Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Instruments
2.3. Computational Analysis
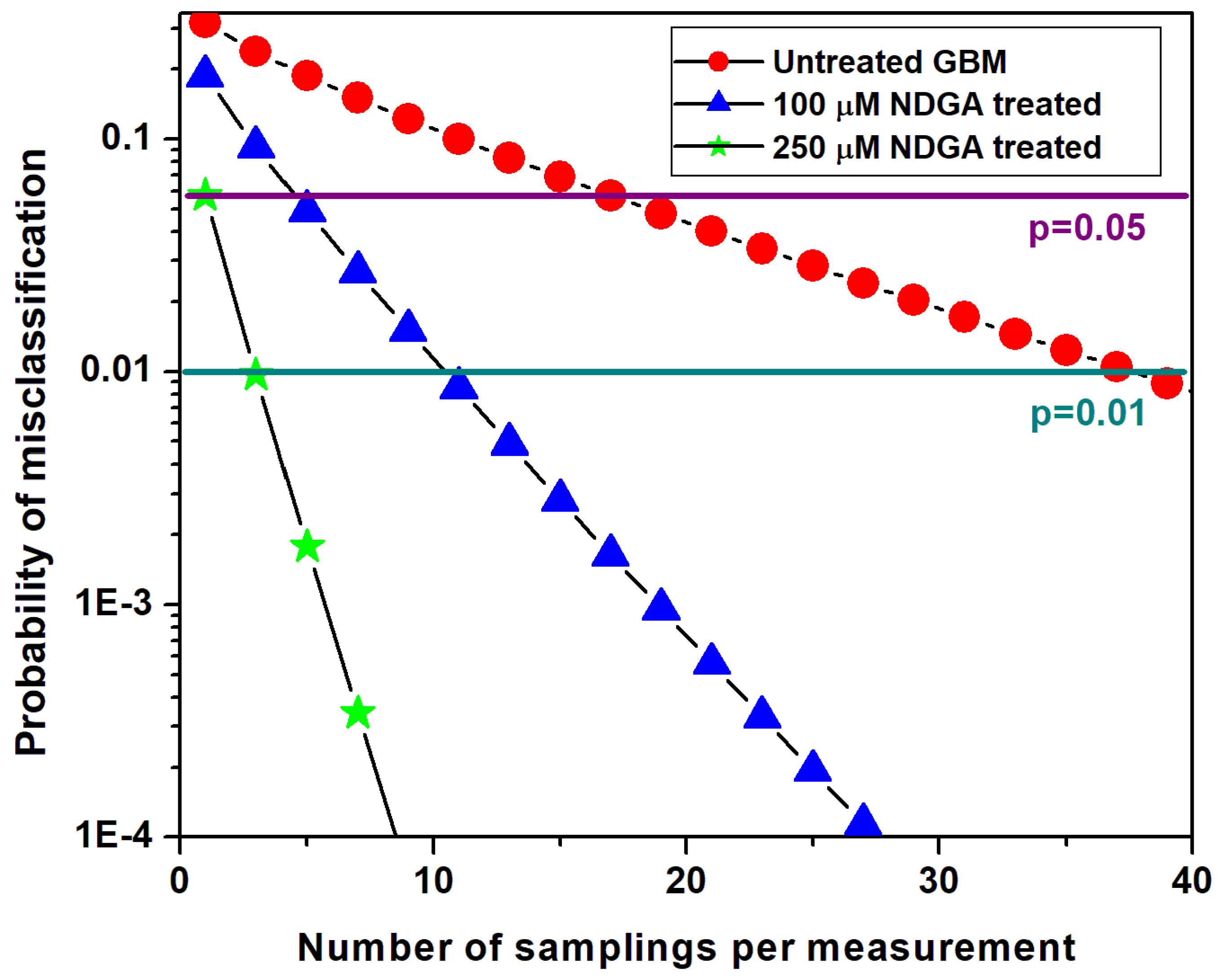
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ward, E.M.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Siegel, D.A.; Feuer, E.J.; Firth, A.U.; Kohler, B.A.; Scott, S.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J. Natl. Cancer Inst. 2019, 111, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C.; Massagué, J. Surviving at a distance: Organ specific metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; von Winterfeld, M.; Klauschen, F.; Bockmayr, M.; Lennerz, J.K.; Denkert, C.; Wolf, T.; Warth, A.; Dietel, M.; Anagnostopoulos, I.; et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget 2015, 6, 570–583. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Levin, V.A.; Leivel, S.; Gutin, P.H. Neoplasms of the central nervous system. In Cancer: Principles and Practice of Oncology, 9th ed.; DeVita, V.T., Hellman, S.J., Rosemberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 1700–1749. [Google Scholar]
- Sze, G. Diseases of the intracranial meninges: MR imaging features. Am. J. Roentgenol. 1993, 160, 727–733. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Garner, J.M.; Ellison, D.W.; Finkelstein, D.; Ganguly, D.; Du, Z.; Sims, M.; Yang, C.H.; Interiano, R.B.; Davidoff, A.M.; Pfeffer, L.M. Molecular heterogeneity in a patient-derived glioblastoma xenoline is regulated by different cancer stem cell populations. PLoS ONE 2015, 10, e0125838. [Google Scholar]
- Wong, E.T.; Hess, K.R.; Gleason, M.J.; Jaeckle, K.A.; Kyritsis, A.P.; Prados, M.D.; Levin, V.A.; Yung, W.A. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J. Clin. Oncol. 1999, 17, 2572–2578. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tachibana, O.; Sata, K.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996, 6, 217–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.H.; Sun, Y.; Pu, Y.; Boydston-White, S.; Liu, Y.; Alfano, R.R. Human brain cancer studied by resonance Raman spectroscopy. J. Biomed. Opt. 2012, 17, 116021. [Google Scholar] [CrossRef]
- Kong, K.; Rowlands, C.J.; Varma, S.; Perkins, W.; Leach, I.H.; Koloydenko, A.A.; Williams, H.C.; Notingher, I. Diagnosis of tumors during tissue-conserving surgery with integrated autofluorescence and Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 15189–15194. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Mercier, J.; St-Arnaud, K.; Guiot, M.C.; Leblond, F.; Petrecca, K. Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans. Biomed. Opt. Express 2016, 7, 5129–5137. [Google Scholar] [CrossRef]
- Alfano, R.R.; Tata, D.; Cordero, J.; Tomashefsky, P.; Longo, F.; Alfano, M. Laser induced fluorescence spectroscopy from native cancerous and normal tissue. IEEE J. Quant. Electron. 1984, 20, 1507–1511. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A. The biochemical, nanochemical and chemometric signatures of brain cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 8–19. [Google Scholar] [CrossRef]
- Manciu, F.S.; Ciubuc, J.D.; Parra, K.; Manciu, M.; Valenzuela, P.; Sundin, E.M.; Reza, L.; Francia, G. Label-free Raman imaging to monitor breast tumor signatures. Technol. Cancer Res. Treat. 2017, 16, 461–469. [Google Scholar] [CrossRef]
- Bi, X.; Rexer, B.; Arteaga, C.L.; Guo, M.; Mahadevan-Jansen, A. Evaluating HER2 amplification status and acquired drug resistance in breast cancer cells using Raman spectroscopy. J. Biomed. Opt. 2014, 19, 025001. [Google Scholar] [CrossRef] [PubMed]
- Hajjawi, O.S. Human brain biochemistry. Am. J. BioSci. 2014, 2, 122–134. [Google Scholar] [CrossRef]
- Ichimura, T.; Chiu, L.D.; Fujita, K.; Kawata, S.; Watanabe, T.M.; Yanagida, T.; Fujita, H. Visualizing cell state transition using Raman spectroscopy. PLoS ONE 2014, 9, e84478. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Andrade-Catto, A.; Cardenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmac. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Page, J.O. Determination of Nordihydroguaiaretic Acid in Creosote Bush. Anal. Chem. 1955, 27, 1266–1268. [Google Scholar] [CrossRef]
- Perry, C.W.; Kalnins, M.V.; Deitcher, K.H. Synthesis of Lignans. I. Nordihydroguaiaretic Acid. J. Org. Chem. 1972, 37, 4371–4376. [Google Scholar] [CrossRef]
- Sinnott, R.A.; Clark, D.W.; De Boer, K.F. Non-toxic therapeutic extract of Larrea tridentata. PCT Int. Appl. 1998, 27, WO1998015184A1. [Google Scholar]
- John, G.S.M.; Vuttarafhi, V.K.; Takeuchi, S.; Pitani, R.S.; Venkatraman, G.; Rayala, S.K. Facile synthesis and nanoscale features of a nanostructured nordihydroguaiaretic acid analog for therapeutic applications. J. Nanobiotechn. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Manda, G.; Rojo, A.I.; Martínez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic acid: From herbal medicine to clinical development for cancer and chronic diseases. Front. Pharmacol. 2020, 11, 151–172. [Google Scholar] [CrossRef]
- Li, F.; Pham, J.D.; Anderson, M.O.; Youngren, J.F. Nordihydroguaiaretic acid inhibits transforming growth factor β type 1 receptor activity and downstream signaling. Eur. J. Pharmacol. 2009, 616, 31–37. [Google Scholar] [CrossRef]
- Manciu, F.S.; Ciubuc, J.D.; Ochoa, K.; Dacha, P.; Subedi, M.; Guerrero, J.; Eastman, M.; Hodges, D.R.; Bennet, K.E. Comparative spectroscopic analysis of nordihydroguaiaretic acid and related natural products to inhibition of calcium oxalate calculi. Biointerface Res. Appl. Chem. 2019, 9, 3942–3948. [Google Scholar]
- Luo, J.; Chuang, T.; Cheung, J.; Quan, J.; Tsai, J.; Sullivan, C.; Hector, R.F.; Reed, M.J.; Meszaros, K.; King, S.R.; et al. Masoprocol (nordihydroguaiaretic acid): A new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur. J. Pharmacol. 1998, 346, 77–79. [Google Scholar] [CrossRef]
- Hwu, J.R.; Tseng, W.N.; Gnabre, J.; Giza, P.; Huang, R.C. Antiviral activities of methylated nordihydroguaiaretic acids. 1. Synthesis, structure identification, and inhibition of tat-regulated HIV transactivation. J. Med. Chem. 1998, 41, 2994–3000. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Dorr, R.T.; Timmermann, B.N. Nordihydroguaiaretic Acid: A Review of Its Numerous and Varied Biological Activities. Pharm. Biol. 2004, 42, 149–158. [Google Scholar] [CrossRef]
- Daniels, M.J.; Brucker Nourse, J., Jr.; Kim, H.; Sainati, V.; Schiavina, M.; Murrali, M.G.; Pan, B.; Ferrie, J.J.; Haney, C.M.; Moons, R.; et al. Cyclized NDGA modifies dynamic α-synuclein monomers preventing aggregation and toxicity. Sci. Rep. 2019, 9, 2937–2954. [Google Scholar] [CrossRef]
- Lü, J.M.; Nurko, J.; Weakley, S.M.; Jiang, J.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: An update. Med. Sci. Monit. 2010, 16, RA93–RA100. [Google Scholar]
- Ono, K.; Hasegawa, K.; Yoshiike, Y.; Takashima, A.; Yamada, M.; Naiki, H. Nordihydroguaiaretic acid potently breaks down pre-formed Alzheimer’s β-amyloid fibrils in vitro. J. Neurochem. 2002, 81, 434–440. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Weinberg, V.K.; Huang, Y.; Mi, J.; Formaker, C.G.; Small, E.J.; Harzstark, A.L.; Lin, A.M.; Fong, L.; Ryan, C.J. A phase II study of insulin-like growth factor receptor inhibition with nordihydroguaiaretic acid in men with non-metastatic hormone-sensitive prostate cancer. Oncol. Rep. 2012, 27, 3–9. [Google Scholar] [CrossRef][Green Version]
- Manciu, F.S.; Guerrero, J.; Rivera, D.; Chang, S.Y.; Bennet, K.E. Combined Theoretical and Experimental Study of Nordihydroguaiaretic Acid—From Traditional Medicine to Modern Spectroscopic Research. Biointerface Res. Appl. Chem. 2020, 10, 6728–6743. [Google Scholar]
- Seufferlein, T.; Seckl, M.J.; Schwarz, E.; Beil, M.; Baust, H.; Lührs, H.; Schmid, R.M.; Adler, G. Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br. J. Cancer 2002, 86, 1188–1196. [Google Scholar] [CrossRef]
- Sun, Y.; Giacalone, N.J.; Lu, B. Terameprocol (tetra-O-methyl nordihydroguaiaretic acid), an inhibitor of Sp1-mediated survivin transcription, induces radiosensitization in non-small cell lung carcinoma. J. Thorac. Oncol. 2011, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.L.; Pokorny, J.L.; Schroeder, M.A.; Sarkaria, J.N. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograsft models for translational biology studies and drug discovery. Curr. Protoc. Pharmacol. 2011, 52, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Gueron, G.; Anselmino, N.; Chiarella, P.; Ortiz, E.G.; Lage Vickers, S.; Paez, A.V.; Giudice, J.; Contin, M.D.; Leonardi, D.; Jaworski, F.; et al. Game-changing restraint of ROS-damaged phenylalanine, upon tumor metastasis. Cell Death Dis. 2018, 9, 140. [Google Scholar] [CrossRef]
- Manciu, M.; Cardenas, M.; Bennet, K.E.; Maran, A.; Yaszemski, M.J.; Maldonado, T.A.; Manciu, F.S. Assessment of Renal Osteodystrophy via Computational Analysis of Label-free Raman Detection of Multiple Biomarkers. Diagnostics 2020, 10, 79. [Google Scholar] [CrossRef]
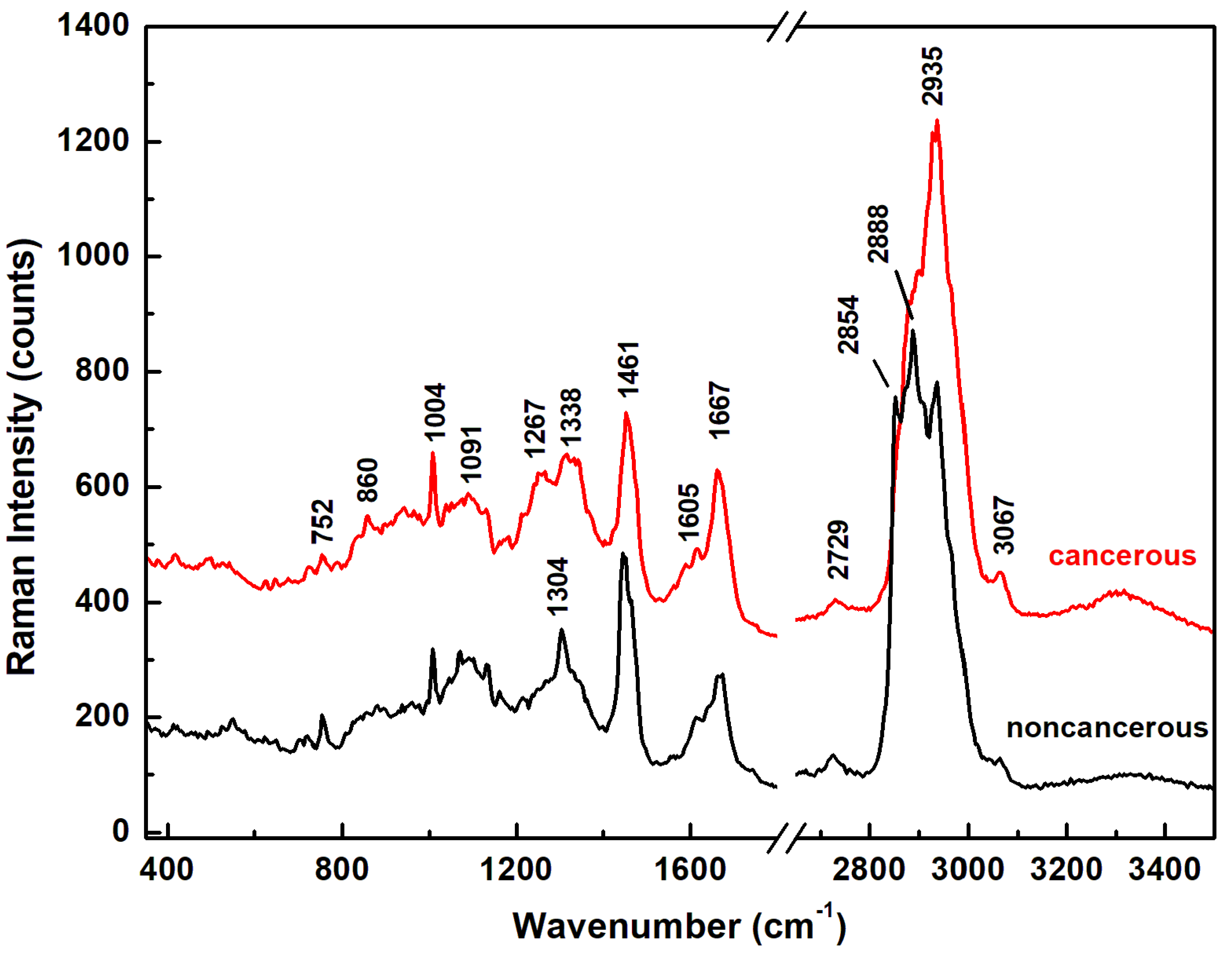
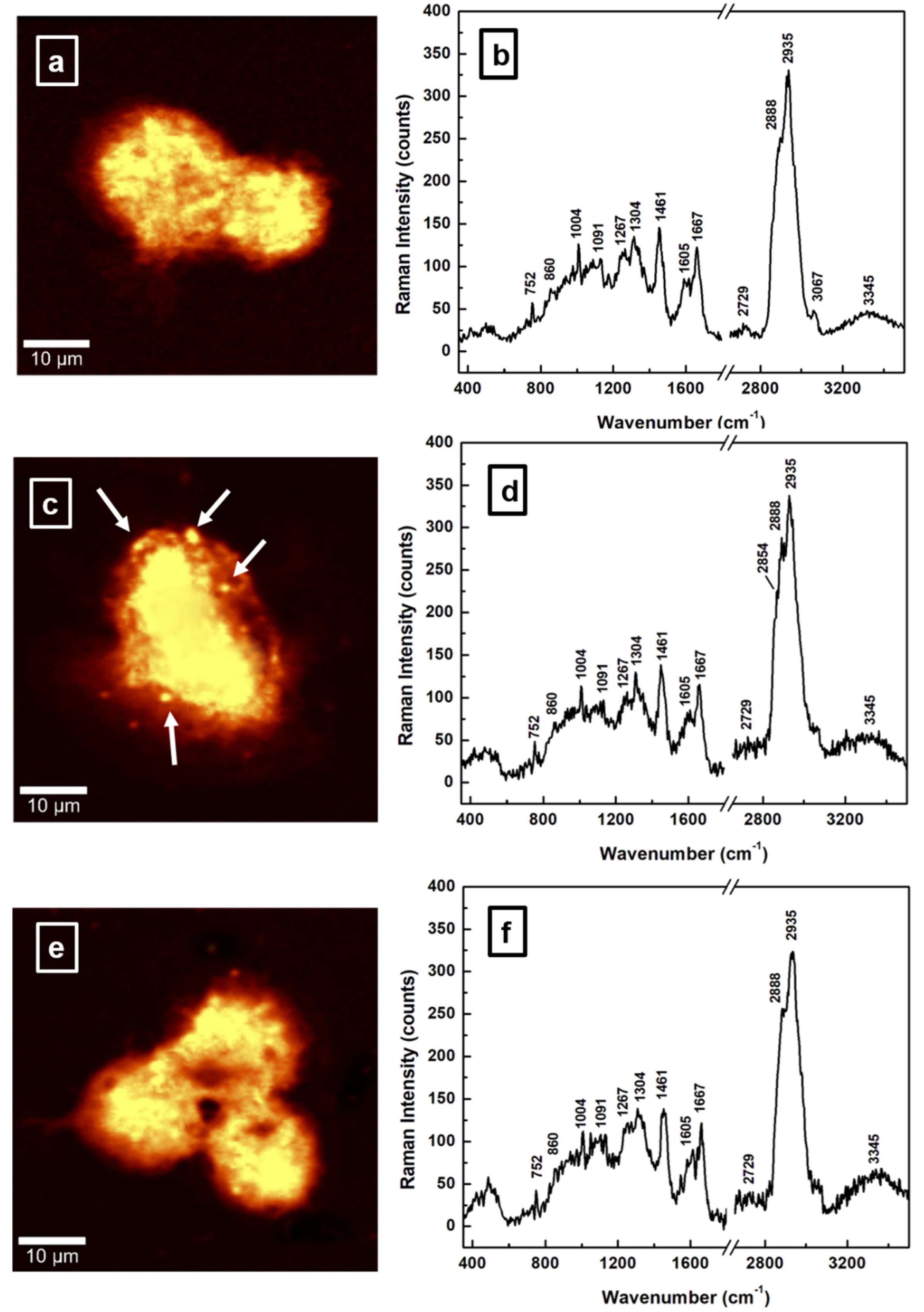
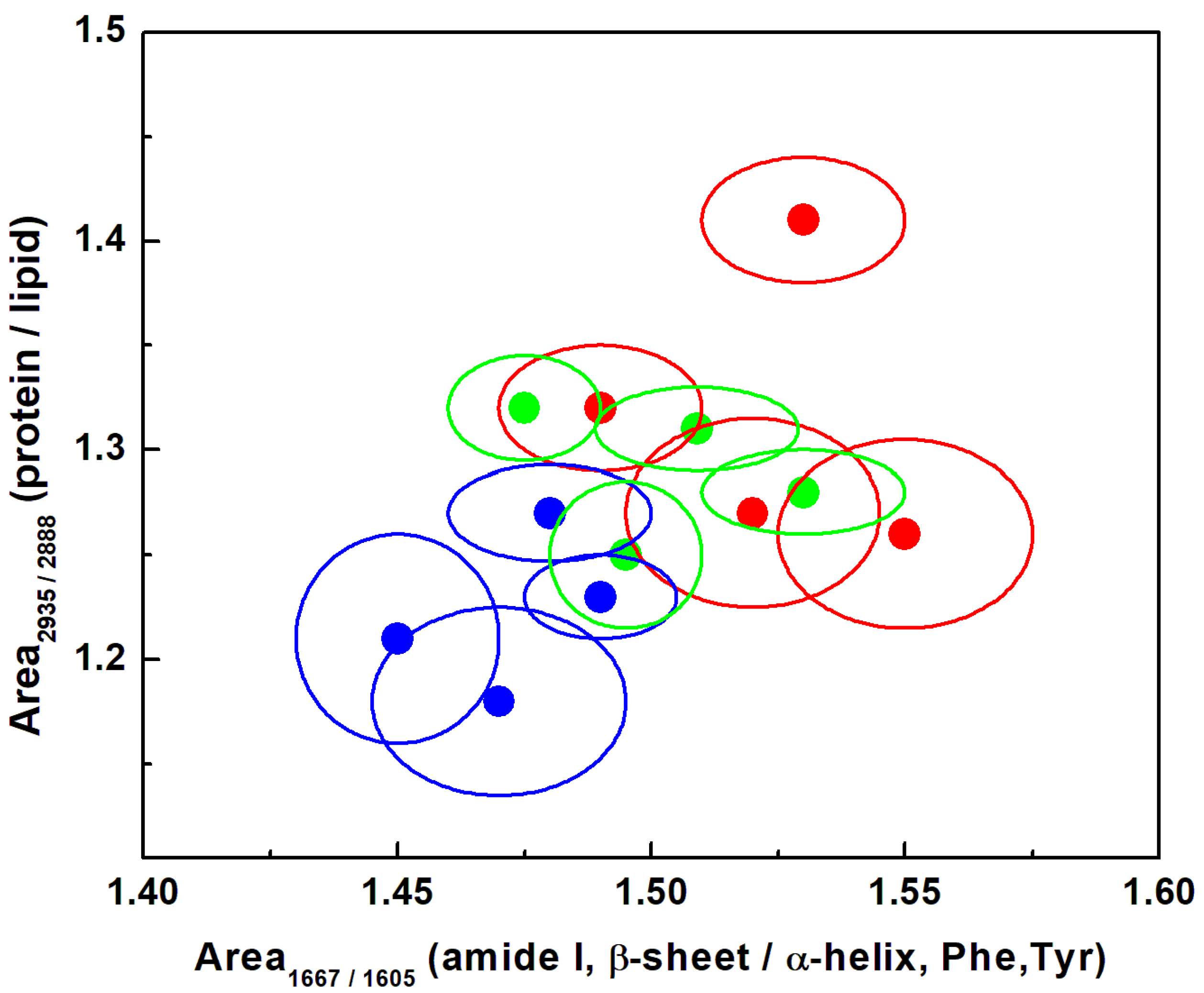
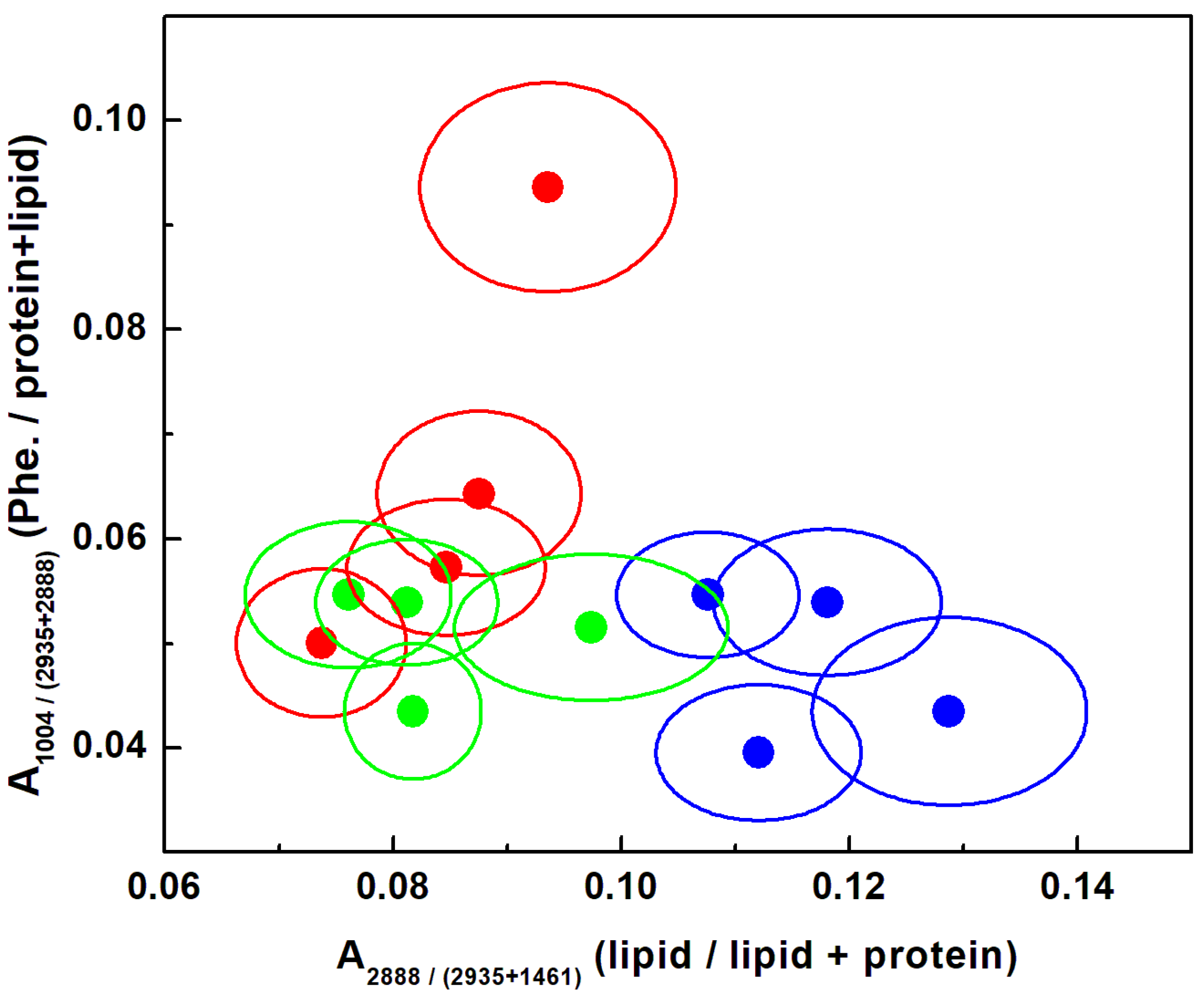

| Raman Wavenumber cm−1 | Assignment a,b | Tentative Attribution a,b |
|---|---|---|
| 752 | CH2 rocking, symmetric breathing | Tryptophan, cytochrome c, mitochondria a |
| Nucleic acids, tryptophan b | ||
| 860 | CC stretch | Tyrosine, proline, glycogen b |
| 1004 | Symmetric CC aromatic ring breathing | Phenylalanine, collagen IV, I a Phenylalanine b |
| 1091 | CC skeletal stretch, PO2 symmetric stretch | Protein, phospholipids, glycogen, collagen IV, I a Phospholipids, nucleic acids b |
| 1267 | Amide III, =C–H bend, P=O asymmetric stretch | Homo polypeptide a, Fatty acids b |
| 1304 | Amide III, N–H bend, α-helix, C–N stretch, and CH3 bend, C–H2 twist | Bending and stretching coupled in-phase, collagen IV, I a Lipids, phospholipids, collagen, protein, DNA b |
| 1338 | CH2 deformation | Protein, A and G of DNA/RNA a Tryptophan b |
| 1461 | CH2 or CH3 out-of-phase deformation, CN bend | Lipid, protein a Protein b |
| 1605 | Amide I α-helix, CO stretch, C=C bend | Protein, phenylalanine, tyrosine a Unsaturated fatty acids, triglycerides b |
| 1667 | Amide I β-sheet, C=O stretch | Unordered or random structure, collagen IV, I a Proteins, cholesterol esters b |
| 2729 | CH3 in-phase deformation overtone | |
| 2854 | CH2 symmetric stretch | Fatty acids, triglycerides a,b |
| 2888 | CH2 asymmetric stretch | Lipids a,b |
| 2935 | CH3 symmetric stretch | Proteins a,b |
| 3067 | CH3–(C=O), C–H aromatic | Nucleic acids, proteins b |
| True | Predicted | |||
| Untreated GBM | 100 µM NDGA-Treated | 250 µM NDGA-Treated | ||
| Untreated GBM | 68.2% | 31.8% | 0% | |
| 100 µM NDGA-treated | 16.8% | 81.2% | 2.0% | |
| 250 µM NDGA-treated | 0 % | 4.8% | 95.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manciu, F.S.; Guerrero, J.; Bennet, K.E.; Chang, S.-Y.; Rahman, M.; Martinez Lopez, L.V.; Chantigian, S.; Castellanos, M.; Manciu, M. Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme. Sensors 2022, 22, 2643. https://doi.org/10.3390/s22072643
Manciu FS, Guerrero J, Bennet KE, Chang S-Y, Rahman M, Martinez Lopez LV, Chantigian S, Castellanos M, Manciu M. Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme. Sensors. 2022; 22(7):2643. https://doi.org/10.3390/s22072643
Chicago/Turabian StyleManciu, Felicia S., Jose Guerrero, Kevin E. Bennet, Su-Youne Chang, Masum Rahman, Lizbeth V. Martinez Lopez, Siobhan Chantigian, Mariana Castellanos, and Marian Manciu. 2022. "Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme" Sensors 22, no. 7: 2643. https://doi.org/10.3390/s22072643
APA StyleManciu, F. S., Guerrero, J., Bennet, K. E., Chang, S.-Y., Rahman, M., Martinez Lopez, L. V., Chantigian, S., Castellanos, M., & Manciu, M. (2022). Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme. Sensors, 22(7), 2643. https://doi.org/10.3390/s22072643






