Motion Artifacts Correction from Single-Channel EEG and fNIRS Signals Using Novel Wavelet Packet Decomposition in Combination with Canonical Correlation Analysis
1. Introduction
2. Theoretical Background
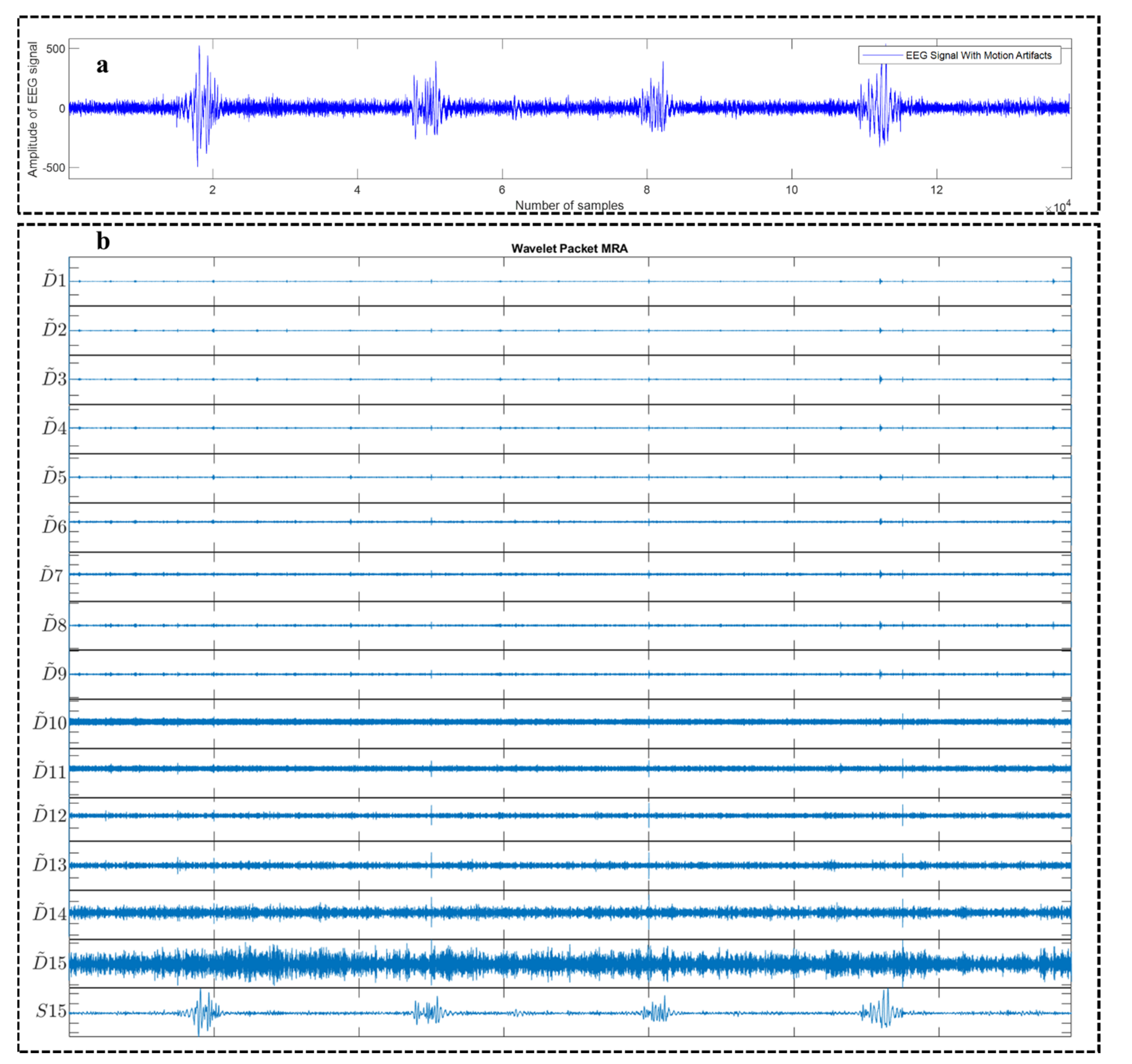
2.1. Wavelet Packet Decomposition (WPD)
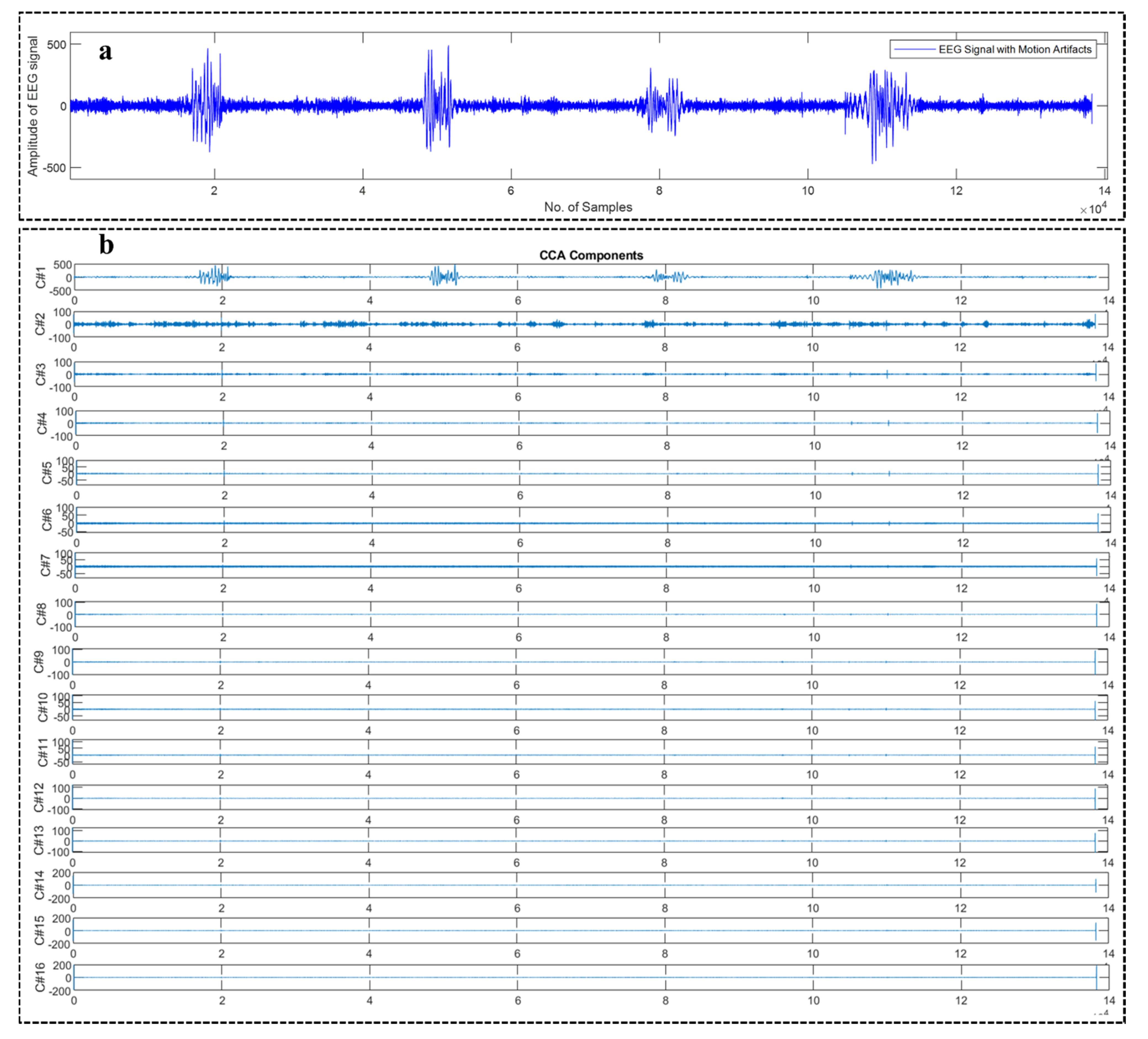
2.2. Canonical Correlation Analysis (CCA)
2.3. WPD-CCA
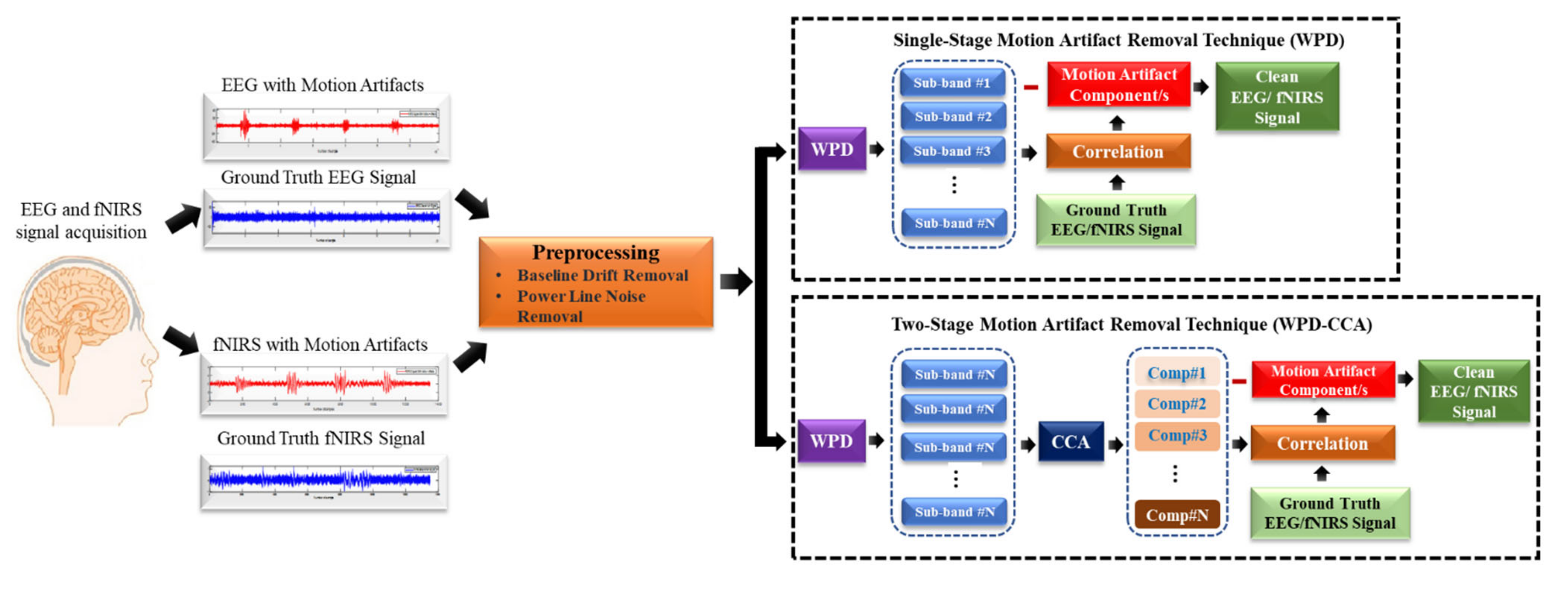
3. Methods
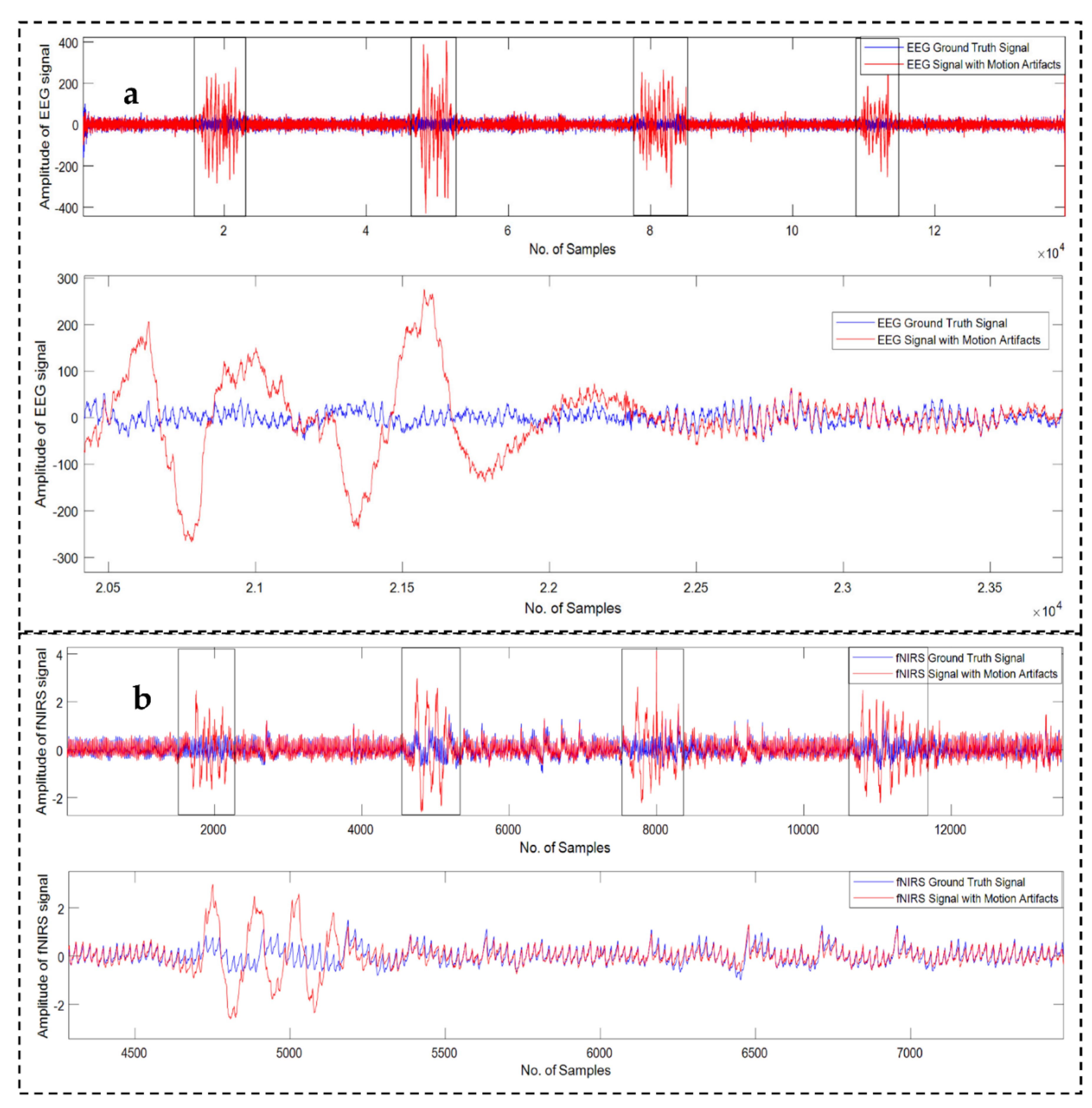
3.1. Dataset Description
3.2. Signal Preprocessing
3.3. Study Design
3.4. Removal of Motion Artifact Components Using “Reference Ground Truth” Method
3.5. Performance Metrics
4. Results
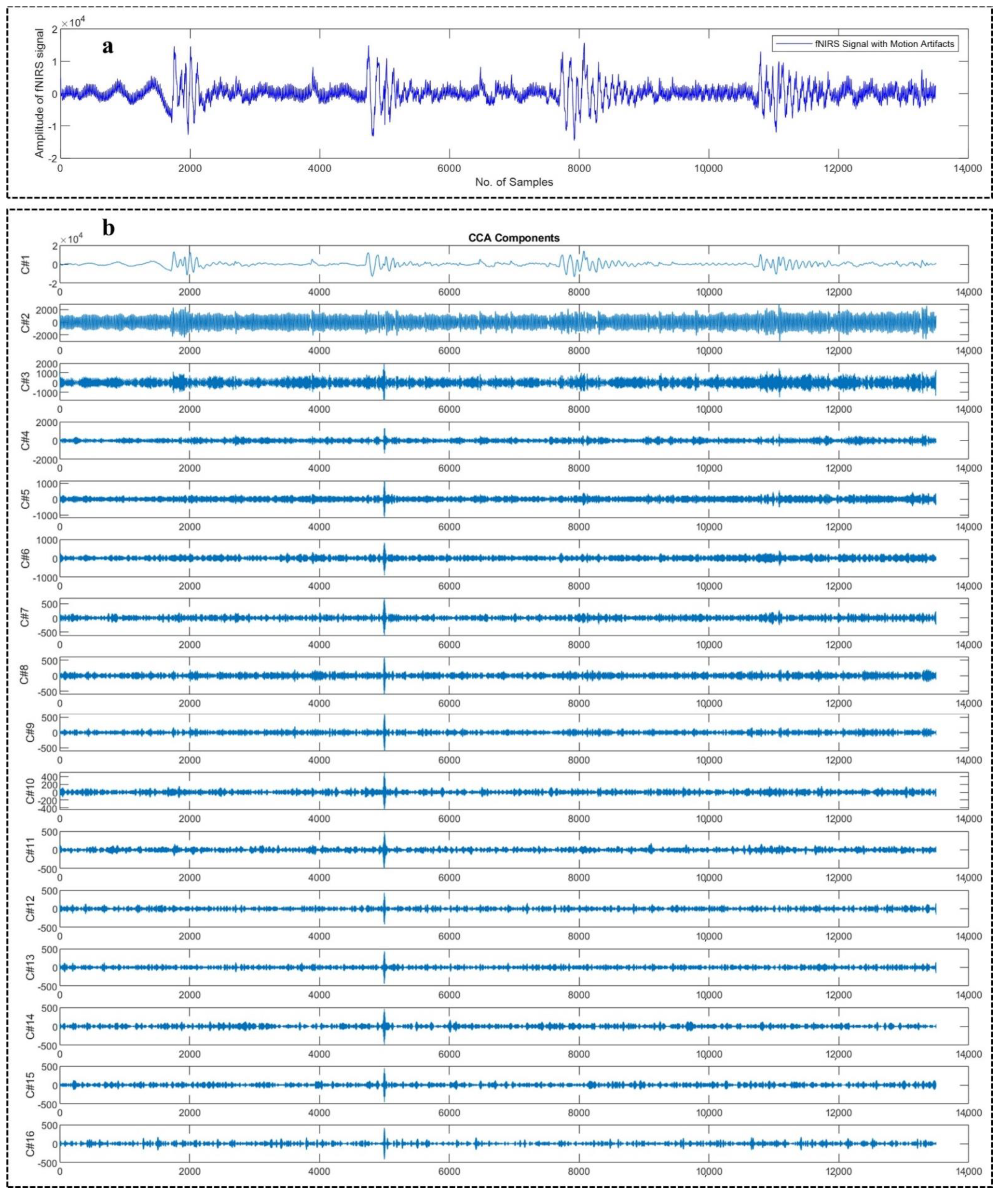
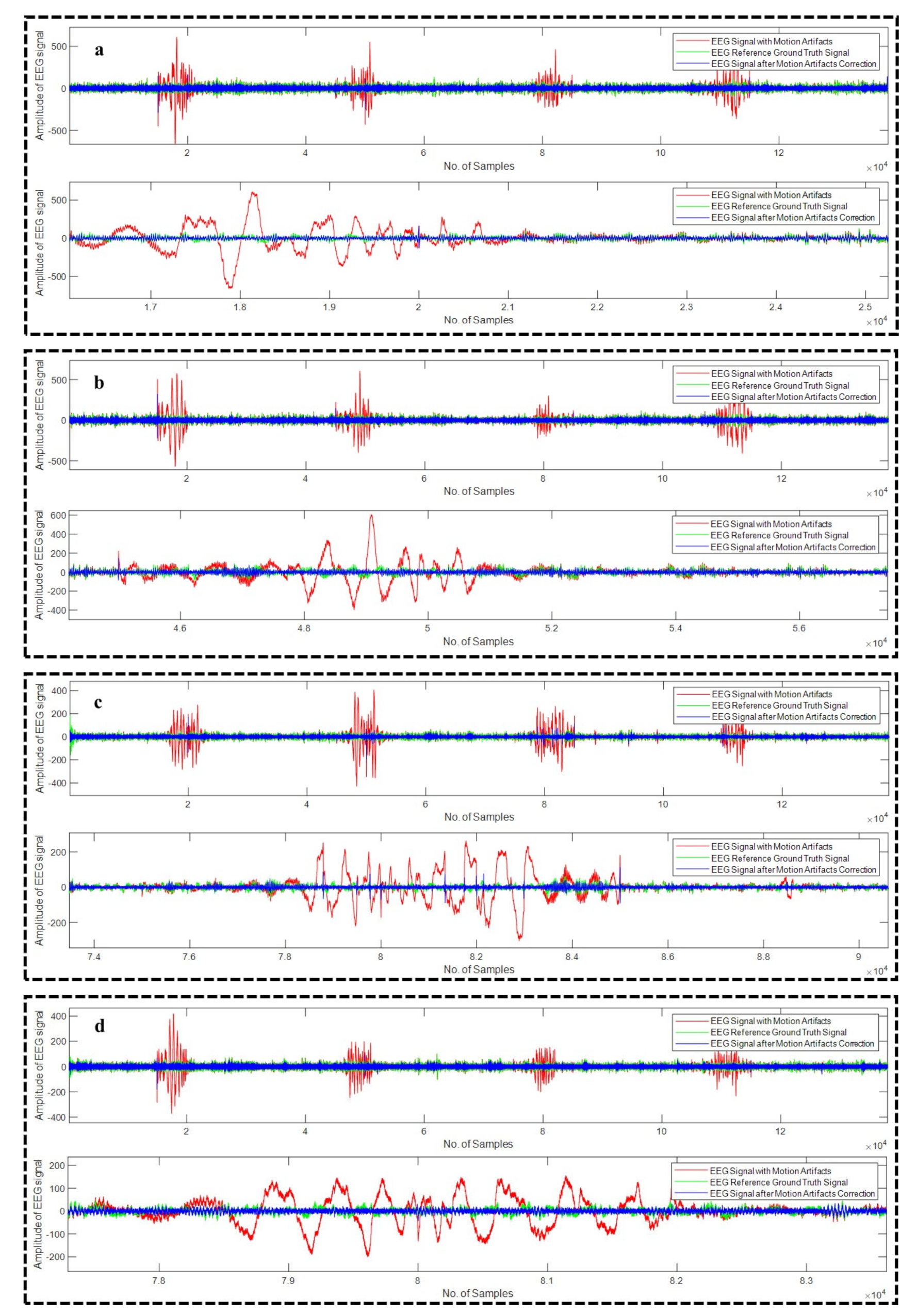
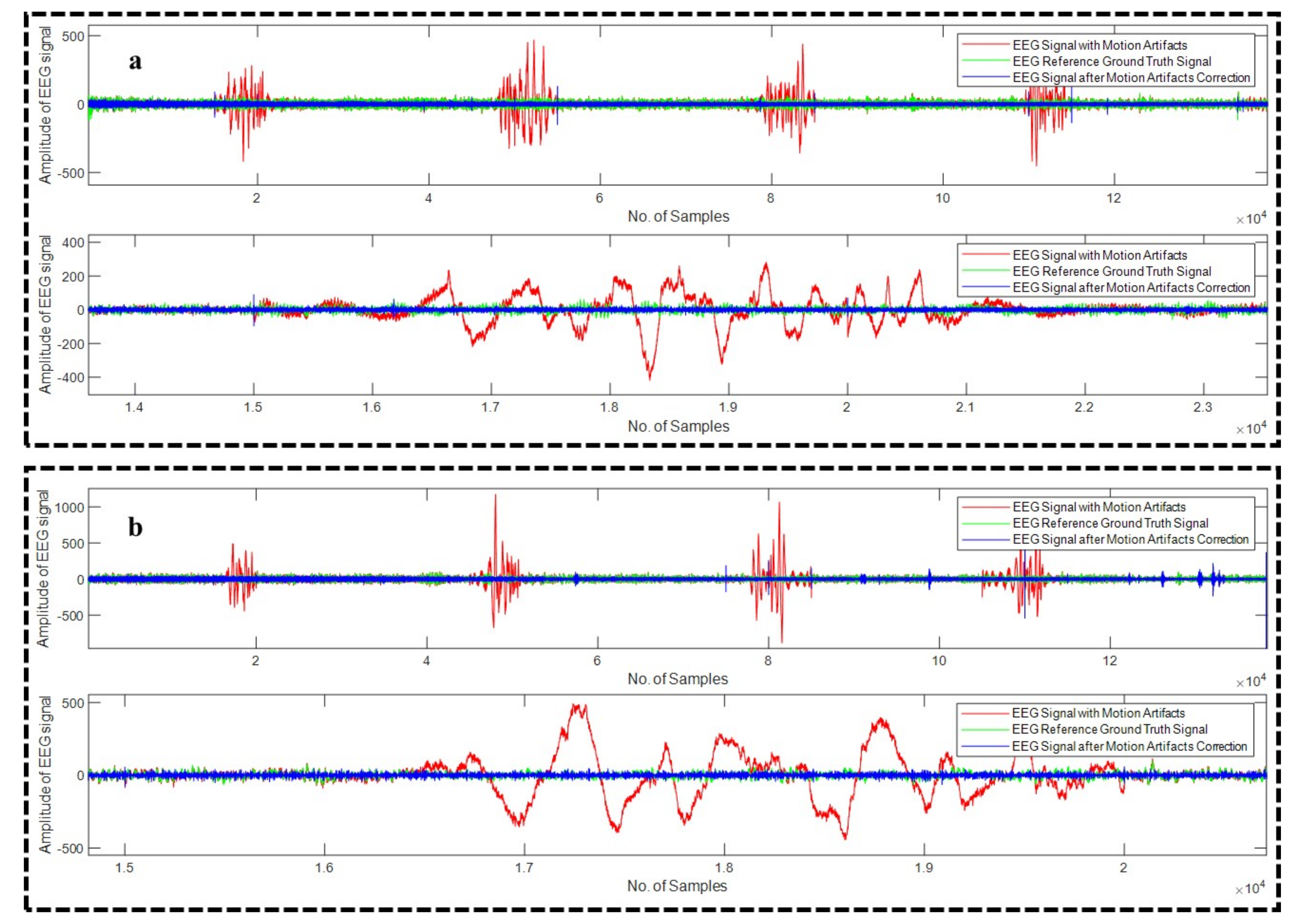
4.1. Motion Artifact Correction from EEG Data
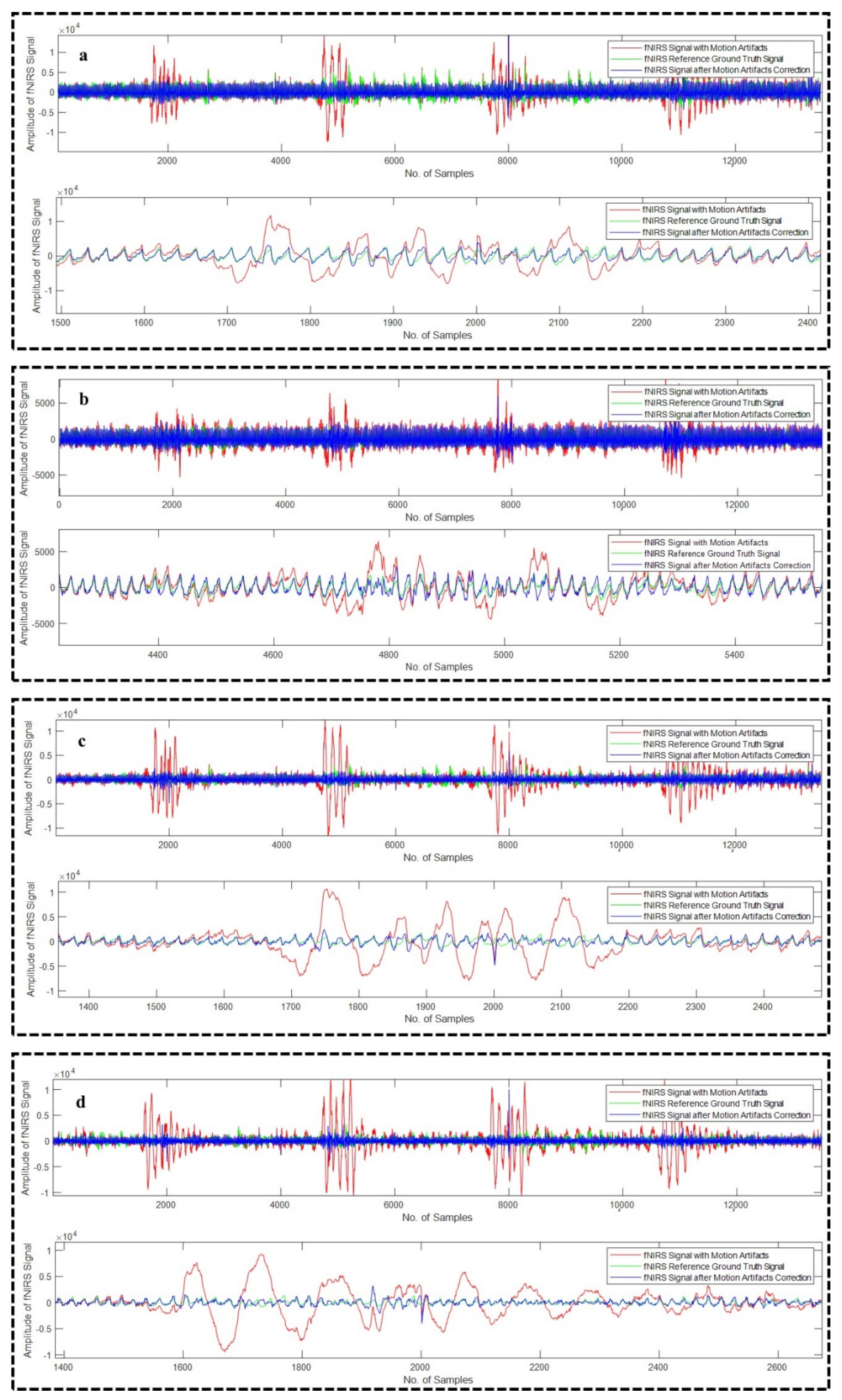
4.2. Motion Artifact Correction from fNIRS Data
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, J.C. Electroencephalography: Basic principles, clinical applications, and related fields. Neurology 2006, 67, 2092–2092-a. [Google Scholar] [CrossRef]
- Nuwer, M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology 1997, 49, 277–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoeb, A.; Guttag, J.; Schachter, S.; Schomer, D.; Bourgeois, B.; Treves, S.T. Detecting seizure onset in the ambulatory setting: Demonstrating feasibility. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 3546–3550. [Google Scholar]
- Sharma, R.R.; Varshney, P.; Pachori, R.B.; Vishvakarma, S.K. Automated system for epileptic EEG detection using iterative filtering. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Berka, C.; Levendowski, D.J.; Westbrook, P.; Davis, G.; Lumicao, M.N.; Olmstead, R.E.; Popovic, M.; Zivkovic, V.T.; Ramsey, C.K. EEG quantification of alertness: Methods for early identification of individuals most susceptible to sleep deprivation. In Proceedings of the Biomonitoring for Physiological and Cognitive Performance during Military Operations, Orlando, FL, USA, 23 May 2005; pp. 78–89. [Google Scholar]
- Berka, C.; Levendowski, D.J.; Cvetinovic, M.M.; Petrovic, M.M.; Davis, G.; Lumicao, M.N.; Zivkovic, V.T.; Popovic, M.V.; Olmstead, R. Real-time analysis of EEG indexes of alertness, cognition, and memory acquired with a wireless EEG headset. Int. J. Hum. Comput. Interact. 2004, 17, 151–170. [Google Scholar] [CrossRef]
- Papadelis, C.; Kourtidou-Papadeli, C.; Bamidis, P.D.; Chouvarda, I.; Koufogiannis, D.; Bekiaris, E.; Maglaveras, N. Indicators of sleepiness in an ambulatory EEG study of night driving. In Proceedings of the 2006 the International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6201–6204. [Google Scholar]
- Tripathy, R.; Acharya, U.R. Use of features from RR-time series and EEG signals for automated classification of sleep stages in deep neural network framework. Biocybern. Biomed. Eng. 2018, 38, 890–902. [Google Scholar] [CrossRef]
- Gupta, V.; Chopda, M.D.; Pachori, R.B. Cross-subject emotion recognition using flexible analytic wavelet transform from EEG signals. IEEE Sens. J. 2018, 19, 2266–2274. [Google Scholar] [CrossRef]
- Stevens, R.; Galloway, T.; Berka, C. Integrating EEG models of cognitive load with machine learning models of scientific problem solving. Augment. Cogn. Past Present Future 2006, 2, 55–65. [Google Scholar]
- Bell, C.J.; Shenoy, P.; Chalodhorn, R.; Rao, R.P. Control of a humanoid robot by a noninvasive brain–computer interface in humans. J. Neural Eng. 2008, 5, 214. [Google Scholar] [CrossRef]
- Lee, J.C.; Tan, D.S. Using a low-cost electroencephalograph for task classification in HCI research. In Proceedings of the 19th Annual ACM Symposium on User Interface Software and Technology, Montreux, Switzerland, 15–18 October 2006; pp. 81–90. [Google Scholar]
- Sullivan, T.J.; Deiss, S.R.; Jung, T.-P.; Cauwenberghs, G. A brain-machine interface using dry-contact, low-noise EEG sensors. In Proceedings of the 2008 IEEE International Symposium on Circuits and Systems, Seattle, WA, USA, 18–21 May 2008; pp. 1986–1989. [Google Scholar]
- Wolpaw, J.R.; McFarland, D.J. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 17849–17854. [Google Scholar] [CrossRef] [Green Version]
- Gaur, P.; Pachori, R.B.; Wang, H.; Prasad, G. A multi-class EEG-based BCI classification using multivariate empirical mode decomposition based filtering and Riemannian geometry. Expert Syst. Appl. 2018, 95, 201–211. [Google Scholar] [CrossRef]
- Gaur, P.; Pachori, R.B.; Wang, H.; Prasad, G. An automatic subject specific intrinsic mode function selection for enhancing two-class EEG-based motor imagery-brain computer interface. IEEE Sens. J. 2019, 19, 6938–6947. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, M.E.; Khandakar, A.; Kiranyaz, S.; Zaman, K.S.; Reaz, M.B.I.; Islam, M.T.; Ezeddin, M.; Kadir, M.A. Multimodal EEG and Keystroke Dynamics Based Biometric System Using Machine Learning Algorithms. IEEE Access 2021, 9, 94625–94643. [Google Scholar] [CrossRef]
- Sangani, S.; Lamontagne, A.; Fung, J. Cortical mechanisms underlying sensorimotor enhancement promoted by walking with haptic inputs in a virtual environment. Prog. Brain Res. 2015, 218, 313–330. [Google Scholar] [PubMed]
- Bunce, S.C.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Pourrezaei, K. Functional near-infrared spectroscopy. IEEE Eng. Med. Biol. Mag. 2006, 25, 54–62. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [Green Version]
- Holper, L.; Muehlemann, T.; Scholkmann, F.; Eng, K.; Kiper, D.; Wolf, M. Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near-infrared spectroscopy (fNIRS). J. Neuroeng. Rehabil. 2010, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Izzetoglu, K.; Bunce, S.; Onaral, B.; Pourrezaei, K.; Chance, B. Functional optical brain imaging using near-infrared during cognitive tasks. Int. J. Hum. Comput. Interact. 2004, 17, 211–227. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [Green Version]
- Matthews, F.; Pearlmutter, B.A.; Wards, T.E.; Soraghan, C.; Markham, C. Hemodynamics for brain-computer interfaces. IEEE Signal Process. Mag. 2007, 25, 87–94. [Google Scholar] [CrossRef]
- Khan, M.J.; Hong, K.-S. Passive BCI based on drowsiness detection: An fNIRS study. Biomed. Opt. Express 2015, 6, 4063–4078. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.-S.; Naseer, N.; Kim, Y.-H. Classification of prefrontal and motor cortex signals for three-class fNIRS–BCI. Neurosci. Lett. 2015, 587, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.E.; Khandakar, A.; Hossain, B.; Alzoubi, K. Effects of the phantom shape on the gradient artefact of electroencephalography (EEG) data in simultaneous EEG–fMRI. Appl. Sci. 2018, 8, 1969. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.E.; Khandakar, A.; Mullinger, K.; Hossain, B.; Al-Emadi, N.; Antunes, A.; Bowtell, R. Reference layer artefact subtraction (RLAS): Electromagnetic simulations. IEEE Access 2019, 7, 17882–17895. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Khandakar, A.; Mullinger, K.J.; Al-Emadi, N.; Bowtell, R. Simultaneous EEG-fMRI: Evaluating the effect of the EEG cap-cabling configuration on the gradient artifact. Front. Neurosci. 2019, 13, 690. [Google Scholar] [CrossRef]
- Nguyen, H.-D.; Yoo, S.-H.; Bhutta, M.R.; Hong, K.-S. Adaptive filtering of physiological noises in fNIRS data. Biomed. Eng. Online 2018, 17, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.K.; Rastegarnia, A.; Yang, Z. Methods for artifact detection and removal from scalp EEG: A review. Neurophysiol. Clin. Clin. Neurophysiol. 2016, 46, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.T.; Ward, T.E.; McLoone, S.F. Artifact removal in physiological signals—Practices and possibilities. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.T.; McLoone, S.F.; Ward, T.E. The use of ensemble empirical mode decomposition with canonical correlation analysis as a novel artifact removal technique. IEEE Trans. Biomed. Eng. 2012, 60, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Akansu, A.N.; Haddad, R.A.; Haddad, P.A.; Haddad, P.R. Multiresolution Signal Decomposition: Transforms, Subbands, and Wavelets; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.-C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Maddirala, A.K.; Shaik, R.A. Motion artifact removal from single channel electroencephalogram signals using singular spectrum analysis. Biomed. Signal Process. Control 2016, 30, 79–85. [Google Scholar] [CrossRef]
- Vautard, R.; Yiou, P.; Ghil, M. Singular-spectrum analysis: A toolkit for short, noisy chaotic signals. Phys. D Nonlinear Phenom. 1992, 58, 95–126. [Google Scholar] [CrossRef]
- Kumar, P.S.; Arumuganathan, R.; Sivakumar, K.; Vimal, C. Removal of ocular artifacts in the EEG through wavelet transform without using an EOG reference channel. Int. J. Open Probl. Compt. Math 2008, 1, 188–200. [Google Scholar]
- Gajbhiye, P.; Tripathy, R.K.; Bhattacharyya, A.; Pachori, R.B. Novel approaches for the removal of motion artifact from EEG recordings. IEEE Sens. J. 2019, 19, 10600–10608. [Google Scholar] [CrossRef]
- Gajbhiye, P.; Mingchinda, N.; Chen, W.; Mukhopadhyay, S.C.; Wilaiprasitporn, T.; Tripathy, R.K. Wavelet Domain Optimized Savitzky–Golay Filter for the Removal of Motion Artifacts From EEG Recordings. IEEE Trans. Instrum. Meas. 2020, 70, 1–11. [Google Scholar] [CrossRef]
- Hossain, M.S.; Reaz, M.B.; Chowdhury, M.E.; Ali, S.H.; Bakar, A.A.A.; Kiranyaz, S.; Khandakar, A.; Alhatou, M.; Habib, R. Motion Artifacts Correction from EEG and fNIRS Signals using Novel Multiresolution Analysis. IEEE Access 2022, 10, 29760–29777. [Google Scholar] [CrossRef]
- Dragomiretskiy, K.; Zosso, D. Variational mode decomposition. IEEE Trans. Signal Process. 2013, 62, 531–544. [Google Scholar] [CrossRef]
- Robertson, F.C.; Douglas, T.S.; Meintjes, E.M. Motion artifact removal for functional near infrared spectroscopy: A comparison of methods. IEEE Trans. Biomed. Eng. 2010, 57, 1377–1387. [Google Scholar] [CrossRef]
- Cooper, R.; Selb, J.; Gagnon, L.; Phillip, D.; Schytz, H.W.; Iversen, H.K.; Ashina, M.; Boas, D.A. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012, 6, 147. [Google Scholar] [CrossRef] [Green Version]
- Brigadoi, S.; Ceccherini, L.; Cutini, S.; Scarpa, F.; Scatturin, P.; Selb, J.; Gagnon, L.; Boas, D.A.; Cooper, R.J. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 2014, 85, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, K.T.; Ayaz, H.; Ward, T.E.; Izzetoglu, M.; McLoone, S.F.; Onaral, B. A methodology for validating artifact removal techniques for physiological signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 918–926. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express 2013, 4, 1366–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, A.M.; Maclin, E.L.; Fabiani, M.; Gratton, G. A kurtosis-based wavelet algorithm for motion artifact correction of fNIRS data. NeuroImage 2015, 112, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, M.R.; Marquez, J.S.; Atri, R.; Ramon, R.; Perry Mayrand, R.; Bai, O. Movement artefact removal from NIRS signal using multi-channel IMU data. Biomed. Eng. Online 2018, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Jahani, S.; Setarehdan, S.K.; Boas, D.A.; Yücel, M.A. Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky–Golay filtering. Neurophotonics 2018, 5, 015003. [Google Scholar] [CrossRef] [Green Version]
- Mallat, S. A Wavelet Tour of Signal Processing; Mallat, S., Ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Jaffard, S.; Meyer, Y.; Ryan, R.D. Wavelets: Tools for Science and Technology; Society For Industrial & Applied (SIAM): Philadelphia, PA, USA, 2001. [Google Scholar]
- Farooq, O.; Datta, S. Mel filter-like admissible wavelet packet structure for speech recognition. IEEE Signal Process. Lett. 2001, 8, 196–198. [Google Scholar] [CrossRef]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of movement artifact from high-density EEG recorded during walking and running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef] [Green Version]
- Hotelling, H. Relations between two sets of variates. In Breakthroughs in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 162–190. [Google Scholar]
- James, C.J.; Hesse, C.W. Independent component analysis for biomedical signals. Physiol. Meas. 2004, 26, R15. [Google Scholar] [CrossRef]
- De Clercq, W.; Vergult, A.; Vanrumste, B.; Van Paesschen, W.; Van Huffel, S. Canonical correlation analysis applied to remove muscle artifacts from the electroencephalogram. IEEE Trans. Biomed. Eng. 2006, 53, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; Knutsson, H. A canonical correlation approach to blind source separation. In Report LiU-IMT-EX-0062; Department of Biomedical Engineering, Linkoping University: Linköping, Sweden, 2001. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsipouras, M.G. Spectral information of EEG signals with respect to epilepsy classification. EURASIP J. Adv. Signal Process. 2019, 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Boudaoud, S.; Terrien, J.; Karlsson, B.; Marque, C. Combination of canonical correlation analysis and empirical mode decomposition applied to denoising the labor electrohysterogram. IEEE Trans. Biomed. Eng. 2011, 58, 2441–2447. [Google Scholar] [CrossRef] [PubMed]


| Type | Technique | EEG (23 Records) | fNIRS (16 Records) | ||
|---|---|---|---|---|---|
| Average ∆SNR (in dB) | (in %) | Average ∆SNR (in dB) | (in %) | ||
| Single-stage motion artifact correction techniques | WPD(db1) | 29.26 (10.29) | 53.48 (33.35) * | 16.03 (4.31) | 26.21 (26.38) |
| WPD(db2) | 29.44 (9.93) * | 51.40 (33.59) | 15.99 (4.49) | 25.92 (28.86) | |
| WPD(db3) | 29.37 (10.01) | 50.74 (33.55) | 15.71 (4.52) | 26.05 (29.11) | |
| WPD(sym4) | 29.27 (10.05) | 50.40 (33.50) | 15.54 (4.55) | 26.14 (29.18) | |
| WPD(sym5) | 29.19 (10.09) | 50.20 (33.47) | 15.43 (4.57) | 26.17 (29.22) | |
| WPD(sym6) | 29.11 (10.12) | 50.05 (33.43) | 15.35 (4.59) | 26.16 (29.24) | |
| WPD(coif1) | 29.43 (9.94) | 51.34 (33.59) | 15.97 (4.49) | 25.94 (28.88) | |
| WPD(coif2) | 29.25 (10.06) | 50.35 (33.49) | 15.51 (4.56) | 26.15 (29.19) | |
| WPD(coif3) | 29.08 (10.13) | 50.00 (33.42) | 15.33 (4.60) | 26.15 (29.25) | |
| WPD(fk4) | 29.21 (9.87) | 52.58 (33.48) | 16.11 (4.42) * | 26.40 (27.53) * | |
| WPD(fk6) | 29.32 (10.03) | 50.55 (33.51) | 15.59 (4.54) | 26.20 (29.08) | |
| WPD(fk8) | 29.15 (10.10) | 50.15 (33.45) | 15.38 (4.58) | 26.25 (29.18) | |
| Two-stage motion artifact correction techniques | WPD(db1)-CCA | 30.76 (12.29) * | 59.51(25.99) * | 16.55 (6.29) * | 36.58 (11.22) |
| WPD(db2)-CCA | 30.35 (12.50) | 57.57 (25.89) | 14.50 (5.85) | 39.62 (10.59) | |
| WPD(db3)-CCA | 29.42 (12.57) | 56.52 (25.71) | 13.72 (5.82) | 40.39 (10.60) | |
| WPD(fk4)-CCA | 30.36 (12.65) | 58.83 (25.93) | 14.97 (6.25) | 38.32 (10.90) | |
| WPD(fk6)-CCA | 29.12 (13.00) | 56.81 (25.16) | 13.81 (5.70) | 40.48 (10.43) | |
| WPD(fk8)-CCA | 28.86 (12.77) | 55.88 (25.10) | 12.41 (5.51) | 41.40 (10.08) * | |
| Type | Method | EEG (21 Records) | |
|---|---|---|---|
| Average ∆SNR (in dB) | (in %) | ||
| Single-stage motion artifact correction techniques | WPD(db1) | 26.20 (6.35) | 60.22 (21.79) * |
| WPD(sym4) | 26.46 (6.56) | 57.23 (22.11) | |
| WPD(coif1) | 26.70 (6.54)* | 58.19 (22.04) | |
| WPD(fk4) | 26.36 (6.36) | 59.37 (21.90) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Chowdhury, M.E.H.; Reaz, M.B.I.; Ali, S.H.M.; Bakar, A.A.A.; Kiranyaz, S.; Khandakar, A.; Alhatou, M.; Habib, R.; Hossain, M.M. Motion Artifacts Correction from Single-Channel EEG and fNIRS Signals Using Novel Wavelet Packet Decomposition in Combination with Canonical Correlation Analysis. Sensors 2022, 22, 3169. https://doi.org/10.3390/s22093169
Hossain MS, Chowdhury MEH, Reaz MBI, Ali SHM, Bakar AAA, Kiranyaz S, Khandakar A, Alhatou M, Habib R, Hossain MM. Motion Artifacts Correction from Single-Channel EEG and fNIRS Signals Using Novel Wavelet Packet Decomposition in Combination with Canonical Correlation Analysis. Sensors. 2022; 22(9):3169. https://doi.org/10.3390/s22093169
Chicago/Turabian StyleHossain, Md Shafayet, Muhammad E. H. Chowdhury, Mamun Bin Ibne Reaz, Sawal Hamid Md Ali, Ahmad Ashrif A. Bakar, Serkan Kiranyaz, Amith Khandakar, Mohammed Alhatou, Rumana Habib, and Muhammad Maqsud Hossain. 2022. "Motion Artifacts Correction from Single-Channel EEG and fNIRS Signals Using Novel Wavelet Packet Decomposition in Combination with Canonical Correlation Analysis" Sensors 22, no. 9: 3169. https://doi.org/10.3390/s22093169
APA StyleHossain, M. S., Chowdhury, M. E. H., Reaz, M. B. I., Ali, S. H. M., Bakar, A. A. A., Kiranyaz, S., Khandakar, A., Alhatou, M., Habib, R., & Hossain, M. M. (2022). Motion Artifacts Correction from Single-Channel EEG and fNIRS Signals Using Novel Wavelet Packet Decomposition in Combination with Canonical Correlation Analysis. Sensors, 22(9), 3169. https://doi.org/10.3390/s22093169








