| August | Symptoms: further brachialis muscle atrophy, worsening of pain after exercise (pain 8/10 NPRS). Assessment: no assessment. Diagnosis: no diagnosis. Treatment: patient’s decision: nonsteroidal anti-inflammatory drugs (pain reduction 4/10 NPRS) Effects: pain reduction (4/10 NPRS). |
| September | Symptoms: further brachialis muscle atrophy, worsening of pain after exercise (pain 8/10 NPRS). Assessment: no assessment. Diagnosis: no diagnosis. Treatment: patient’s decision: nonsteroidal anti-inflammatory drugs (pain reduction 4/10 NPRS) Effects: pain reduction (4/10 NPRS). |
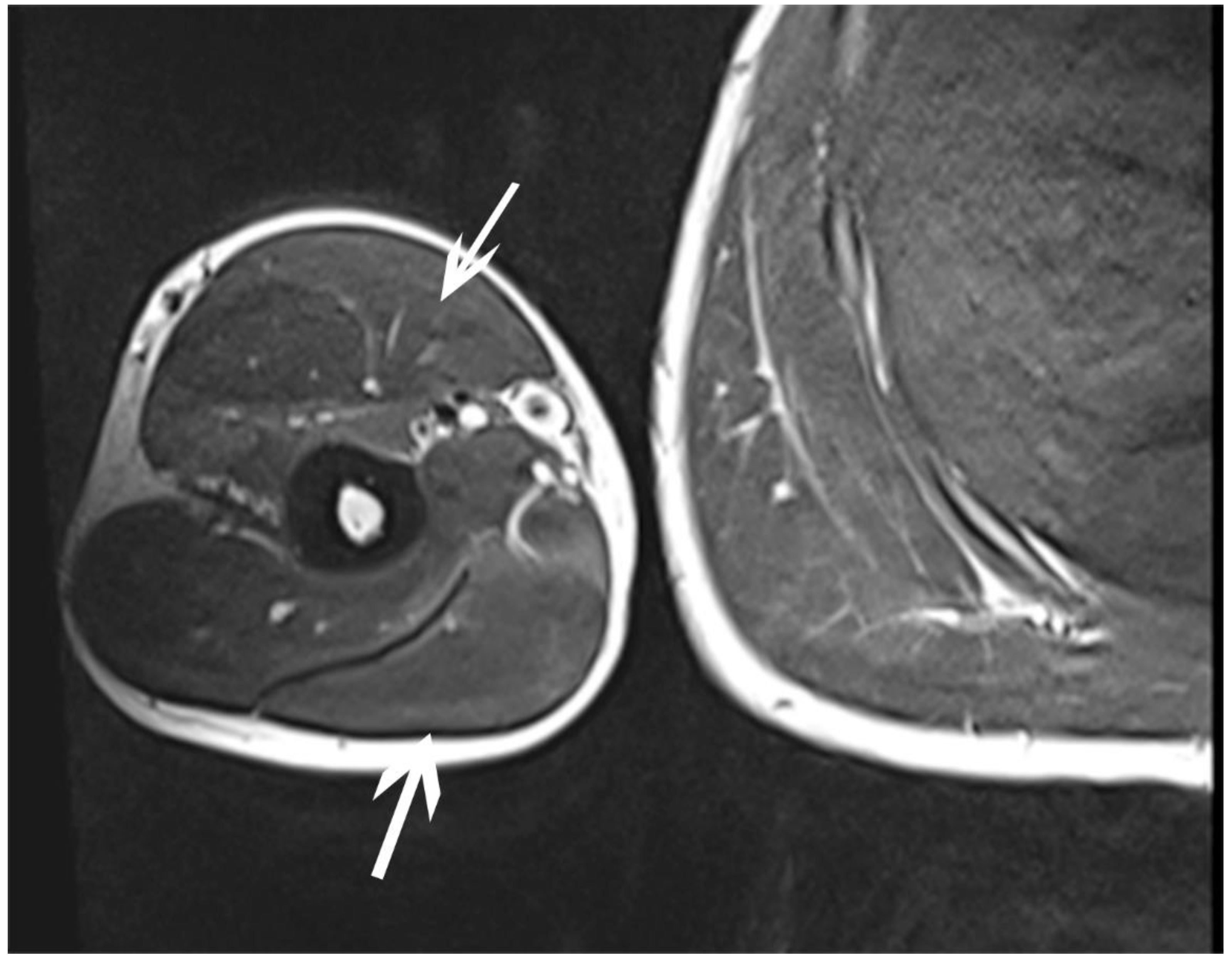
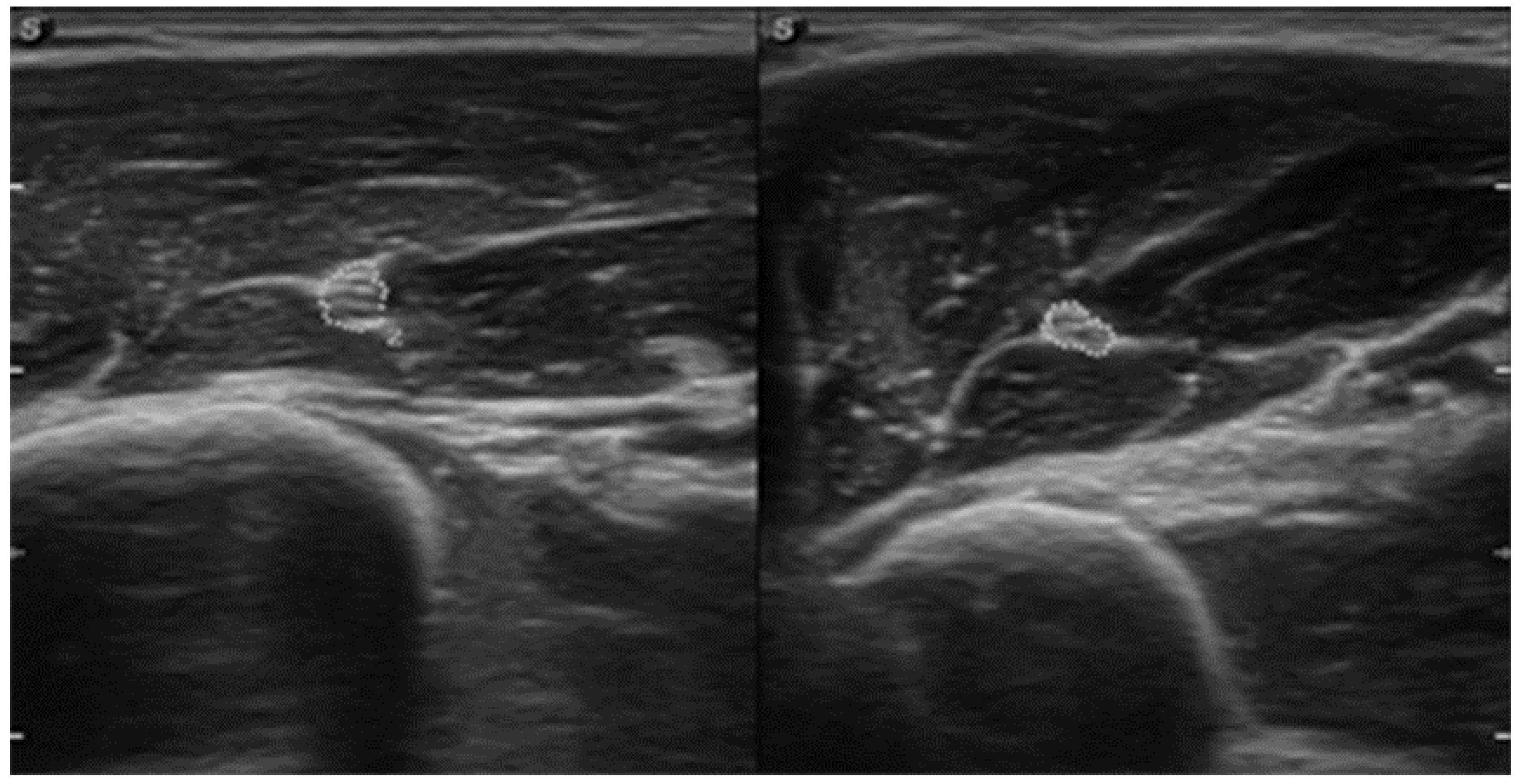
| October | Symptoms: episodic of more severe pain, paraesthesia, tingling, numbness and burning sensations of right upper limb, (pain 3/10 NPRS). Assessment: medical history, physical examination (another orthopaedic surgeon), shoulder (Figure 3) and elbow MRI order (the same orthopaedic surgeon), cervical spine MRI order, electromyography, nerve conduction study (Table 2 and Table 3) (another orthopaedic surgeon), medical history, physical examination, ultrasound (another physiotherapist). Diagnosis: mononeuropathy of the upper limb, unspecified (G56.9 ICD) (orthopaedic surgeon), PTS suggestion (physiotherapist) Treatment: orthopedy surgeon: physiotherapy (massage, muscle relaxation, electrostimulation, exercises); physiotherapist recommendation: neurodynamic techniques. Effects: no positive effect. |
| November | Symptoms: no new symptoms, (pain 3/10 NPRS). Assessment: medical history, physical examination and tests performed to date (neurologist). Diagnosis: confirmation of the diagnosis of PTS. Treatment: neurologist: pharmacological treatment. Effects: No positive therapeutic effect (4/10 NPRS). |
3. Physiotherapy Management
3.1. Baseline Assessment
3.2. Intervention
- Median nerve neurodynamic technique (Supplement Material—Figure S1):
- Neurodynamic sequence: arm adduction to 90°, arm external rotation, wrist and fingers extension, forearm supination, and elbow extension.
- Tension techniques were performed in the proximal and distal directions: 1-direction proximal tension mobilisation (movement—elbow extension—small amplitude of motion at the end of the movement), and 1-direction distal tension mobilisation (movement—wrist extension—small amplitude of motion).
- Ulnar nerve neurodynamic technique (Supplement Material—Figure S2):
- Neurodynamic sequence: arm adduction to 90°, arm internal rotation, wrist and fingers extension, forearm pronation, and elbow extension.
- Tension techniques were performed in the proximal and distal directions: 1-direction proximal tension mobilisation (movement—shoulder depression—small amplitude of motion at the end of the movement), and 1-direction distal tension mobilisation (movement—wrist adduction—small amplitude of motion).
- Radial nerve neurodynamic technique (Supplement Material—Figure S3):
- Neurodynamic sequence: arm extension, arm adduction to 45°, arm internal rotation, wrist and fingers flexion, forearm pronation, and elbow extension.
- Tension techniques were performed in the proximal and distal directions: 1-direction proximal tension mobilisation (movement—elbow extension—small amplitude of motion at the end of the movement), and 1-direction distal tension mobilisation (movement—wrist flexion—small amplitude of motion).
- Musculocutaneus nerve neurodynamic technique (Supplement Material—Figure S4):
- Neurodynamic sequence: arm extension, arm adduction to 45°, arm internal rotation, wrist ulnar deviation and thumb flexion, forearm intermediate, and elbow extension.
- Tension techniques were performed in the proximal and distal directions: 1-direction proximal tension mobilisation (movement—elbow extension—small amplitude of motion at the end of the movement), and 1-direction distal tension mobilisation (movement—wrist ulnar deviation—small amplitude of motion).
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Alfen, N.; van Engelen, B.G. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain 2006, 129, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Kwolek, A.; Majka-Sibiga, M.; Zwolińska, J.; Korab, D. Parsonage-Turner syndrome (neuralgic amyotrophy)—Case report. Przegląd Med. Uniw. Rzesz. Rzesz. 2006, 3, 223–226. [Google Scholar]
- Sathasivam, S.; Lecky, B.; Manohar, R.; Selvan, A. Neuralgic amyotrophy. J. Bone Jt. Surg. Br. 2008, 90, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Tsairis, P.; Dyck, P.J.; Mulder, D.W. Natural history of brachial plexus neuropathy. Report on 99 patients. Arch. Neurol. 1972, 27, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, C.M.; Helms, C.A. Parsonage-Turner syndrome: MR imaging findings and clinical information of 27 patients. Radiology 2006, 240, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Winalski, C.S.; Sundaram, M. Neuralgic amyotrophy (Parsonage Turner syndrome). Orthopedics 2014, 37, 130–133. [Google Scholar] [CrossRef]
- Gstoettner, C.; Mayer, J.A.; Rassam, S.; Hruby, S.A. Neuralgic amyotrophy: A paradigm shift in diagnosis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 879–888. [Google Scholar] [CrossRef]
- Tomczykiewicz, K.; Stępień, A.; Staszewski, J. Personage-Turner syndrome—Case report. Pol. Merkur. Lekarski. 2011, 182, 100–102. [Google Scholar]
- van Alfen, N.; van der Werf, S.P.; van Engelen, B.G. Long-term pain, fatigue, and impairment in neuralgic amyotrophy. Arch Phys. Med. Rehabil. 2009, 90, 435–439. [Google Scholar] [CrossRef]
- Pennella, D.; Giagio, S.; Maselli, F.; Giovannico, G.; Roncone, A.; Fiorentino, F.; Brindisino, F. Red flags useful to screen for gastrointestinal and hepatic diseases in patients with shoulder pain: A scoping review. Musculoskelet. Care 2022, 20, 721–730. [Google Scholar] [CrossRef]
- Brindisino, F.; Passudetti, V.; Pennella, D.; Giovannico, G.; Heick, J.D. Recognition of pulmonary pathology in a patient presenting with shoulder pain. Physiother. Theory Pract. 2022, 38, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Kotevoglu, N.; Gulbahce-Saglam, S. Ultrasound imaging in the diagnosis of carpal tunnel syndrome and its relevance to clinical evaluation. Jt. Bone Spine 2005, 72, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.; Royuela, A.; Perez de Vargas, A.; Lopez, A.; Cepeda, Y.; Martinelli, G. Carpal Tunnel Syndrome: Diagnostic Usefulness of Ultrasound Measurement of the Median Nerve Area and Quantitative Elastographic Measurement of the Median Nerve Stiffness. J. Ultrasound Med. 2020, 39, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Babusiaux, D.; Laulan, J.; Bouilleau, L.; Martin, A.; Adrien, C.; Aubertin, A.; Rabarin, F. Contribution of static and dynamic ultrasound in cubital tunnel syndrome. Orthop. Traumatol. Surg. Res. 2014, 100, 209–212. [Google Scholar] [CrossRef]
- Chang, K.V.; Wu, W.T.; Han, D.-S.; Ozcakar, L. Ulnar Nerve Cross-Sectional Area for the Diagnosis of Cubital Tunnel Syndrome: A Meta-Analysis of Ultrasonographic Measurements. Arch. Phys. Med. Rehabil. 2018, 99, 743–757. [Google Scholar] [CrossRef] [PubMed]
- De Burca, N. Brachial neuritis (Parsonnage-Turner syndrome)—A case study. Man. Ther. 2009, 14, 567–571. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Neurodynamic Techniques Versus “Sham” Therapy in the Treatment of Carpal Tunnel Syndrome: A Randomized Placebo-Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 843–854. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Is manual therapy based on neurodynamic techniques effective in the treatment of carpal tunnel syndrome? A randomized controlled trial. Clin. Rehabil. 2019, 33, 408–417. [Google Scholar]
- Wolny, T.; Linek, P. Long-term patient observation after conservative treatment of carpal tunnel syndrome: A summary of two randomised controlled trials. PeerJ 2019, 7, e8012. [Google Scholar] [CrossRef]
- Oskay, B.; Meriç, A.; Kirdi, N.; Firat, T.; Ayhan, C.; Leblebicioğlu, G. Neurodynamic mobilization in the conservative treatment of cubital tunnel syndrome: Long-term follow-up of 7 cases. J. Manip. Physiol. Ther. 2010, 33, 156–163. [Google Scholar] [CrossRef]
- Jensen, M.P.; Turner, J.A.; Romano, J.M.; Fisher, L.D. Comparative reliability and validity of chronic pain intensity measures. Pain 1999, 83, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wolny, T.; Linek, P.; Michalski, P. Inter-rater reliability of two-point discrimination in acute stroke patients. NeuroRehabilitation 2017, 41, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wolny, T.; Linek, P. Reliability of two-point discrimination test in carpal tunnel syndrome patients. Physiother. Theory Pract. 2019, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Katz, J.N.; Fossel, A.H.; Wright, J.G.; Tarasuk, V.; Bombardier, C. Measuring the Whole or the Parts? Validity, Reliability, and Responsiveness of the Disabilities of the Arm, Shoulder and Hand Outcome Measure in Different Regions of the Upper Extremity. J. Hand Ther. 2001, 14, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Jerath, V.P.; Mahajan, V.K. Parsonage-Turner syndrome: A firsthand experience of an uncommon malady. Am. J. Neurodegener. Dis. 2021, 10, 34–37. [Google Scholar]
- Rix, G.D.; Rothman, D.C.; Robinson, A. Idiopathic neuralgic amyotrophy: An illustrative case report. J. Manip. Physiol. Ther. 2006, 29, 52–59. [Google Scholar] [CrossRef]
- Pessa, M.E.; Verriello, L.; Valente, M.; Gigli, G.L. A rare case of pure sensitive Parsonage-Turner syndrome. Neurol. Sci. 2019, 40, 1499–1501. [Google Scholar] [CrossRef]
- Van Eijk, J.J.J.; Groothuis, J.T.; Van Alfen, N. Neuralgic amyotrophy: An update on diagnosis, pathophysiology, and treatment: Neuralgic amyotrophy update. Muscle Nerve 2016, 53, 337–350. [Google Scholar] [CrossRef]
- Dill-Macky, M.J.; Song, S.; Silbert, P.L. Magnetic resonance imaging features of subacute idiopathic brachial neuritis. Australas. Radiol. 2000, 44, 98–100. [Google Scholar] [CrossRef]
- Lieba-Samal, D.; Jengojan, S.; Kasprian, G.; Wöber, C.; Bodner, G. Neuroimaging of classic neuralgic amyotrophy: Imaging of neuralgic amyotrophy. Muscle Nerve 2016, 54, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Alexander, M.; Kane, D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology 2015, 54, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.W. Diagnosis of carpal tunnel syndrome. The gold standard. Am. J. Phys. Med. Rehabil. 1993, 72, 1. [Google Scholar] [CrossRef] [PubMed]
- Duman, I.; Guvenc, I.; Kalyon, T.A. Neuralgic amyotrophy, diagnosed with magnetic resonance neurography in acute stage: A case report and review of the literaturę. Neurologist 2007, 13, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Manzano, G.; Cancela-Cilleruelo, I.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Thoomes-de-Graaf, M.; Ortega-Santiago, R. Effects of Adding a Neurodynamic Mobilization to Motor Control Training in Patients with Lumbar Radiculopathy Due to Disc Herniation: A Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 124–132. [Google Scholar] [CrossRef]
- Cabrera-Martos, I.; Rodríguez-Torres, J.; López-López, L.; Prados-Román, E.; Granados-Santiago, M.; Valenza, M.C. Effects of an active intervention based on myofascial release and neurodynamics in patients with chronic neck pain: A randomized controlled trial. Physiother. Theory Pract. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, R.; Krivitsky, M.; Nicola, M.; Zarour, C. Atypical Presentation of Parsonage-Turner Syndrome. Cureus 2020, 12, e8892. [Google Scholar] [CrossRef]
| Test | Stimulated Points | Latency [ms] | Amplitude [mV] | Duration [ms] | Area [mV × ms] | Stimulation [mA] | Stimulation [ms] | Distance [mm] | Time [ms] | Velocity [m/s] |
|---|---|---|---|---|---|---|---|---|---|---|
| Right, Musculus biceps brachii, nervus musculocutaneus, C5-C6 | ||||||||||
| 4 | Erb’s point | 4.3 | 14.1 | 6.2 | 43.3 | 60 | 0.1 | 250 | - | - |
| Right, Musculus brachialis, nervus musculocutaneus, C5-C6 | ||||||||||
| 6 | 1 | 5.0 | 14.5 | 10.5 | 37.2 | 60 | 0.1 | 240 | - | - |
| Right, Musculus Triceps brachii, nervus radialis, C6-Th1 | ||||||||||
| 5 | 1 | 4.6 | 60 | 0.1 | 250 | - | - | |||
| Right, Musculus Abductor digiti minimi, nervus ulnaris, C6-Th1 | ||||||||||
| 2 | Wrist | 2.5 | 7.0 | 6.45 | 20.9 | 50 | 0.1 | 55 | - | - |
| Elbow | 7.2 | 6.6 | 6.7 | 23.5 | 50 | 0.1 | 280 | 4.7 | 59.6 | |
| Min. | Max. | Mean | Disparity | Disp. Factor [%] | |
|---|---|---|---|---|---|
| Lat. [ms] | 23.8 | 26.6 | 25.0 | 2.78 | 11.1 |
| Ampl. F [µV] | 121 | 313 | 218 | 192 | 88.2 |
| Ampl. F/M [%] | 0.804 | 2.09 | 1.45 | ||
| V prox. [m/s] | 55.6 | 65.7 | 61.7 | 10.2 | 16.6 |
| Examination | Pre-Therapy | Post-Therapy | |
|---|---|---|---|
| Pain | permanent | 3 | 0 |
| after exercise | 6 | 0 | |
| 2PD | affected | 33.33 mm | 22.43 mm |
| non-affected | 20.98 mm | 21.44 mm | |
| CSA | affected | 0.09 cm2 | 0.06 cm2 |
| non-affected | 0.06 cm2 | 0.06 cm2 | |
| THss BM | affected | 11.61 mm | 11.77 mm |
| non-affected | 18.79 mm | 18.74 mm | |
| DASH | 76 pkt. | 43 pkt. | |
| DASH “sport” | 16 pkt. | 8 pkt. | |
| SF-36 | PF | 75% | 85% |
| RF | 25% | 75% | |
| BP | 45% | 67.5% | |
| GH | 45.8% | 58.3% | |
| VT | 50% | 70% | |
| SF | 87.5% | 100% | |
| RE | 66.6% | 100% | |
| MH | 68% | 80% | |
| PCS | 47.7% | 71.4% | |
| MCS | 68% | 87.5% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolny, T.; Glibov, K.; Granek, A.; Linek, P. Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage–Turner Syndrome—A Case Report. Sensors 2023, 23, 501. https://doi.org/10.3390/s23010501
Wolny T, Glibov K, Granek A, Linek P. Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage–Turner Syndrome—A Case Report. Sensors. 2023; 23(1):501. https://doi.org/10.3390/s23010501
Chicago/Turabian StyleWolny, Tomasz, Katarzyna Glibov, Arkadiusz Granek, and Paweł Linek. 2023. "Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage–Turner Syndrome—A Case Report" Sensors 23, no. 1: 501. https://doi.org/10.3390/s23010501
APA StyleWolny, T., Glibov, K., Granek, A., & Linek, P. (2023). Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage–Turner Syndrome—A Case Report. Sensors, 23(1), 501. https://doi.org/10.3390/s23010501







