Step-Counting Accuracy of a Commercial Smartwatch in Mild-to-Moderate PD Patients and Effect of Spatiotemporal Gait Parameters, Laterality of Symptoms, Pharmacological State, and Clinical Variables
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Accuracy of GV4 vs. Manual Step Counting
4.2. Effect of Side and Pharmacological State
4.3. Association of Step-Count Error, Spatiotemporal Gait Parameters, and Clinical Variables
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacKinnon, C.D. Sensorimotor Anatomy of Gait, Balance, and Falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Toth, L.P.; LaMunion, S.R.; Crouter, S.E. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sport. Med. 2017, 47, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo Cruz, B.; Ahmadi, M.N.; Lee, I.-M.; Stamatakis, E. Prospective Associations of Daily Step Counts and Intensity With Cancer and Cardiovascular Disease Incidence and Mortality and All-Cause Mortality. JAMA Intern. Med. 2022, 182, 1139. [Google Scholar] [CrossRef]
- Master, H.; Annis, J.; Huang, S.; Beckman, J.A.; Ratsimbazafy, F.; Marginean, K.; Carroll, R.; Natarajan, K.; Harrell, F.E.; Roden, D.M.; et al. Association of Step Counts over Time with the Risk of Chronic Disease in the All of Us Research Program. Nat. Med. 2022, 28, 2301–2308. [Google Scholar] [CrossRef]
- Paluch, A.E.; Gabriel, K.P.; Fulton, J.E.; Lewis, C.E.; Schreiner, P.J.; Sternfeld, B.; Sidney, S.; Siddique, J.; Whitaker, K.M.; Carnethon, M.R. Steps per Day and All-Cause Mortality in Middle-Aged Adults in the Coronary Artery Risk Development in Young Adults Study. AMA Netw. Open 2021, 4, e2124516. [Google Scholar] [CrossRef]
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.-M.; et al. Daily Steps and All-Cause Mortality: A Meta-Analysis of 15 International Cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef]
- Del Pozo Cruz, B.; Ahmadi, M.; Naismith, S.L.; Stamatakis, E. Association of Daily Step Count and Intensity With Incident Dementia in 78 430 Adults Living in the UK. JAMA Neurol. 2022, 79, 1059–1063. [Google Scholar] [CrossRef]
- Straiton, N.; Alharbi, M.; Bauman, A.; Neubeck, L.; Gullick, J.; Bhindi, R.; Gallagher, R. The Validity and Reliability of Consumer-Grade Activity Trackers in Older, Community-Dwelling Adults: A Systematic Review. Maturitas 2018, 112, 85–93. [Google Scholar] [CrossRef]
- Degroote, L.; De Bourdeaudhuij, I.; Verloigne, M.; Poppe, L.; Crombez, G. The Accuracy of Smart Devices for Measuring Physical Activity in Daily Life: Validation Study. JMIR Mhealth Uhealth 2018, 6, e10972. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Canning, C.G.; Hausdorff, J.M.; Lord, S.; Rochester, L. Falls in Parkinson’s Disease: A Complex and Evolving Picture. Mov. Disord. 2017, 32, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive Decline in Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The Role of Executive Function and Attention in Gait. Mov. Disord. 2008, 23, 329–342, quiz 472. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Welzel, J.; Wendtland, D.; Warmerdam, E.; Romijnders, R.; Elshehabi, M.; Geritz, J.; Berg, D.; Hansen, C.; Maetzler, W. Step Length Is a Promising Progression Marker in Parkinson’s Disease. Sensors 2021, 21, 2292. [Google Scholar] [CrossRef]
- Wilson, J.; Alcock, L.; Yarnall, A.J.; Lord, S.; Lawson, R.A.; Morris, R.; Taylor, J.-P.; Burn, D.J.; Rochester, L.; Galna, B. Gait Progression Over 6 Years in Parkinson’s Disease: Effects of Age, Medication, and Pathology. Front. Aging Neurosci. 2020, 12, 577435. [Google Scholar] [CrossRef]
- Varghese, J.; van Alen, C.M.; Fujarski, M.; Schlake, G.S.; Sucker, J.; Warnecke, T.; Thomas, C. Sensor Validation and Diagnostic Potential of Smartwatches in Movement Disorders. Sensors 2021, 21, 3139. [Google Scholar] [CrossRef]
- Skidmore, F.M.; Mackman, C.A.; Pav, B.; Shulman, L.M.; Garvan, C.; Macko, R.F.; Heilman, K.M. Daily Ambulatory Activity Levels in Idiopathic Parkinson Disease. J. Rehabil. Res. Dev. 2008, 45, 1343–1348. [Google Scholar] [CrossRef]
- Christiansen, C.; Moore, C.; Schenkman, M.; Kluger, B.; Kohrt, W.; Delitto, A.; Berman, B.; Hall, D.; Josbeno, D.; Poon, C.; et al. Factors Associated With Ambulatory Activity in De Novo Parkinson Disease. J. Neurol. Phys. 2017, 41, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Ellis, T.D.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Capturing Ambulatory Activity Decline in Parkinson’s Disease. J. Neurol. Phys. 2012, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Handlery, R.; Stewart, J.C.; Pellegrini, C.; Monroe, C.; Hainline, G.; Flach, A.; Handlery, K.; Fritz, S. Physical Activity in De Novo Parkinson Disease: Daily Step Recommendation and Effects of Treadmill Exercise on Physical Activity. Phys. Ther. 2021, 101, pzab174. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Sasaki, J.E.; Jeng, B.; Cederberg, K.L.; Bamman, M.M.; Motl, R.W. Accuracy and Precision of Three Consumer-Grade Motion Sensors During Overground and Treadmill Walking in People With Parkinson Disease: Cross-Sectional Comparative Study. JMIR Rehabil. Assist. Technol. 2020, 7, e14059. [Google Scholar] [CrossRef]
- Lamont, R.M.; Daniel, H.L.; Payne, C.L.; Brauer, S.G. Accuracy of Wearable Physical Activity Trackers in People with Parkinson’s Disease. Gait Posture 2018, 63, 104–108. [Google Scholar] [CrossRef]
- Wendel, N.; Macpherson, C.E.; Webber, K.; Hendron, K.; DeAngelis, T.; Colon-Semenza, C.; Ellis, T. Accuracy of Activity Trackers in Parkinson Disease: Should We Prescribe Them? Phys. Ther. 2018, 98, 705–714. [Google Scholar] [CrossRef]
- Fokkema, T.; Kooiman, T.J.M.; Krijnen, W.P.; VAN DER Schans, C.P.; DE Groot, M. Reliability and Validity of Ten Consumer Activity Trackers Depend on Walking Speed. Med. Sci. Sport. Exerc. 2017, 49, 793–800. [Google Scholar] [CrossRef]
- Svarre, F.R.; Jensen, M.M.; Nielsen, J.; Villumsen, M. The Validity of Activity Trackers Is Affected by Walking Speed: The Criterion Validity of Garmin Vivosmart® HR and StepWatchTM 3 for Measuring Steps at Various Walking Speeds under Controlled Conditions. PeerJ 2020, 8, e9381. [Google Scholar] [CrossRef]
- Chow, J.J.; Thom, J.M.; Wewege, M.A.; Ward, R.E.; Parmenter, B.J. Accuracy of Step Count Measured by Physical Activity Monitors: The Effect of Gait Speed and Anatomical Placement Site. Gait Posture 2017, 57, 199–203. [Google Scholar] [CrossRef]
- Kim, D.W.; Hassett, L.M.; Nguy, V.; Allen, N.E. A Comparison of Activity Monitor Data from Devices Worn on the Wrist and the Waist in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2019, 6, 693–699. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression and Mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Peto, V.; Jenkinson, C.; Fitzpatrick, R.; Greenhall, R. The Development and Validation of a Short Measure of Functioning and Well Being for Individuals with Parkinson’s Disease. Qual. Life Res. 1995, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The Functional Independence Measure: A New Tool for Rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Vítečková, S.; Horáková, H.; Poláková, K.; Krupička, R.; Růžička, E.; Brožová, H. Agreement between the GAITRite® System and the Wearable Sensor BTS G-Walk® for Measurement of Gait Parameters in Healthy Adults and Parkinson’s Disease Patients. PeerJ 2020, 8, e8835. [Google Scholar] [CrossRef]
- CTA 2056-2016 (ANSI)—Physical Activity Monitoring for Fitness Wearables: Step Counting. Available online: https://webstore.ansi.org/Standards/ANSI/cta20562016ansi (accessed on 19 October 2022).
- Evenson, K.R.; Spade, C.L. Review of Validity and Reliability of Garmin Activity Trackers. J. Meas Phys. Behav. 2020, 3, 170–185. [Google Scholar] [CrossRef]
- Tk, K.; My, L. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Godfrey, A.; Morris, R.; Hickey, A.; Del Din, S. Beyond the Front End: Investigating a Thigh Worn Accelerometer Device for Step Count and Bout Detection in Parkinson’s Disease. Med. Eng. Phys. 2016, 38, 1524–1529. [Google Scholar] [CrossRef]
- Miller-Patterson, C.; Buesa, R.; McLaughlin, N.; Jones, R.; Akbar, U.; Friedman, J.H. Motor Asymmetry over Time in Parkinson’s Disease. J. Neurol. Sci. 2018, 393, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, K.L.J.; Jeng, B.; Sasaki, J.E.; Lai, B.; Bamman, M.; Motl, R.W. Accuracy and Precision of Wrist-Worn Actigraphy for Measuring Steps Taken during over-Ground and Treadmill Walking in Adults with Parkinson’s Disease. Park. Relat. Disord. 2021, 88, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-D.; Kuo, C.-C.; Pellegrini, C.A.; Hsu, M.-J. Accuracy of Wristband Activity Monitors during Ambulation and Activities. Med. Sci. Sport. Exerc. 2016, 48, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.R. Epidemiology, Diagnosis and Differential Diagnosis in Parkinson’s Disease Tremor. Park. Relat. Disord. 2012, 18 (Suppl. S1), S90–S92. [Google Scholar] [CrossRef]
- Warmerdam, E.; Romijnders, R.; Hansen, C.; Elshehabi, M.; Zimmermann, M.; Metzger, F.G.; von Thaler, A.-K.; Berg, D.; Schmidt, G.; Maetzler, W. Arm Swing Responsiveness to Dopaminergic Medication in Parkinson’s Disease Depends on Task Complexity. NPJ Park. Dis. 2021, 7, 89. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Morris, R.; Martini, D.N.; Smulders, K.; Kelly, V.E.; Zabetian, C.P.; Poston, K.; Hiller, A.; Chung, K.A.; Yang, L.; Hu, S.-C.; et al. Cognitive Associations with Comprehensive Gait and Static Balance Measures in Parkinson’s Disease. Park. Relat. Disord. 2019, 69, 104–110. [Google Scholar] [CrossRef]
- Geritz, J.; Welzel, J.; Hansen, C.; Maetzler, C.; Hobert, M.A.; Elshehabi, M.; Sobczak, A.; Kudelka, J.; Stiel, C.; Hieke, J.; et al. Does Executive Function Influence Walking in Acutely Hospitalized Patients With Advanced Parkinson’s Disease: A Quantitative Analysis. Front. Neurol. 2022, 13, 852725. [Google Scholar] [CrossRef]
- Hillel, I.; Gazit, E.; Nieuwboer, A.; Avanzino, L.; Rochester, L.; Cereatti, A.; Croce, U.D.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; et al. Is Every-Day Walking in Older Adults More Analogous to Dual-Task Walking or to Usual Walking? Elucidating the Gaps between Gait Performance in the Lab and during 24/7 Monitoring. Eur. Rev. Aging Phys. Act. 2019, 16, 6. [Google Scholar] [CrossRef]

| PD (N = 47) | HS (N = 47) | |
|---|---|---|
| Age (years) | 66.3 ± 8.2 | 42.0 ± 15.3 |
| Females | 14 (33%) | 33 (63%) |
| Weight (kg) | 77.6 ± 12.2 | 71.0 ± 13.8 |
| Height (cm) | 173.9 ± 8.7 | 170.6 ± 7.6 |
| BMI | 25.6 ± 2.9 | 24.4 ± 4.3 |
| H&Y | 2 (2–2) | - |
| Disease duration (years) | 6.1 ± 5.1 | - |
| LEDD (mg) | 576 ± 317 | - |
| HS-SE | HS-SL | PD | PD ON | PD OFF | |
|---|---|---|---|---|---|
| ICC [1,2] | 0.901 (0.856–0.933) | 0.686 (0.535–0.789) | 0.658 (0.306–0.825) | 0.749 (0.407–0.881) | 0.305 (0.039–0.535) |
| ICC [1,2] (MA) | - | - | 0.644 (0.247–0.824) | 0.719 (0.424–0.856) | 0.184 (−0.085–0.434) |
| ICC [1,2] (LA) | - | - | 0.632 (0.344–0.795) | 0.755 (0.392–0.887) | 0.328 (0.042–0.563) |
| HS-SE | HS-SL | PD | PD ON | PD OFF | HS-SE vs. PD | ON vs. OFF | |
|---|---|---|---|---|---|---|---|
| MAPE | 2.38 (1.80–2.95) | 8.07 (4.97–11.18) | 6.26 (4.48–8.04) | 5.53 (3.91–7.16) | 7.14 (4.68–9.61)) | p < 0.001 | p = 0.047 |
| MAPE (MA) | - | - | 6.78 (5.06–8.50) | 6.04 (4.42–7.66) | 7.54 (5.05–10.02) | NS | |
| MAPE (LA) | - | - | 5.57 (3.54–7.60) | 5.03 (3.28–6.78) | 6.96 (4.24–9.69) | p = 0.018 | |
| MA vs. LA | - | - | p = 0.029 | NS | NS | ||
| MPE | −0.46 ± 2.98 | 4.38 ± 11.60 | −4.67 ± 7.01 | −4.39 ± 6.48 | −4.74 ± 9.40 | ||
| MPE (MA) | - | - | −5.16 ± 7.27 | −4.07 ± 7.02 | −5.78 ± 9.78 | ||
| MPE (LA) | - | - | −4.32 ± 7.59 | −4.48 ± 6.40 | −3.93 ± 10.96 | ||
| MA vs. LA | - | - | NS | NS | NS |
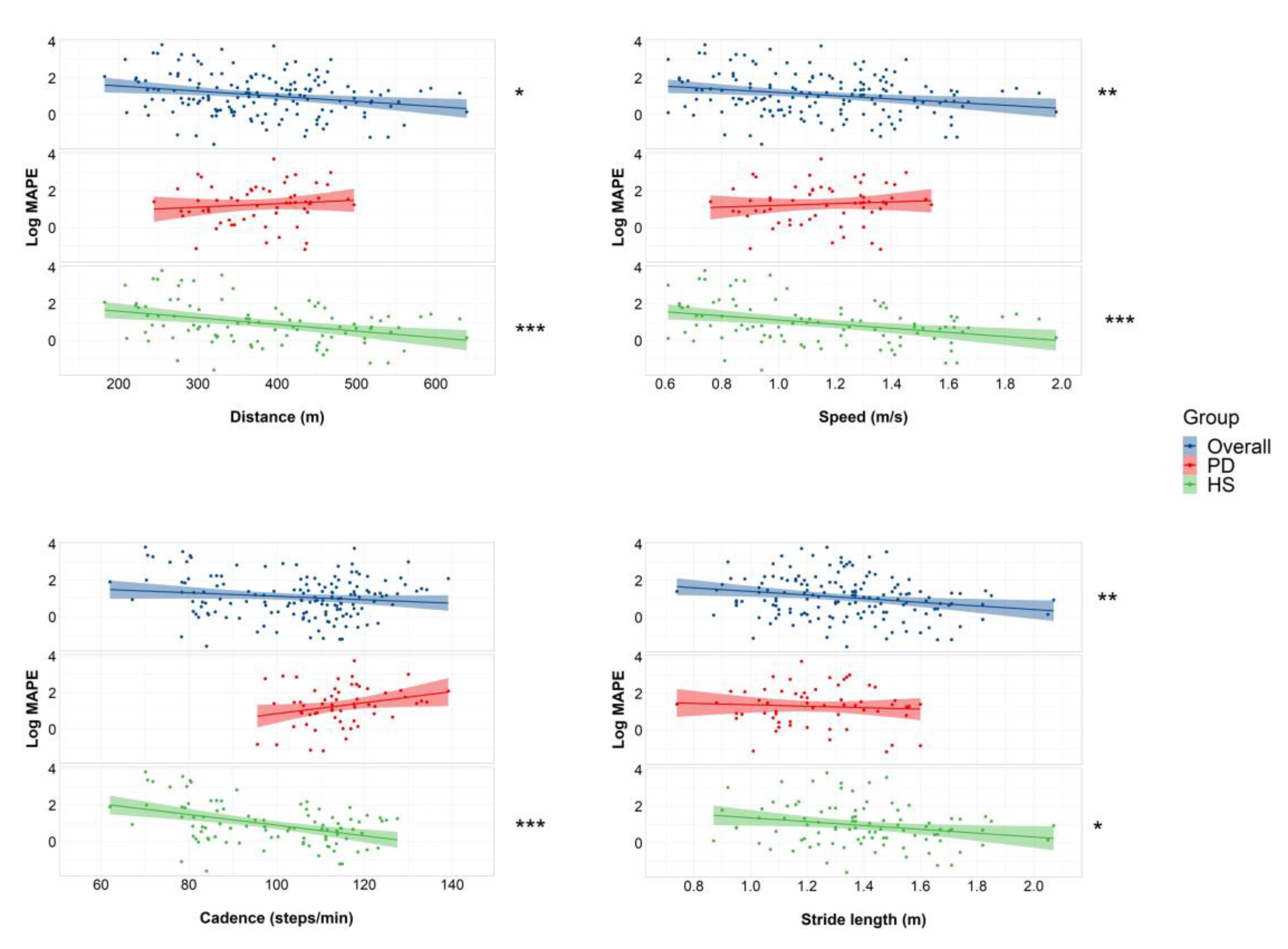
| Distance (m) | Speed (m/s) | Cadence (Steps/min) | Stride Length (m) | ||
|---|---|---|---|---|---|
| MAPE | Overall | −0.199 * | −0.213 ** | −0.048 | −0.224 ** |
| PD | 0.169 | 0.106 | 0.256 | −0.018 | |
| HS | −0.345 *** | −0.341 *** | −0.367 *** | −0.230 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, E.; Caliò, B.; Alborghetti, M.; Rinaldi, D.; Hansen, C.; Vuillerme, N.; Maetzler, W.; Pontieri, F.E. Step-Counting Accuracy of a Commercial Smartwatch in Mild-to-Moderate PD Patients and Effect of Spatiotemporal Gait Parameters, Laterality of Symptoms, Pharmacological State, and Clinical Variables. Sensors 2023, 23, 214. https://doi.org/10.3390/s23010214
Bianchini E, Caliò B, Alborghetti M, Rinaldi D, Hansen C, Vuillerme N, Maetzler W, Pontieri FE. Step-Counting Accuracy of a Commercial Smartwatch in Mild-to-Moderate PD Patients and Effect of Spatiotemporal Gait Parameters, Laterality of Symptoms, Pharmacological State, and Clinical Variables. Sensors. 2023; 23(1):214. https://doi.org/10.3390/s23010214
Chicago/Turabian StyleBianchini, Edoardo, Bianca Caliò, Marika Alborghetti, Domiziana Rinaldi, Clint Hansen, Nicolas Vuillerme, Walter Maetzler, and Francesco E. Pontieri. 2023. "Step-Counting Accuracy of a Commercial Smartwatch in Mild-to-Moderate PD Patients and Effect of Spatiotemporal Gait Parameters, Laterality of Symptoms, Pharmacological State, and Clinical Variables" Sensors 23, no. 1: 214. https://doi.org/10.3390/s23010214
APA StyleBianchini, E., Caliò, B., Alborghetti, M., Rinaldi, D., Hansen, C., Vuillerme, N., Maetzler, W., & Pontieri, F. E. (2023). Step-Counting Accuracy of a Commercial Smartwatch in Mild-to-Moderate PD Patients and Effect of Spatiotemporal Gait Parameters, Laterality of Symptoms, Pharmacological State, and Clinical Variables. Sensors, 23(1), 214. https://doi.org/10.3390/s23010214









