Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Assessment of Eligibility
2.3. Study Design
2.4. Trial Visits
2.5. Statistical Analysis
3. Results
3.1. Median Absolute Relative Difference (MedARD)
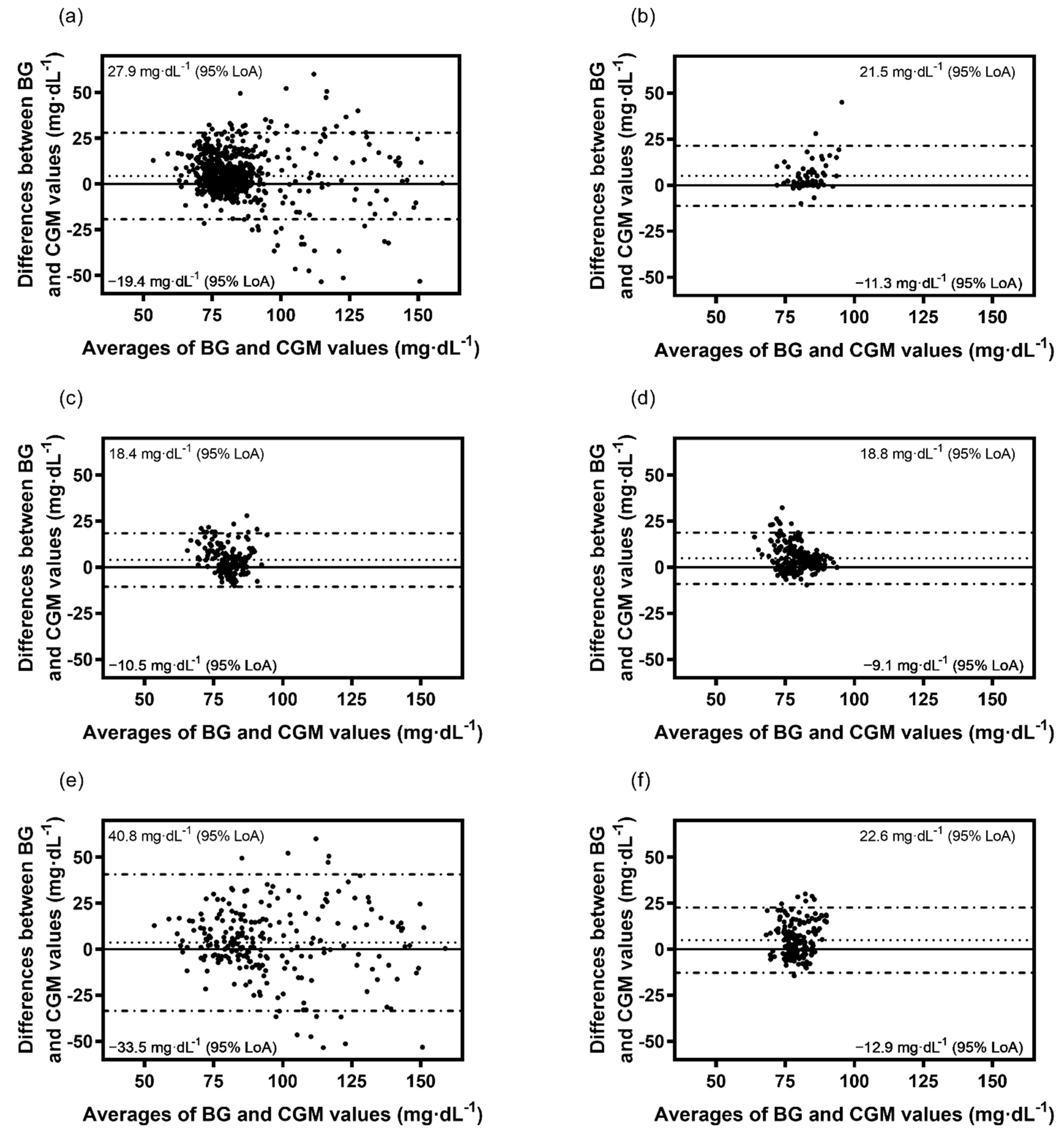
3.2. Bland-Altman Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freckmann, G.; Hagenlocher, S.; Baumstark, A.; Jendrike, N.; Gillen, R.C.; Rössner, K.; Haug, C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J. Diabetes Sci. Technol. 2007, 1, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Beck, R.W.; Close, K.L.; Grunberger, G.; Sacks, D.B.; Kowalski, A.; Brown, A.S.; Heinemann, L.; Aleppo, G.; Ryan, D.B.; et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018, 41, 2275–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, O.; Tripolt, N.; Pferschy, P.; Obermayer, A.; Kojzar, H.; Mueller, A.; Yildirim, H.; Sourij, C.; Eckstein, M.; Sourij, H. Performance of the Intermittently Scanned Continuous Glucose Monitoring (isCGM) System during a High Oral Glucose Challenge in Adults with Type 1 Diabetes-A Prospective Secondary Outcome Analysis. Biosensors 2021, 11, 22. [Google Scholar] [CrossRef]
- Moser, O.; Sternad, C.; Eckstein, M.L.; Szadkowska, A.; Michalak, A.; Mader, J.K.; Ziko, H.; Elsayed, H.; Aberer, F.; Sola-Gazagnes, A.; et al. Performance of intermittently scanned continuous glucose monitoring systems in people with type 1 diabetes: A pooled analysis. Diabetes Obes. Metab. 2021, 3, 522–529. [Google Scholar] [CrossRef]
- Moser, O.; Pandis, M.; Aberer, F.; Kojzar, H.; Hochfellner, D.; Elsayed, H.; Motschnig, M.; Augustin, T.; Kreuzer, P.; Pieber, T.R.; et al. A head-to-head comparison of personal and professional continuous glucose monitoring systems in people with type 1 diabetes: Hypoglycaemia remains the weak spot. Diabetes Obes. Metab. 2019, 21, 1043–1048. [Google Scholar] [CrossRef]
- Moser, O.; Yardley, J.E.; Bracken, R.M. Interstitial glucose and physical exercise in type 1 diabetes: Integrative physiology, technology, and the gap in-between. Nutrients 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: https://randomizer.org/%0Aabout/ (accessed on 12 June 2021).
- Pleus, S.; Schoemaker, M.; Morgenstern, K.; Schmelzeisen-Redeker, G.; Haug, C.; Link, M.; Zschornack, E.; Freckmann, G. Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J. Diabetes Sci. Technol. 2015, 9, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Wadwa, R.P.; Laffel, L.M.; Shah, V.N.; Garg, S.K. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol. Ther. 2018, 20, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Segev, N.; Hornung, L.N.; Tellez, S.E.; Courter, J.D.; Lawson, S.A.; Nathan, J.D.; Abu-El-Haija, M.; Elder, D.A. Continuous glucose monitoring in the intensive care unit following total pancreatectomy with islet autotransplantation in children: Establishing accuracy of the Dexcom G6 model. J. Clin. Med. 2021, 10, 1893. [Google Scholar] [CrossRef]
- Nagl, K.; Berger, G.; Aberer, F.; Ziko, H.; Weimann, K.; Bozic, I.; Rami-Merhar, B.; Mader, J.K. Performance of three different continuous glucose monitoring systems in children with type 1 diabetes during a diabetes summer camp. Pediatr. Diabetes 2021, 22, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Guillot, F.H.; Jacobs, P.G.; Wilson, L.M.; El Youssef, J.; Gabo, V.B.; Branigan, D.L.; Tyler, N.S.; Ramsey, K.; Riddell, M.C.; Castle, J.R. Accuracy of the Dexcom G6 glucose sensor during aerobic, resistance, and interval exercise in adults with type 1 diabetes. Biosensors 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Castorino, K.; Polsky, S.; O’malley, G.; Levister, C.; Nelson, K.; Farfan, C.; Brackett, S.; Puhr, S.; Levy, C.J. Performance of the Dexcom G6 Continuous Glucose Monitoring System in Pregnant Women with Diabetes. Diabetes Technol. Ther. 2020, 22, 943–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.B.; Zhang, X.; Puhr, S.A.; Johnson, T.K.; Walker, T.C.; Balo, A.K.; Price, D. Performance of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System in Pediatric Participants with Type 1 Diabetes. J. Diabetes Sci. Technol. 2019, 13, 254–258. [Google Scholar] [CrossRef]
- Shah, V.N.; Laffel, L.M.; Wadwa, R.P.; Garg, S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol. Ther. 2018, 20, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.L.; Hildebrand, K.L.; McCormick, S.A.; Bedel, M.J. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth. Analg. 1999, 88, 999–1003. [Google Scholar]
- Ryu, T. Fluid management in patients undergoing neurosurgery. Anesth. Pain Med. 2021, 16, 215–224. [Google Scholar] [CrossRef]
- Heer, M.; Baisch, F.; Kropp, J.; Gerzer, R.; Drummer, C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am. J. Physiol.-Ren. Physiol. 2000, 278, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Jaklevic, M.C. Start-Ups Tout Continuous Glucose Monitoring for People without Diabetes. JAMA 2021, 325, 2140–2142. Available online: https://pubmed.ncbi.nlm.nih.gov/33978710/ (accessed on 23 February 2022). [CrossRef]
- Moser, O.; Eckstein, M.L.; Mueller, A.; Tripolt, N.J.; Yildirim, H.; Abbas, F.; Pferschy, P.N.; Goswami, N.; Aberer, F.; Obermayer, A.; et al. Impact of a Single 36 Hours Prolonged Fasting Period in Adults with Type 1 Diabetes—A Cross-Over Controlled Trial. Front. Endocrinol. 2021, 12, 656346. [Google Scholar] [CrossRef]
- Nowotny, B.; Nowotny, P.J.; Strassburger, K.; Roden, M. Precision and accuracy of blood glucose measurements using three different instruments. Diabet. Med. 2012, 29, 260–265. [Google Scholar] [CrossRef] [PubMed]
| rtCGM System Accuracy, Median Absolute Relative Difference * [IQR], % | n | |
|---|---|---|
| Overall | 7.1 [3.3–10.8] | 803 |
| Overall Baseline | 2.6 [0.8–9.3] | 186 |
| Control | 5.9 [2.7–10.8] | 186 |
| Sodium chloride | 5.0 [2.7–10.2] | 204 |
| 5% Glucose | 11.0 [5.3–21.6] | 227 |
| Ringer’s solution | 7.5 [3.1–13.2] | 186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schierbauer, J.R.; Günther, S.; Haupt, S.; Zimmer, R.T.; Zunner, B.E.M.; Zimmermann, P.; Wachsmuth, N.B.; Eckstein, M.L.; Aberer, F.; Sourij, H.; et al. Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors 2022, 22, 3104. https://doi.org/10.3390/s22093104
Schierbauer JR, Günther S, Haupt S, Zimmer RT, Zunner BEM, Zimmermann P, Wachsmuth NB, Eckstein ML, Aberer F, Sourij H, et al. Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors. 2022; 22(9):3104. https://doi.org/10.3390/s22093104
Chicago/Turabian StyleSchierbauer, Janis R., Svenja Günther, Sandra Haupt, Rebecca T. Zimmer, Beate E. M. Zunner, Paul Zimmermann, Nadine B. Wachsmuth, Max L. Eckstein, Felix Aberer, Harald Sourij, and et al. 2022. "Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial" Sensors 22, no. 9: 3104. https://doi.org/10.3390/s22093104
APA StyleSchierbauer, J. R., Günther, S., Haupt, S., Zimmer, R. T., Zunner, B. E. M., Zimmermann, P., Wachsmuth, N. B., Eckstein, M. L., Aberer, F., Sourij, H., & Moser, O. (2022). Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors, 22(9), 3104. https://doi.org/10.3390/s22093104








