Comparison of Diagnostic Test Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis for Breast Cancer: A Systematic Review and Meta-Analysis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Collection Process
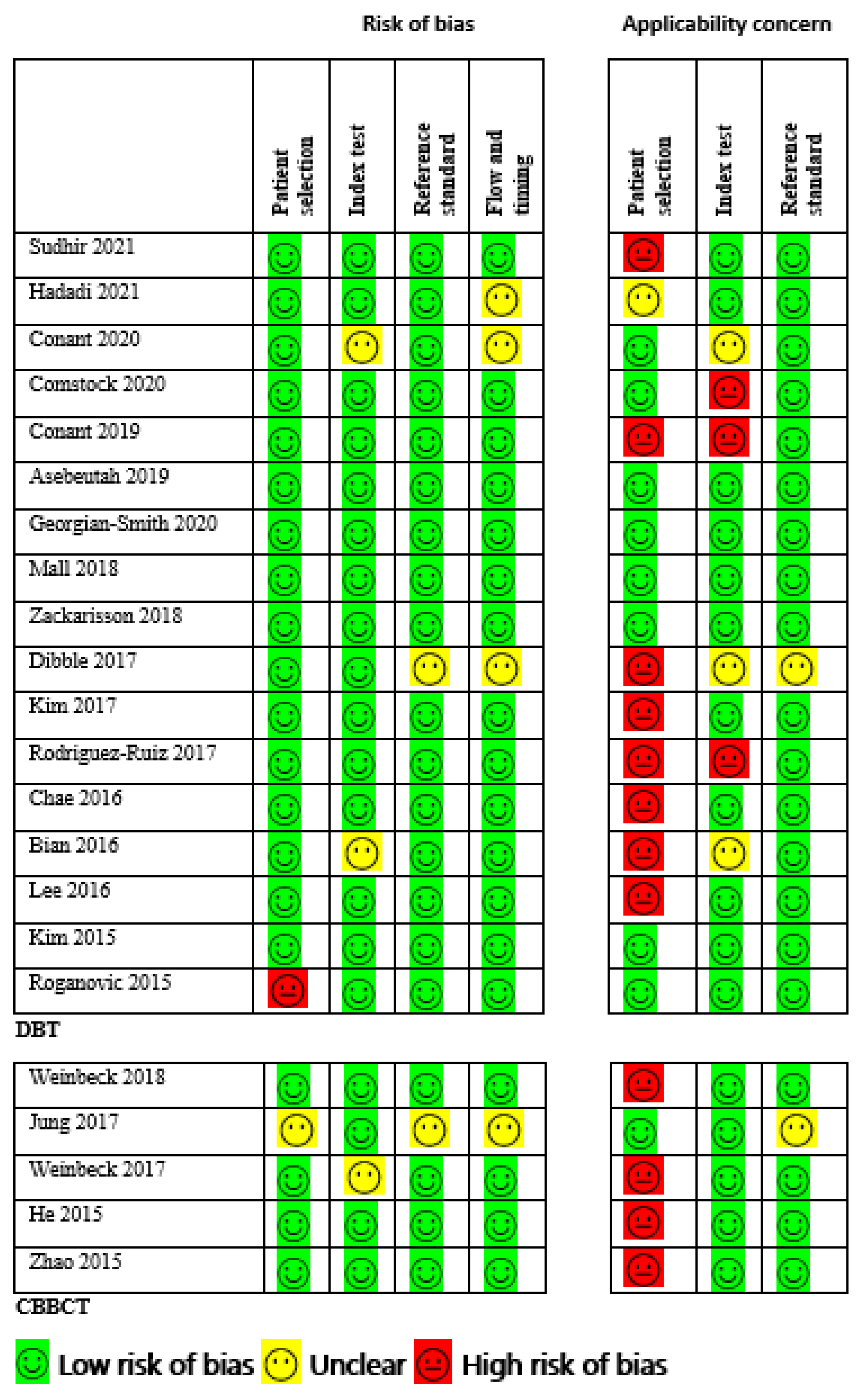
2.5. Risk of Bias and Quality Appraisal
2.6. Data Analysis
3. Results
3.1. Study Inclusion
3.2. Overview of Included Studies
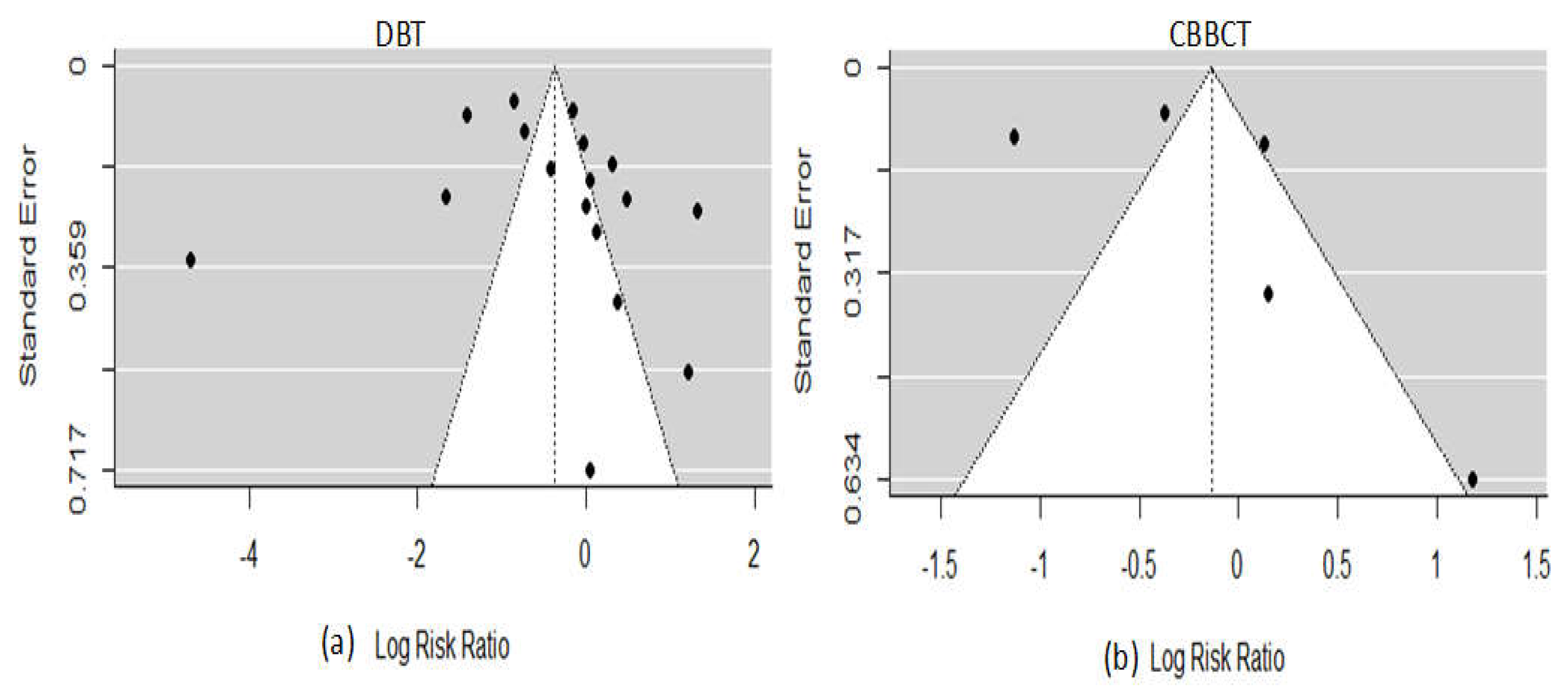
3.3. Quality Assessment and Publication Bias
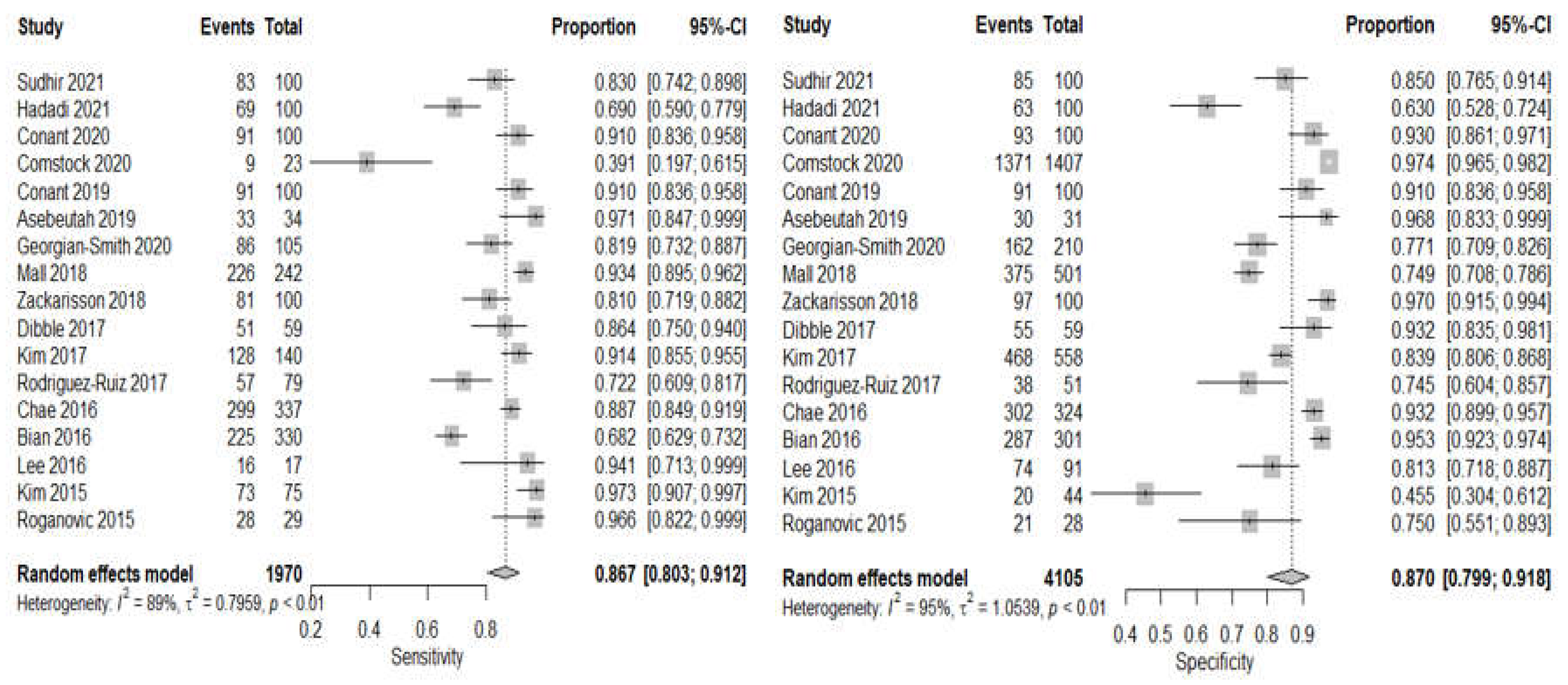
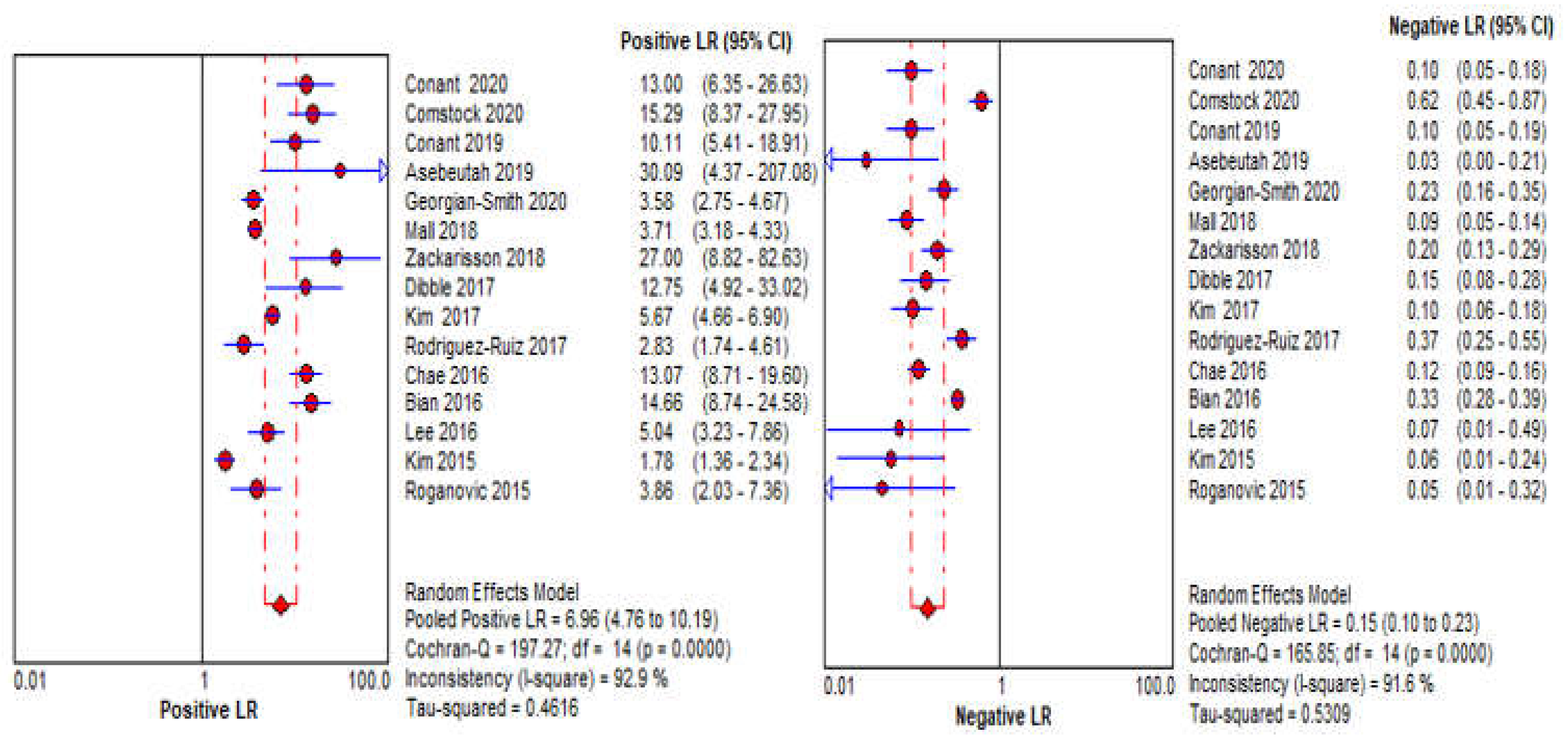
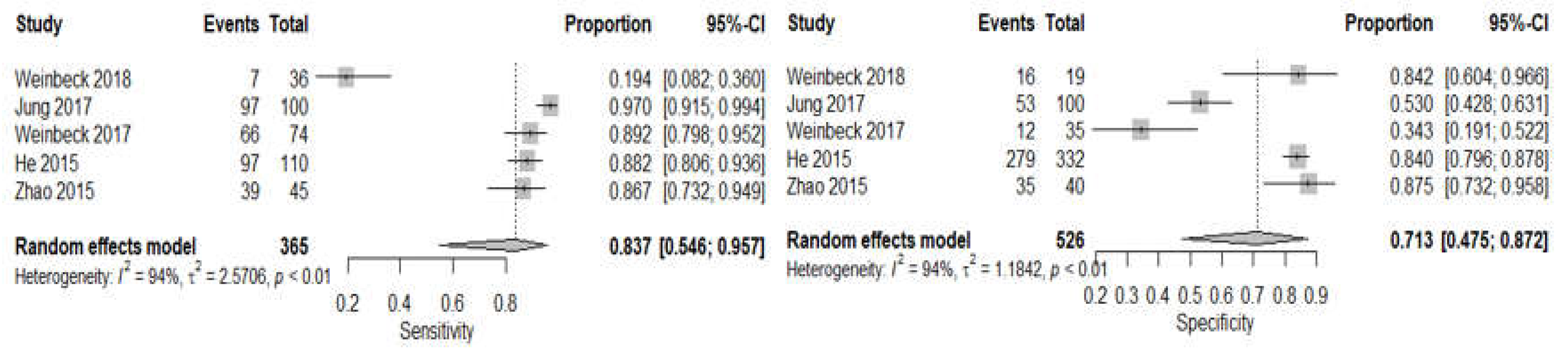
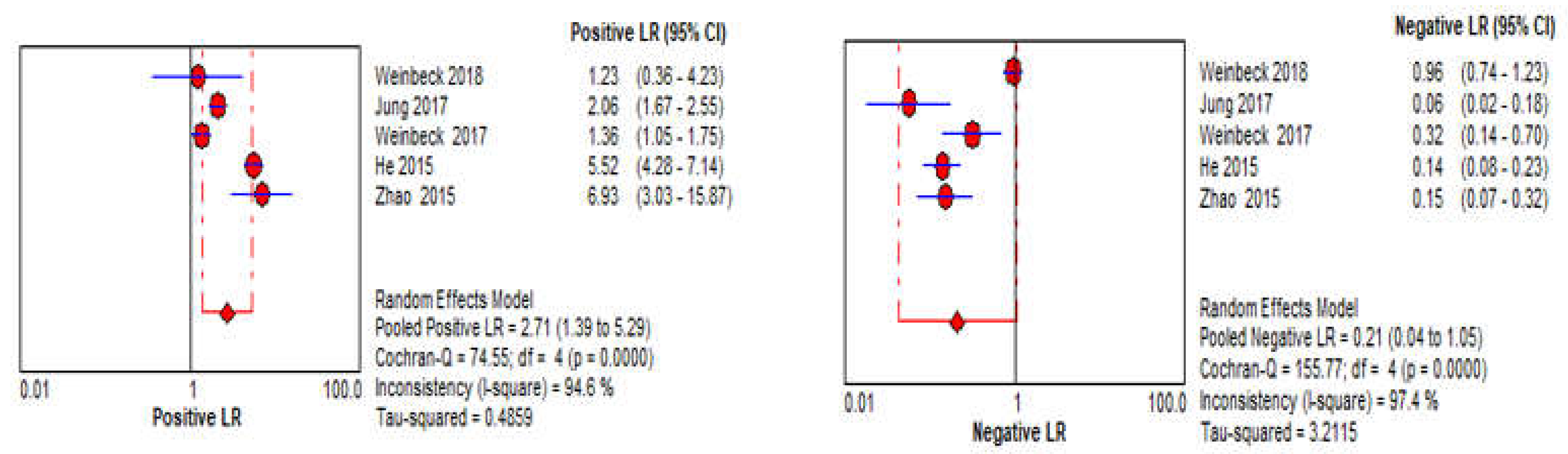
3.4. DBT Meta-Analysis
3.5. CBBCT Meta-Analysis
| Study | Country | Equipment | Total No. of Patients | (Mean Age ± SD) Years | No. of Radiol. (Mean Years) | Gland. Dose (mGy) | Sens. | Specf. | Benign Cases (%) | Malig. Cases (%) | Study Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Digital Breast Tomosynthesis | |||||||||||
| Sudhir et al. [50] | India | N/A | 130 | 45 ± 12 | 2 (N/A) | N/A | 82.8/100 | 84.8/100 | N/A | N/A | DM vs. DBT vs. US+DBT vs. CEDM a |
| Hadadi et al. [51] | Australia | N/A | 35 | N/A | 7 (2) | N/A | 69/100 | 63/100 | N/A | N/A | DBT vs. DM a |
| Conant et al. [34] | USA | Hologic Selenia Dimensions | 56839 | 54 ± NA | N/A | N/A | 91.2/100 | 92.6/100 | N/A | N/A | DBTvs. DM a |
| Comstock al. [35] | USA/Germany | N/A | 1444 | 54.9 ± 0.85 | 2 (N/A) | N/A | 9/23 | 1371/1407 | 0.6 | 99.4 | One-view DBT vs. DM b |
| Conant et al. [36] | USA | Hologic Selenia Dimensions | 50971 | 54.6 ± 8.9 | 13 (N/A) | N/A | 90.6/100 | 91.3/100 | N/A | N/A | DBT vs. DM b,e |
| Asbeutah et al. [37] | Kuwait | GE Senographe Essential | 58 | 54.3 ±12.6 | 1 (>10) | N/A | 33/34 | 30/31 | 47.7 | 52.3 | DBT vs. DM b,f |
| Georgian-Smith et al. [38] | USA | Siemens Mammomat Inspiration system | 330 | 56.3 ± 9.8 | 31 (4–38) | N/A | 86/105 | 162/210 | 63.6 | 31.8 | DBT vs. DM b,e |
| Mall et al. [10] | Australia | Hologic Selenia Dimensions | 144 | N/A | 15 (16) | N/A | 226/242 | 375/501 | 66.7 | 33.3 | DBT vs. DM b,d |
| Zackrisson et al. [39] | Sweden | Siemens Mammomat Inspiration system | 14848 | 57.0 ± 10.0 | 7 (2–14) | 2.30 | 81.1/100 | 97.2/100 | N/A | N/A | DBT vs. DM b,d,f |
| Dibble et al. [40] | USA | Hologic Selenia Dimensions | 59 | 58.9 ± N/A | 3 (6–16) | N/A | 51/59 | 55/59 | N/A | N/A | DBT vs. DM a |
| Kim et al. [41] | Korea | Hologic Selenia Dimensions | 698 | 48.7 ± 11.2 | 12 (9.3) | 1.30 | 128/140 | 468/558 | 79.9 | 20.1 | DBT vs. US b,f |
| Rodriguez-Ruiz et al. [42] | Netherlands | N/A | 181 | 52 ± N/A | 6 (23) | 2.41 | 57/79 | 38/51 | 39.2 | 60.8 | DBT vs. DM a,f |
| Chae et al. [43] | Korea | GE Senographe Essential | 319 | 49.0 ± N/A | 3 (8–18) | N/A | 299/337 | 302/324 | 11.1 | 88.9 | DBT vs. DM b,e |
| Bian et al. [44] | China | Hologic Selenia Dimensions | 631 | 45.0 ± N/A | 3 (3–20) | N/A | 225/330 | 287/301 | 47.7 | 52.3 | DBT vs. DM a |
| Lee et al. [45] | Korea | Hologic Selenia Dimensions | 108 | 46.3 ± 7.8 | 3 (N/A) | 1.50 | 17/17 | 74/91 | 84.3 | 15.7 | DBT vs. US a,f |
| Kim et al. [46] | Korea | Hologic Selenia Dimensions | 113 | 49.6 ± N/A | 3 (>13) | N/A | 73/75 | 20/44 | 37.0 | 63.0 | DBT vs. US a,f |
| Roganovic et al. [47] | Bosnia and Herzegovina | Hologic Selenia Dimensions | N/A | 53.2 ± N/A | 1(10) | 2.3 | 29/29 | 21/28 | 49.1 | 50.9 | DBT vs. DM vs. MRI b,f |
| Cone-Beam Breast Computed Tomography | |||||||||||
| Weinbeck et al. [12] | Germany | Koning (CBCT 1000) Breast CT | 41 | 67.8 ± N/A | 2 (>7) | 5.85-7.5 | 7/36 | 16/19 | 43.0 | 51.0 | CBBCT vs. MRI vs. DM a,e |
| Jung et al. [48] | N/A | N/A | 30 | 30 ± N/A | 4 (7) | N/A | 97/100 | 53/100 | 76.5 | 23.5 | CBBCT a,c |
| Weinbeck et al. [11] | Germany | Koning (CBCT 1000) Breast CT | 59 | N/A | 2 (18.5) | 5.8–16.6 | 66/74 | 12/35 | 31.3 | 66.1 | CBBCT vs. DM a,c |
| He et al. [49] | China | Koning (CBCT 1000) Breast CT | 212 | 48 ± N/A | 2 (>10) | 8 ± 1.6 | 97/110 | 279/332 | 75.1 | 24.9 | CBBCT vs. DM vs. US b |
| Zhao et al. [13] | USA | Koning (CBCT 1000) Breast CT | 65 | 55.6 ± 9.8 | 2 (>7) | 5.8–24.84 | 39/45 | 35/40 | 47.1 | 52.9 | CBBCT vs. DM b,e |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A

References
- O’Connell, A.; Conover, D.L.; Zhang, Y.; Seifert, P.; Logan-Young, W.; Lin, C.F.; Sahler, L.; Ning, R. Cone-beam CT for breast imaging: Radiation dose, breast coverage, and image quality. AJR Am. J. Roentgenol. 2010, 195, 496–509. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustamante, M.; Rienzo, A.; Osorio, R.; Lefranc, E.; Duarte-Mermoud, M.A.; Herrera-Viedma, E.; Lefranc, G. Algorithm for processing mammography: Detection of microcalcifications. IEEE Lat. Am. Trans. 2018, 16, 2460–2466. [Google Scholar] [CrossRef]
- Mellado, M.; Osa, A.M.; Murillo, A.; Bermejo, R.; Burguete, A.; Pons, M.J.; Erdozain, N. Influencia de la mamografía digital en la detección y manejo de microcalcificaciones [Impact of digital mammography in the detection and management of microcalcifications]. Radiologia 2013, 55, 142–147. [Google Scholar] [CrossRef]
- Mann, R.M.; Hooley, R.; Barr, R.G.; Moy, L. Novel approaches to screening for breast cancer. Radiology 2020, 297, 266–285. [Google Scholar] [CrossRef]
- Zhu, Y.; O’Connell, A.M.; Ma, Y.; Liu, A.; Li, H.; Zhang, Y.; Zhang, X.; Ye, Z. Dedicated breast CT: State of the art—Part II. Clinical application and future outlook. Eur. Radiol. 2022, 32, 2286–2300. [Google Scholar] [CrossRef]
- Conant, E.F.; Beaber, E.F.; Sprague, B.L.; Herschorn, S.D.; Weaver, D.L.; Onega, T.; Tosteson, A.N.A.; McCarthy, A.M.; Poplack, S.P.; Haas, J.; et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: A cohort study within the PROSPR consortium. Breast Cancer Res. Treat. 2016, 156, 109–116. [Google Scholar] [CrossRef]
- Fontaine, M.; Tourasse, C.; Pages, E.; Laurent, N.; Laffargue, G.; Millet, I.; Molinari, N.; Taourel, P. Local Tumor Staging of Breast Cancer: Digital Mammography versus Digital Mammography Plus Tomosynthesis. Radiology 2019, 291, 594–603. [Google Scholar] [CrossRef]
- Iotti, V.; Rossi, P.G.; Nitrosi, A.; Ravaioli, S.; Vacondio, R.; Campari, C.; Marchesi, V.; Ragazzi, M.; Bertolini, M.; Besutti, G.; et al. Comparing two visualization protocols for tomosynthesis in screening: Specificity and sensitivity of slabs versus planes plus slabs. Eur. Radiol. 2019, 29, 3802–3811. [Google Scholar] [CrossRef]
- Mall, S.; Noakes, J.; Kossoff, M.; Lee, W.; McKessar, M.; Goy, A.; Duncombe, J.; Roberts, M.; Giuffre, B.; Miller, A.; et al. Can digital breast tomosynthesis perform better than standard digital mammography work-up in breast cancer assessment clinic? Eur. Radiol. 2018, 28, 5182–5194. [Google Scholar] [CrossRef]
- Wienbeck, S.; Uhlig, J.; Luftner-Nagel, S.; Zapf, A.; Surov, A.; von Fintel, E.; Stahnke, V.; Lotz, J.; Fischer, U. The role of cone-beam breast-CT for breast cancer detection relative to breast density. Eur. Radiol. 2017, 27, 5185–5195. [Google Scholar] [CrossRef] [PubMed]
- Wienbeck, S.; Fischer, U.; Luftner-Nagel, S.; Lotz, J.; Uhlig, J. Contrast-enhanced cone-beam breast-CT (CBBCT): Clinical performance compared to mammography and MRI. Eur. Radiol. 2018, 28, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, X.; Cai, W.; Conover, D.; Ning, R. Cone beam breast CT with multiplanar and three dimensional visualization in differentiating breast masses compared with mammography. Eur. J. Radiol. 2015, 84, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Movik, E.; Dalsbø, T.K.; Fagelund, B.C.; Friberg, E.G.; Håheim, L.L.; Skår, Å. Digital Breast Tomosynthesis with Hologic 3D Mammography Selenia Dimensions System for Use in Breast Cancer Screening: A Single Technology Assessment; Report from the Norwegian Institute of Public Health No. 2017–08; Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2017. [Google Scholar]
- Thompson, W.; Argaez, C. Digital Breast Tomosynthesis for the Screening and Diagnosis of Breast Cancer: A Review of the Diagnostic Accuracy, Cost-Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental screening for breast cancer in women with dense breasts: A systematic review for the US Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Phi, X.A.; Tagliafico, A.; Houssami, N.; Greuter, M.J.W.; de Bock, G.H. Digital breast tomosynthesis for breast cancer screening and diagnosis in women with dense breasts-a systematic review and meta-analysis. BMC Cancer 2018, 18, 380. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, J.; Fischer, U.; Biggemann, L.; Lotz, J.; Wienbeck, S. Pre- and post-contrast versus post-contrast cone-beam breast CT: Can we reduce radiation exposure while maintaining diagnostic accuracy? Eur. Radiol. 2019, 29, 3141–3148. [Google Scholar] [CrossRef]
- Uhlig, J.; Uhlig, A.; Biggemann, L.; Fischer, U.; Lotz, J.; Wienbeck, S. Diagnostic accuracy of cone-beam breast computed tomography: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2019, 29, 1194–1202. [Google Scholar] [CrossRef]
- Belair, J.; Zuley, M.; Ganott, M.; Kelly, A.; Shinde, D.; Shah, R.; Catullo, V.; Mishra, M.D.V.; Gur, D. Non-contrast Cone-Beam CT vs Tomosynthesis: Identification and Classification of Benign and Malignant Breast Lesions. In Proceedings of the Radiological Society of North America 2012 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 25–30 November 2012; Available online: http://archive.rsna.org/2012/12022690.html (accessed on 24 December 2021).
- Zuley, M.; Guo, B.; Ganott, M.; Bandos, A.; Catullo, V.; Lu, A.; Kelly, A.E.; Anello, M.L.; Abrams, G.S.; Chough, D. Comparison of Visibility and Diagnostic Accuracy of Cone Beam Computed Tomography, Tomosynthesis, MRI and Digital Mammography for Breast Masses. In Proceedings of the Radiological Society of North America 2013 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 1–6 December 2013; Available online: http://archive.rsna.org/2013/13022530.html (accessed on 24 December 2021).
- Komolafe, T.E.; Olagbaju, O.A.; Li, M.; Zheng, J.; Yang, X. Comparison of Diagnostic Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis: A Systematic Review and Meta-Analysis Approach. PROSPERO2020CRD42020180192. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020180192 (accessed on 5 January 2022).
- Deeks, J.J.; Bossuyt, P.M.M. Chapter 3: Evaluating diagnostic tests. In Cochrane Handbook for Systematic Reviews of Reviews of Diagnostic Test Accuracy Version 2; Deeks, J.J., Bossuyt, P.M.M., Leeflang, M.M.G., Takwoingi, Y., Eds.; Cochrane: London, UK, 2017. [Google Scholar]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; The PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Manikandan, R.; Dorairajan, L.N. How to appraise a diagnostic test. Indian J. Urol. 2011, 27, 513–519. [Google Scholar]
- Komolafe, T.E.; Cao, Y.; Nguchu, B.A.; Monkam, P.; Olaniyi, E.O.; Sun, H.; Zheng, J.; Yang, X. Diagnostic test accuracy of deep learning detection of COVID-19: A systematic review and meta-analysis. Acad. Radiol. 2021, 8, 1507–1523. [Google Scholar] [CrossRef]
- Yang, B.; Mallett, S.; Takwoingi, Y.; Davenport, C.F.; Hyde, C.J.; Whiting, P.F.; Deeks, J.J.; Leeflang, D.M.M.; the QUADAS-C Group. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann. Intern. Med. 2021, 174, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.J.; Lee, J. Diagnostic test accuracy: Application and practice using R software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar] [CrossRef] [PubMed]
- Chappell, F.M.; Raab, G.M.; Wardlaw, J.M. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat. Med. 2009, 28, 2653–2668. [Google Scholar] [CrossRef]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.S.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Liu, J.L. The role of the funnel plot in detecting publication and related biases in meta-analysis. Evid.-Based Dent. 2011, 12, 121–122. [Google Scholar] [CrossRef]
- Conant, E.F.; Zuckerman, S.P.; McDonald, E.S.; Weinstein, S.P.; Korhonen, K.E.; Birnbaum, J.A.; Tobey, J.D.; Schnall, M.D.; Hubbard, R.A. Five Consecutive Years of Screening with Digital Breast Tomosynthesis: Outcomes by Screening Year and Round. Radiology 2020, 295, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women with Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Conant, E.F.; Barlow, W.E.; Herschorn, S.D.; Weaver, D.L.; Beaber, E.F.; Tosteson, A.N.A.; Haas, J.S.; Lowry, K.P.; Stout, N.K.; Trentham-Dietz, A.; et al. Association of Digital Breast Tomosynthesis vs Digital Mammography with Cancer Detection and Recall Rates by Age and Breast Density. JAMA Oncol. 2019, 5, 635–642. [Google Scholar] [CrossRef]
- Asbeutah, A.M.; Karmani, N.; Asbeutah, A.A.; Echreshzadeh, Y.A.; AlMajran, A.A.; Al-Khalifah, K.H. Comparison of Digital Breast Tomosynthesis and Digital Mammography for Detection of Breast Cancer in Kuwaiti Women. Med. Princ. Pract. 2019, 28, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Georgian-Smith, D.; Obuchowski, N.A.; Lo, J.Y.; Brem, R.F.; Baker, J.A.; Fisher, P.R.; Rim, A.; Zhao, W.; Fajardo, L.L.; Mertelmeier, T. Can Digital Breast Tomosynthesis Replace Full-Field Digital Mammography? A Multireader, Multicase Study of Wide-Angle Tomosynthesis. Am. J. Roentgenol. 2019, 212, 1393–1399. [Google Scholar] [CrossRef]
- Zackrisson, S.; Lång, K.; Rosso, A.; Johnson, K.; Dustler, M.; Förnvik, D.; Andersson, I. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): A prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018, 19, 1493–1503. [Google Scholar] [CrossRef]
- Dibble, E.H.; Lourenco, A.P.; Baird, G.L.; Ward, R.C.; Maynard, A.S.; Mainiero, M.B. Comparison of digital mammography and digital breast tomosynthesis in the detection of architectural distortion. Eur. Radiol. 2018, 28, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Chang, J.M.; Lee, J.; Chu, A.J.; Seo, M.; Gweon, H.M.; Förnvik, H.; Sartor, H.; Timberg, P.; Tingberg, A.; et al. Erratum to: Diagnostic performance of tomosynthesis and breast ultrasonography in women with dense breasts: A prospective comparison study. Breast Cancer Res. Treat. 2017, 163, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ruiz, A.; Gubern-Merida, A.; Imhof-Tas, M.; Lardenoije, S.; Wanders, A.J.T.; Andersson, I.; Zackrisson, S.; Lång, K.; Dustler, M.; Karssemeijer, N.; et al. One-view digital breast tomosynthesis as a stand-alone modality for breast cancer detection: Do we need more? Eur. Radiol. 2018, 28, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Chae, E.Y.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Choi, W.J. Detection and characterization of breast lesions in a selective diagnostic population: Diagnostic accuracy study for comparison between one-view digital breast tomosynthesis and two-view full-field digital mammography. Br. J. Radiol. 2016, 89, 20150743. [Google Scholar] [CrossRef] [Green Version]
- Bian, T.; Lin, Q.; Cui, C.; Li, L.; Qi, C.; Fei, J.; Su, X. Digital Breast Tomosynthesis: A New Diagnostic Method for Mass-Like Lesions in Dense Breasts. Breast J. 2016, 22, 535–540. [Google Scholar] [CrossRef]
- Lee, W.K.; Chung, J.; Cha, E.S.; Lee, J.E.; Kim, J.H. Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur. J. Radiol. 2016, 85, 291–296. [Google Scholar] [CrossRef]
- Kim, S.A.; Chang, J.M.; Cho, N.; Yi, A.; Moon, W.K. Characterization of breast lesions: Comparison of digital breast tomosynthesis and ultrasonography. Korean J. Radiol. 2015, 16, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Roganovic, D.; Djilas, D.; Vujnovic, S.; Pavic, D.; Stojanov, D. Breast MRI, digital mammography and breast tomosynthesis: Comparison of three methods for early detection of breast cancer. Bosn. J. Basic Med. Sci. 2015, 15, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.K.; Kuzmiak, C.M.; Kim, K.W.; Choi, N.M.; Kim, H.J.; Langman, E.L.; Yoon, S.; Steen, D.; Zeng, D.; Gao, F. Potential Use of American College of Radiology BI-RADS Mammography Atlas for Reporting and Assessing Lesions Detected on Dedicated Breast CT Imaging: Preliminary Study. Acad. Radiol. 2017, 24, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wu, Y.P.; Kong, Y.; Lv, N.; Huang, Z.M.; Li, S.; Wang, Y.; Geng, Z.-J.; Wu, P.-H.; Wei, W.-D. The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: A prospective study with 212 patients. Eur. J. Radiol. 2016, 85, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, R.; Sannapareddy, K.; Potlapalli, A.; Krishnamurthy, P.B.; Buddha, S.; Koppula, V. Diagnostic accuracy of contrast-enhanced digital mammography in breast cancer detection in comparison to tomosynthesis, synthetic 2D mammography and tomosynthesis combined with ultrasound in women with dense breast. Br. J. Radiol. 2021, 94, 20201046. [Google Scholar] [CrossRef]
- Hadadi, I.; Rae, W.; Clarke, J.; McEntee, M.; Ekpo, E. Breast cancer detection: Comparison of digital mammography and digital breast tomosynthesis across non-dense and dense breasts. Radiography 2021, 27, 1027–1032. [Google Scholar] [CrossRef]
- Deike-Hofmann, K.; Koenig, F.; Paech, D.; Dreher, C.; Delorme, S.; Schlemmer, H.P.; Bickelhaupt, S. Abbreviated MRI Protocols in Breast Cancer Diagnostics. J. Magn. Reson 2019, 49, 647–658. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Shimauchi, A.; Jansen, S.A.; Abe, H.; Jaskowiak, N.; Schmidt, R.A.; Newstead, G.M. Breast cancers not detected at MRI: Review of false-negative lesions. Am. J. Roentgenol 2010, 194, 1674–1679. [Google Scholar] [CrossRef]
- Mango, V.L.; Morris, E.A.; Dershaw, D.D.; Abramson, A.; Fry, C.; Moskowitz, C.S.; Hughes, M.; Kaplan, J.; Jochelson, M.S. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur. J. Radiol. 2015, 84, 65–70. [Google Scholar] [CrossRef]


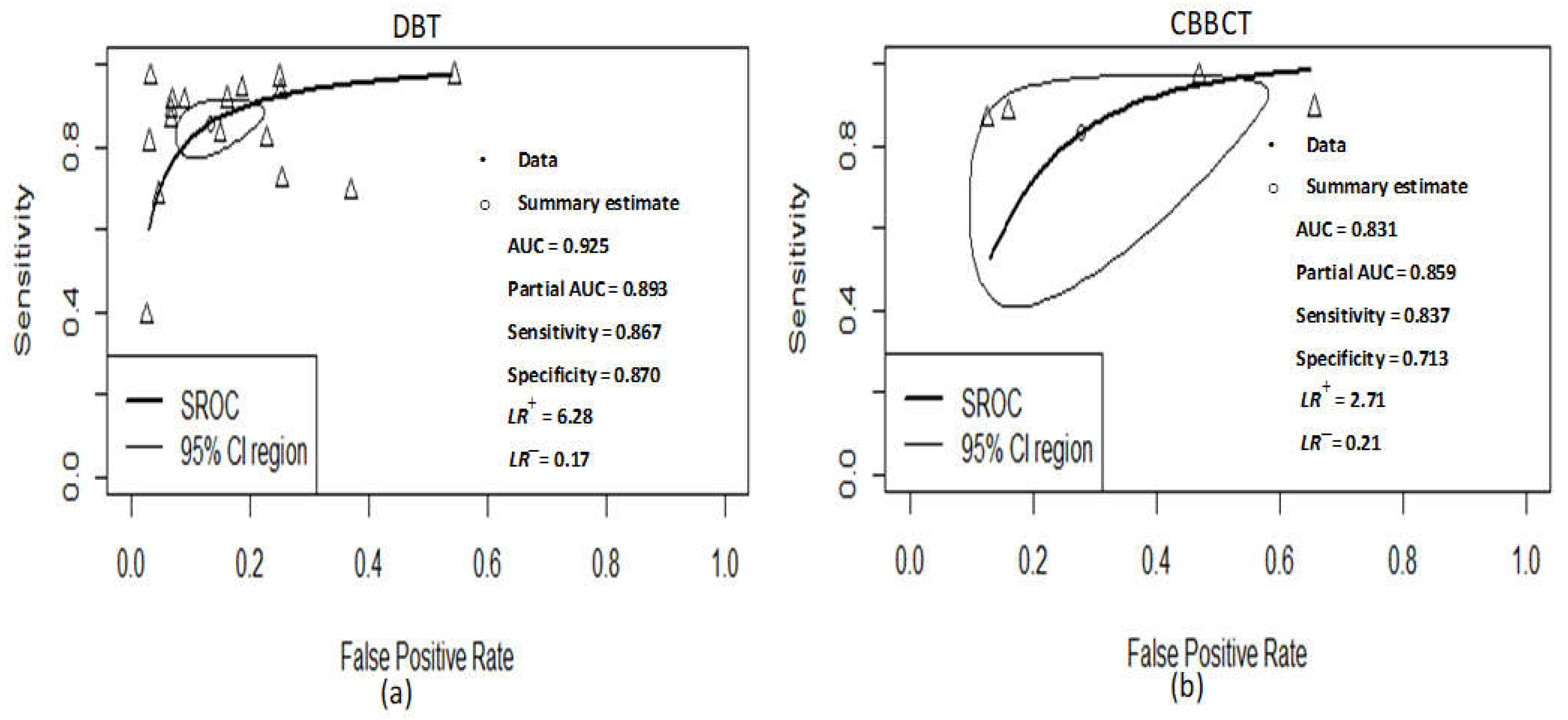
| DOR Parameters | Pooled Value at 95% CI (DBT) | Pooled Value at 95% CI (CBBCT) |
|---|---|---|
| Sensitivity | 86.7% (80.3–91.2, I2 = 89%) | 83.7% (54.6–95.7 I2 = 94%) |
| Specificity | 87.0% (79.9–91.8, I2 = 95%) | 71.3% (47.5–87.2, I2 = 94%) |
| LR+ | 6.28 (4.40–8.96, I2 = 93%) | 2.71 (1.39–5.29, I2 = 95%) |
| LR− | 0.17 (0.12–0.25, I2 = 91%) | 0.21 (0.04–1.05, I2 = 97%) |
| AUC of SROC | 0.925 | 0.831 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komolafe, T.E.; Zhang, C.; Olagbaju, O.A.; Yuan, G.; Du, Q.; Li, M.; Zheng, J.; Yang, X. Comparison of Diagnostic Test Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis for Breast Cancer: A Systematic Review and Meta-Analysis Approach. Sensors 2022, 22, 3594. https://doi.org/10.3390/s22093594
Komolafe TE, Zhang C, Olagbaju OA, Yuan G, Du Q, Li M, Zheng J, Yang X. Comparison of Diagnostic Test Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis for Breast Cancer: A Systematic Review and Meta-Analysis Approach. Sensors. 2022; 22(9):3594. https://doi.org/10.3390/s22093594
Chicago/Turabian StyleKomolafe, Temitope Emmanuel, Cheng Zhang, Oluwatosin Atinuke Olagbaju, Gang Yuan, Qiang Du, Ming Li, Jian Zheng, and Xiaodong Yang. 2022. "Comparison of Diagnostic Test Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis for Breast Cancer: A Systematic Review and Meta-Analysis Approach" Sensors 22, no. 9: 3594. https://doi.org/10.3390/s22093594
APA StyleKomolafe, T. E., Zhang, C., Olagbaju, O. A., Yuan, G., Du, Q., Li, M., Zheng, J., & Yang, X. (2022). Comparison of Diagnostic Test Accuracy of Cone-Beam Breast Computed Tomography and Digital Breast Tomosynthesis for Breast Cancer: A Systematic Review and Meta-Analysis Approach. Sensors, 22(9), 3594. https://doi.org/10.3390/s22093594






