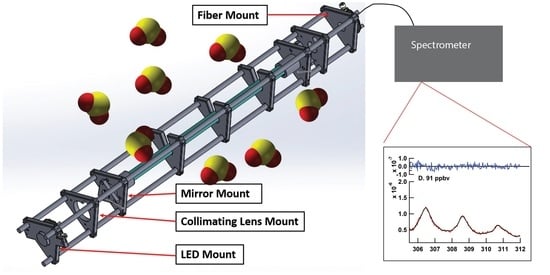
Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS)
Abstract
1. Introduction
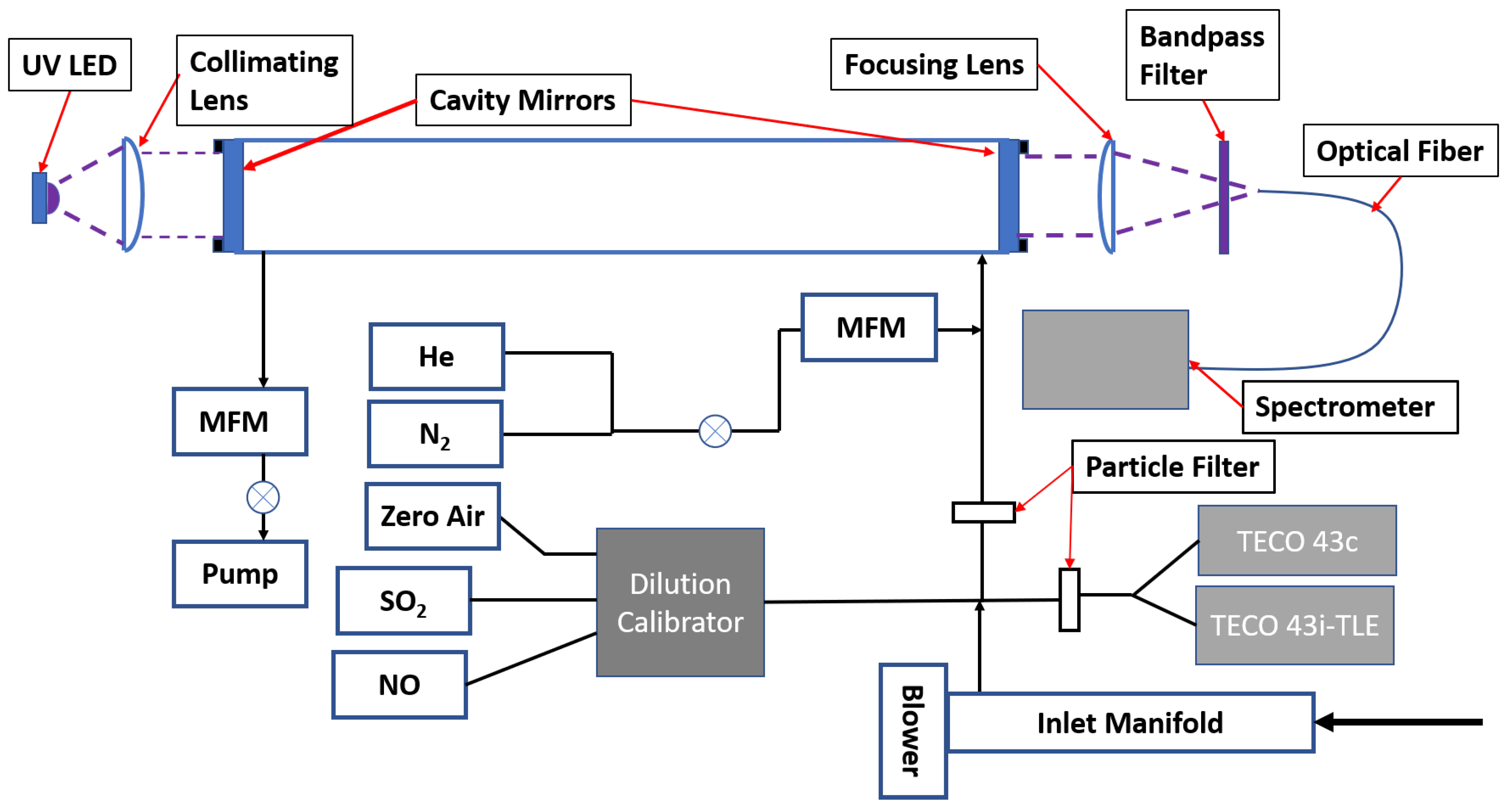
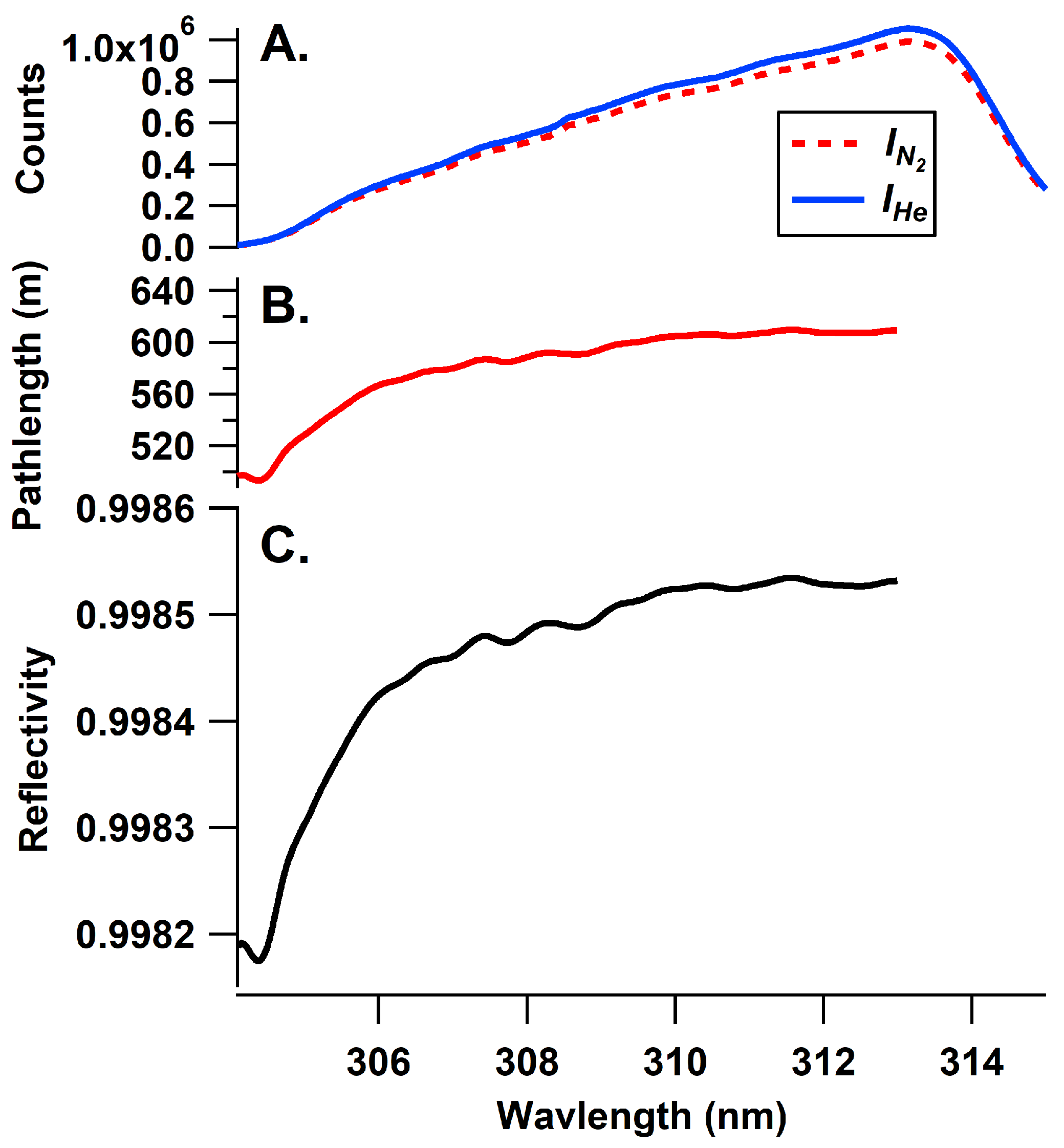
2. Materials and Methods
Comparison of SO2 Measurements
3. Results
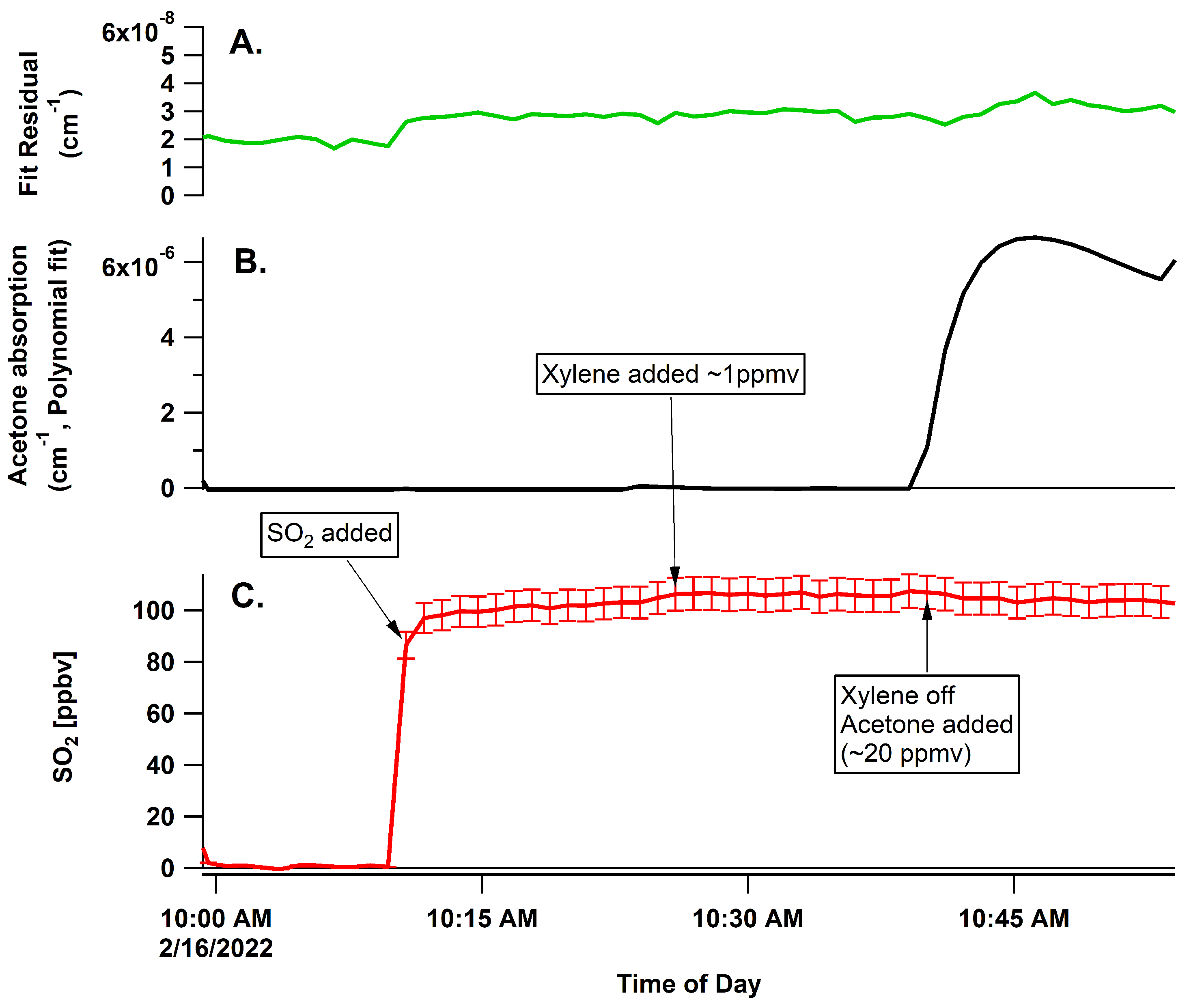
3.1. Comparison to SO2 Standard
3.2. Interferences
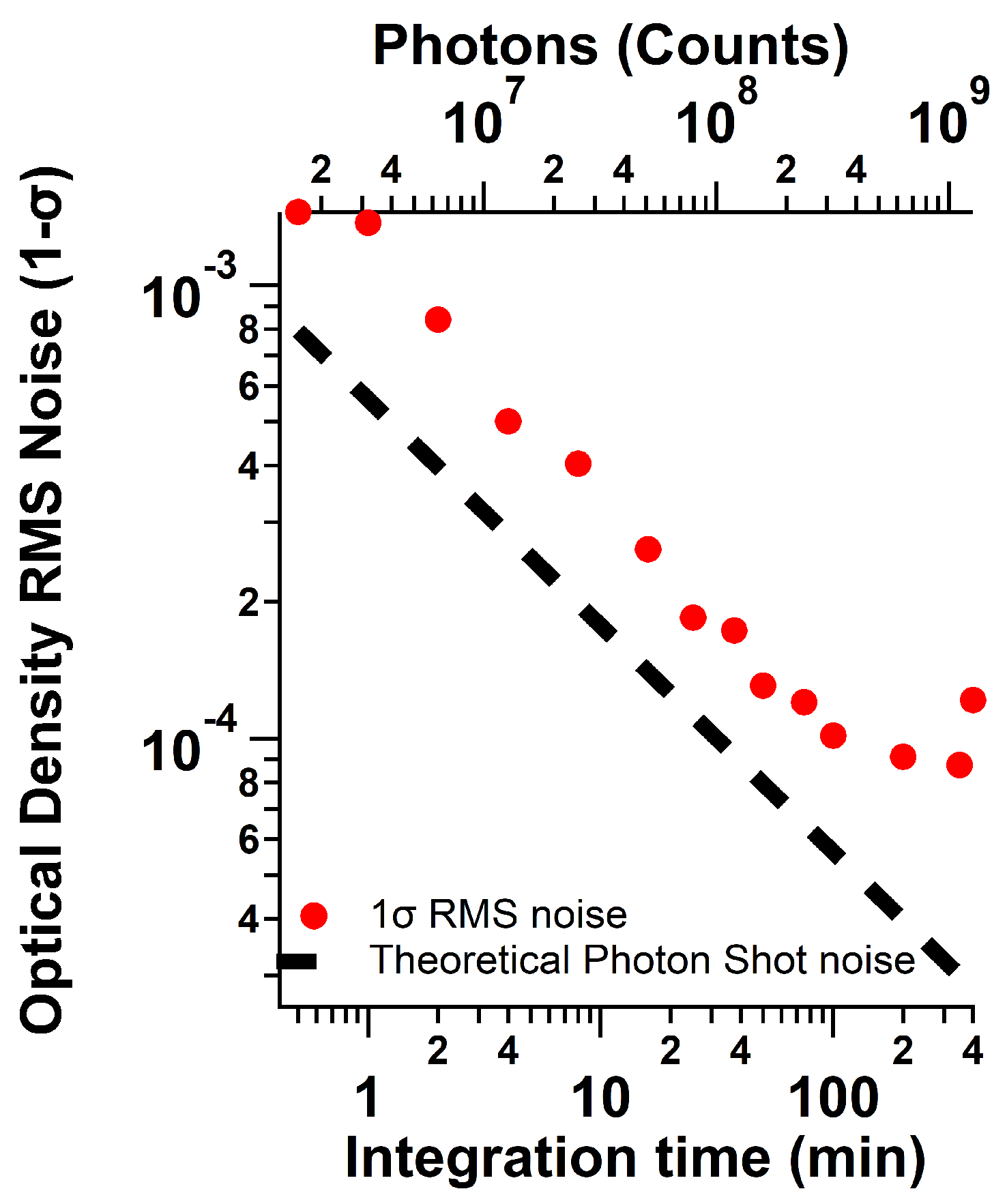
3.3. Signal-Averaging Effect on Precision and Accuracy
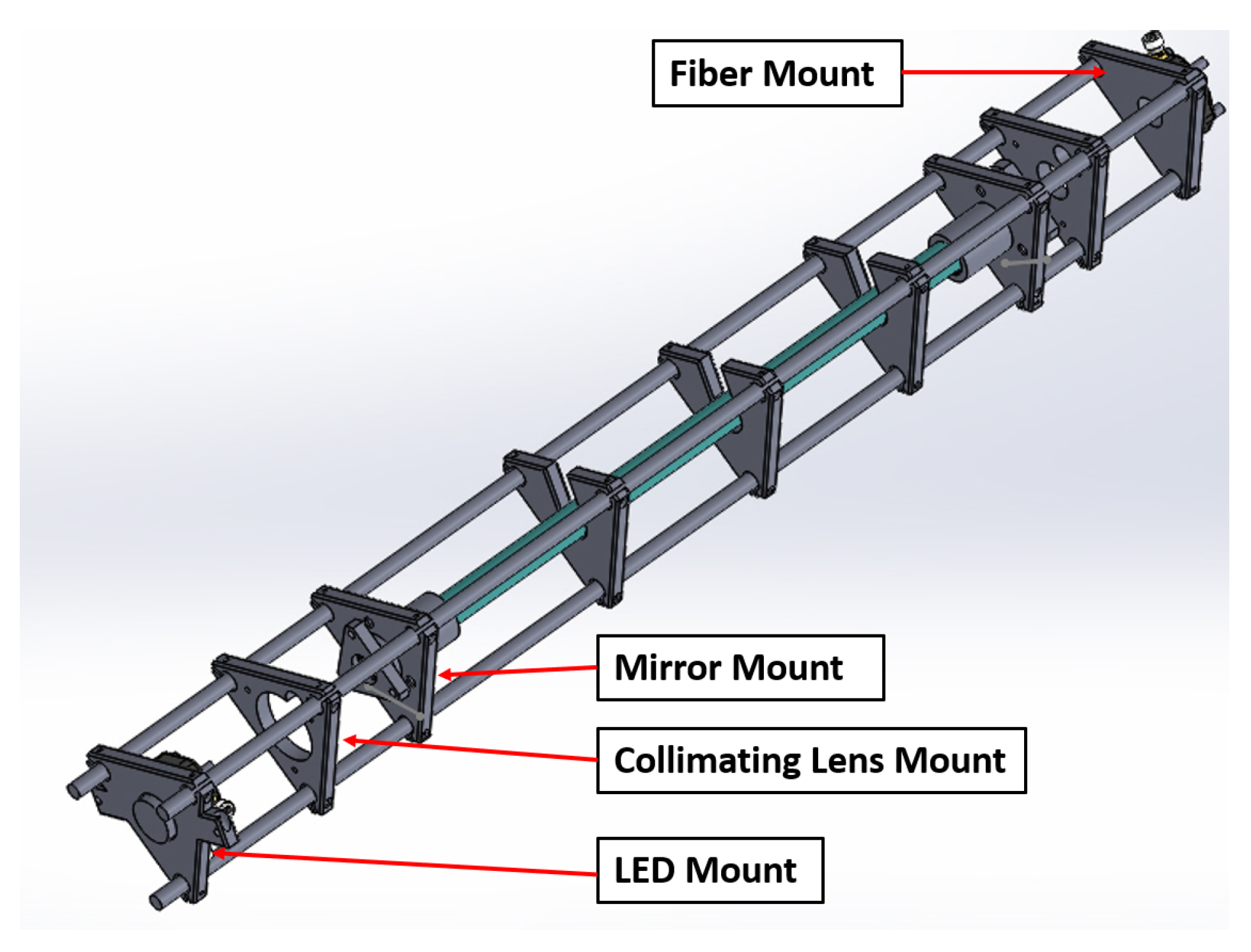
3.4. Performance of 3D-Printed Cage System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBCEAS | Broadband Cavity-Enhanced Absorption Spectroscopy |
| DOAS | Differential Optical Absorption Spectroscospy |
| PLA | Poly-lactic Acid |
| slpm | standard liters per minute |
| sccm | standard cubic centimeters per minute |
| ASA | Acrylic styrene-acrylonitrile |
References
- Schwartz, S.E. Both Sides Now. Ann. N. Y. Acad. Sci. 1987, 502, 83–144. [Google Scholar] [CrossRef]
- Holasek, R.E.; Self, S.; Woods, A.W. Satellite observations and interpretation of the 1991 Mount Pinatubo eruption plumes. J. Geophys. Res. Solid Earth 1996, 101, 27635–27655. [Google Scholar] [CrossRef]
- Logan, J.A.; McElroy, M.B.; Wofsy, S.C.; Prather, M.J. Oxidation of CS2 and COS: Sources for atmospheric SO2. Nature 1979, 281, 185–188. [Google Scholar] [CrossRef]
- Smith, S.J.; van Aardenne, J.; Klimont, Z.; Andres, R.J.; Volke, A.; Delgado Arias, S. Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos. Chem. Phys. 2011, 11, 1101–1116. [Google Scholar] [CrossRef]
- Hidy, G.M.; Blanchard, C. The changing face of lower tropospheric sulfur oxides in the United States. Elem. Sci. Anthr. 2016, 4, 000138. [Google Scholar] [CrossRef]
- EPA. Integrated Science Assessment for Sulfur Oxides-Health Criteria; Technical Report EPA-HQ-ORD-2013-0357; U.S. Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Bluth, G.J.S.; Rose, W.I.; Sprod, I.E.; Krueger, A.J. Stratospheric Loading of Sulfur From Explosive Volcanic Eruptions. J. Geol. 1997, 105, 671–684. [Google Scholar] [CrossRef]
- Rasch, P.J.; Tilmes, S.; Turco, R.P.; Robock, A.; Oman, L.; Chen, C.C.J.; Stenchikov, G.L.; Garcia, R.R. An Overview of Geoengineering of Climate Using Stratospheric Sulphate Aerosols. Philos. Trans. Math. Phys. Eng. Sci. 2008, 366, 4007–4037. [Google Scholar] [CrossRef]
- Visioni, D.; Pitari, G.; Aquila, V. Sulfate geoengineering: A review of the factors controlling the needed injection of sulfur dioxide. Atmos. Chem. Phys. 2017, 17, 3879–3889. [Google Scholar] [CrossRef]
- Parrish, D.D.; Fehsenfeld, F.C. Methods for gas-phase measurements of ozone, ozone precursors and aerosol precursors. Atmos. Environ. 2000, 34, 1921–1957. [Google Scholar] [CrossRef]
- Stecher, H.A., III; Luther, G.W., III; MacTaggart, D.L.; Farwell, S.O.; Crosley, D.R.; Dorko, W.D.; Goldan, P.D.; Beltz, N.; Krischke, U.; Luke, W.T.; et al. Results of the Gas-Phase Sulfur Intercomparison Experiment (GASIE): Overview of experimental setup, results and general conclusions. J. Geophys. Res. Atmos. 1997, 102, 16219–16236. [Google Scholar] [CrossRef]
- West, P.W.; Gaeke, G.C. Fixation of Sulfur Dioxide as Disulfitomercurate (II) and Subsequent Colorimetric Estimation. Anal. Chem. 1956, 28, 1816–1819. [Google Scholar] [CrossRef]
- Gilliam, J.; Hall, E. Reference and Equivalent Methods Used to Measure National Ambient Air Quality Standards (NAAQS) Criteria Air Pollutants; Technical Report EPA/600/R-16/139; U.S. Environmental Protection Agency: Washington, DC, USA, 2016; Volume I. [Google Scholar]
- Yin, X.; Wu, H.; Dong, L.; Li, B.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittel, F.K. ppb-Level SO2 Photoacoustic Sensors with a Suppressed Absorption–Desorption Effect by Using a 7.41 μm External-Cavity Quantum Cascade Laser. ACS Sens. 2020, 5, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.S.; Liu, Y.; Wang, L.; Zhang, J. Detection of Sulfur Dioxide by Cavity Ring-Down Spectroscopy. Environ. Sci. Technol. 2011, 45, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Stutz, J.; Platt, U. Improving long-path differential optical absorption spectroscopy with a quartz-fiber mode mixer. Appl. Opt. 1997, 36, 1105–1115. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kim, Y.J.; Lee, J. Application of a long-path differential optical absorption spectrometer (LP-DOAS) on the measurements of NO2, SO2, O3, and HNO2 in Gwangju, Korea. J. Environ. Manag. 2008, 86, 750–759. [Google Scholar] [CrossRef]
- Speidel, M.; Nau, R.; Arnold, F.; Schlager, H.; Stohl, A. Sulfur dioxide measurements in the lower, middle and upper troposphere: Deployment of an aircraft-based chemical ionization mass spectrometer with permanent in-flight calibration. Atmos. Environ. 2007, 41, 2427–2437. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Zhu, J.; Guo, Y.; Zhang, R.; Liu, Y.; Zhang, Y.; Yu, Q.; Ma, W.; Zhou, B. Surveillance of SO2 and NO2 from ship emissions by MAX-DOAS measurements and the implications regarding fuel sulfur content compliance. Atmos. Chem. Phys. 2019, 19, 13611–13626. [Google Scholar] [CrossRef]
- Ball, S.M.; Langridge, J.M.; Jones, R.L. Broadband cavity enhanced absorption spectroscopy using light emitting diodes. Chem. Phys. Lett. 2004, 398, 68–74. [Google Scholar] [CrossRef]
- Langridge, J.M.; Ball, S.M.; Jones, R.L. A compact broadband cavity enhanced absorption spectrometer for detection of atmospheric NO2 using light emitting diodes. Analyst 2006, 131, 916–922. [Google Scholar] [CrossRef]
- Washenfelder, R.A.; Langford, A.O.; Fuchs, H.; Brown, S.S. Measurement of glyoxal using an incoherent broadband cavity enhanced absorption spectrometer. Atmos. Chem. Phys. 2008, 8, 7779–7793. [Google Scholar] [CrossRef]
- Thalman, R.; Volkamer, R. Inherent calibration of a blue LED-CE-DOAS instrument to measure iodine oxide, glyoxal, methyl glyoxal, nitrogen dioxide, water vapour and aerosol extinction in open cavity mode. Atmos. Meas. Tech. 2010, 3, 1797–1814. [Google Scholar] [CrossRef]
- Venables, D.S.; Gherman, T.; Orphal, J.; Wenger, J.C.; Ruth, A.A. High Sensitivity in Situ Monitoring of NO3 in an Atmospheric Simulation Chamber Using Incoherent Broadband Cavity-Enhanced Absorption Spectroscopy. Environ. Sci. Technol. 2006, 40, 6758–6763. [Google Scholar] [CrossRef] [PubMed]
- Axson, J.L.; Washenfelder, R.A.; Kahan, T.F.; Young, C.J.; Vaida, V.; Brown, S.S. Absolute ozone absorption cross section in the Huggins Chappuis minimum (350–470 nm) at 296 K. Atmos. Chem. Phys. 2011, 11, 11581–11590. [Google Scholar] [CrossRef]
- Thalman, R.; Baeza-Romero, M.T.; Ball, S.M.; Borrás, E.; Daniels, M.J.S.; Goodall, I.C.A.; Henry, S.B.; Karl, T.; Keutsch, F.N.; Kim, S.; et al. Instrument intercomparison of glyoxal, methyl glyoxal and NO2 under simulated atmospheric conditions. Atmos. Meas. Tech. 2015, 8, 1835–1862. [Google Scholar] [CrossRef]
- Dixneuf, S.; Ruth, A.A.; Vaughan, S.; Varma, R.M.; Orphal, J. The time dependence of molecular iodine emission from Laminaria digitata. Atmos. Chem. Phys. 2009, 9, 823–829. [Google Scholar] [CrossRef]
- Vaughan, S.; Gherman, T.; Ruth, A.A.; Orphal, J. Incoherent broad-band cavity-enhanced absorption spectroscopy of the marine boundary layer species I2, IO and OIO. Phys. Chem. Chem. Phys. 2008, 10, 4471–4477. [Google Scholar] [CrossRef]
- Dong, M.; Zhao, W.; Huang, M.; Chen, W.; Hu, C.; Gu, X.; Pei, S.; Huang, W.; Zhang, W. Near-ultraviolet Incoherent Broadband Cavity Enhanced Absorption Spectroscopy for OClO and CH2O in Cl-initiated Photooxidation Experiment. Chin. J. Chem. Phys. 2013, 26, 133–139. [Google Scholar] [CrossRef][Green Version]
- Young, I.A.K.; Jones, R.L.; Pope, F.D. The UV and visible spectra of chlorine peroxide: Constraining the atmospheric photolysis rate. Geophys. Res. Lett. 2014, 41, 1781–1788. [Google Scholar] [CrossRef]
- Hoch, D.J.; Buxmann, J.; Sihler, H.; Pöhler, D.; Zetzsch, C.; Platt, U. An instrument for measurements of BrO with LED-based Cavity-Enhanced Differential Optical Absorption Spectroscopy. Atmos. Meas. Tech. 2014, 7, 199–214. [Google Scholar] [CrossRef]
- Gherman, T.; Venables, D.S.; Vaughan, S.; Orphal, J.; Ruth, A.A. Incoherent Broadband Cavity-Enhanced Absorption Spectroscopy in the near-Ultraviolet: Application to HONO and NO2. Environ. Sci. Technol. 2008, 42, 890–895. [Google Scholar] [CrossRef]
- Washenfelder, R.A.; Attwood, A.R.; Flores, J.M.; Zarzana, K.J.; Rudich, Y.; Brown, S.S. Broadband cavity-enhanced absorption spectroscopy in the ultraviolet spectral region for measurements of nitrogen dioxide and formaldehyde. Atmos. Meas. Tech. 2016, 9, 41–52. [Google Scholar] [CrossRef]
- Wang, M.; Varma, R.; Venables, D.S.; Zhou, W.; Chen, J. A Demonstration of Broadband Cavity-Enhanced Absorption Spectroscopy at Deep-Ultraviolet Wavelengths: Application to Sensitive Real-Time Detection of the Aromatic Pollutants Benzene, Toluene, and Xylene. Anal. Chem. 2022, 94, 4286–4293. [Google Scholar] [CrossRef] [PubMed]
- Thalman, R.; Volkamer, R. Temperature dependent absorption cross-sections of O2–O2 collision pairs between 340 and 630 nm and at atmospherically relevant pressure. Phys. Chem. Chem. Phys. 2013, 15, 15371–15381. [Google Scholar] [CrossRef]
- Brown, S.S. Absorption Spectroscopy in High-Finesse Cavities for Atmospheric Studies. Chem. Rev. 2003, 103, 5219–5238. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, A.; Scherer, J.J.; Paul, J.B. CW Integrated cavity output spectroscopy. Chem. Phys. Lett. 1999, 307, 343–349. [Google Scholar] [CrossRef]
- Kebabian, P.L.; Herndon, S.C.; Freedman, A. Detection of Nitrogen Dioxide by Cavity Attenuated Phase Shift Spectroscopy. Anal. Chem. 2005, 77, 724–728. [Google Scholar] [CrossRef]
- Richard, L.; Ventrillard, I.; Chau, G.; Jaulin, K.; Kerstel, E.; Romanini, D. Optical-feedback cavity-enhanced absorption spectroscopy with an interband cascade laser: Application to SO2 trace analysis. Appl. Phys. B 2016, 122, 247. [Google Scholar] [CrossRef]
- Chen, J.; Venables, D.S. A broadband optical cavity spectrometer for measuring weak near-ultraviolet absorption spectra of gases. Atmos. Meas. Tech. 2011, 4, 425–436. [Google Scholar] [CrossRef]
- Bogumil, K.; Orphal, J.; Homann, T.; Voigt, S.; Spietz, P.; Fleischmann, O.; Vogel, A.; Hartmann, M.; Kromminga, H.; Bovensmann, H.; et al. Measurements of molecular absorption spectra with the SCIAMACHY pre-flight model: Instrument characterization and reference data for atmospheric remote-sensing in the 230–2380 nm region. J. Photochem. Photobiol. A Chem. 2003, 157, 167–184. [Google Scholar] [CrossRef]
- Vandaele, A.; Hermans, C.; Simon, P.; Carleer, M.; Colin, R.; Fally, S.; Mérienne, M.; Jenouvrier, A.; Coquart, B. Measurements of the NO2 absorption cross-section from 42,000 cm−1 to 10,000 cm−1 (238–1000 nm) at 220 K and 294 K. J. Quant. Spectrosc. Radiat. Transf. 1998, 59, 171–184. [Google Scholar] [CrossRef]
- Wilmouth, D.M.; Hanisco, T.F.; Donahue, N.M.; Anderson, J.G. Fourier Transform Ultraviolet Spectroscopy of the A 2Π3/2 ← X 2Π3/2 Transition of BrO. J. Phys. Chem. A 1999, 103, 8935–8945. [Google Scholar] [CrossRef]
- Gierczak, T.; Burkholder, J.B.; Bauerle, S.; Ravishankara, A. Photochemistry of acetone under tropospheric conditions. Chem. Phys. 1998, 231, 229–244. [Google Scholar] [CrossRef]
- Rufus, J.; Stark, G.; Smith, P.L.; Pickering, J.C.; Thorne, A.P. High-resolution photoabsorption cross section measurements of SO2, 2: 220 to 325 nm at 295 K. J. Geophys. Res. Planets 2003, 108. [Google Scholar] [CrossRef]
- Meller, R.; Moortgat, G.K. Temperature dependence of the absorption cross sections of formaldehyde between 223 and 323 K in the wavelength range 225–375 nm. J. Geophys. Res. Atmos. 2000, 105, 7089–7101. [Google Scholar] [CrossRef]
- Barbero, A.; Blouzon, C.; Savarino, J.; Caillon, N.; Dommergue, A.; Grilli, R. A compact incoherent broadband cavity-enhanced absorption spectrometer for trace detection of nitrogen oxides, iodine oxide and glyoxal at levels below parts per billion for field applications. Atmos. Meas. Tech. 2020, 13, 4317–4331. [Google Scholar] [CrossRef]
- Thalman, R.; Zarzana, K.J.; Tolbert, M.A.; Volkamer, R. Rayleigh scattering cross-section measurements of nitrogen, argon, oxygen and air. J. Quant. Spectrosc. Radiat. Transf. 2014, 147, 171–177. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Hese, A.; Ruth, A.A. Incoherent broad-band cavity-enhanced absorption spectroscopy. Chem. Phys. Lett. 2003, 371, 284–294. [Google Scholar] [CrossRef]
- Platt, U.; Stutz, J. Differential Optical Absorption Spectroscopy (DOAS)—Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2008; Volume 15. [Google Scholar] [CrossRef]
- Dankaert, T.; Fayt, C.; Roozendael, M.V.; Smedt, I.D.; Letocart, V.; Merlaud, A.; Pinardi, G. QDOAS Software User Manual, Version 3.2. 2017. Available online: http://uv-vis.aeronomie.be/software/QDOAS (accessed on 31 January 2021).
- Sander, S.P.; Friedl, R.R. Kinetics and product studies of the reaction chlorine monoxide + bromine monoxide using flash photolysis-ultraviolet absorption. J. Phys. Chem. 1989, 93, 4764–4771. [Google Scholar] [CrossRef]
- Coburn, S.; Ortega, I.; Thalman, R.; Blomquist, B.; Fairall, C.W.; Volkamer, R. Measurements of diurnal variations and eddy covariance (EC) fluxes of glyoxal in the tropical marine boundary layer: Description of the Fast LED-CE-DOAS instrument. Atmos. Meas. Tech. 2014, 7, 3579–3595. [Google Scholar] [CrossRef]
- Suhail, K.; George, M.; Chandran, S.; Varma, R.; Venables, D.; Wang, M.; Chen, J. Open path incoherent broadband cavity-enhanced measurements of NO3 radical and aerosol extinction in the North China Plain. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 24–31. [Google Scholar] [CrossRef]
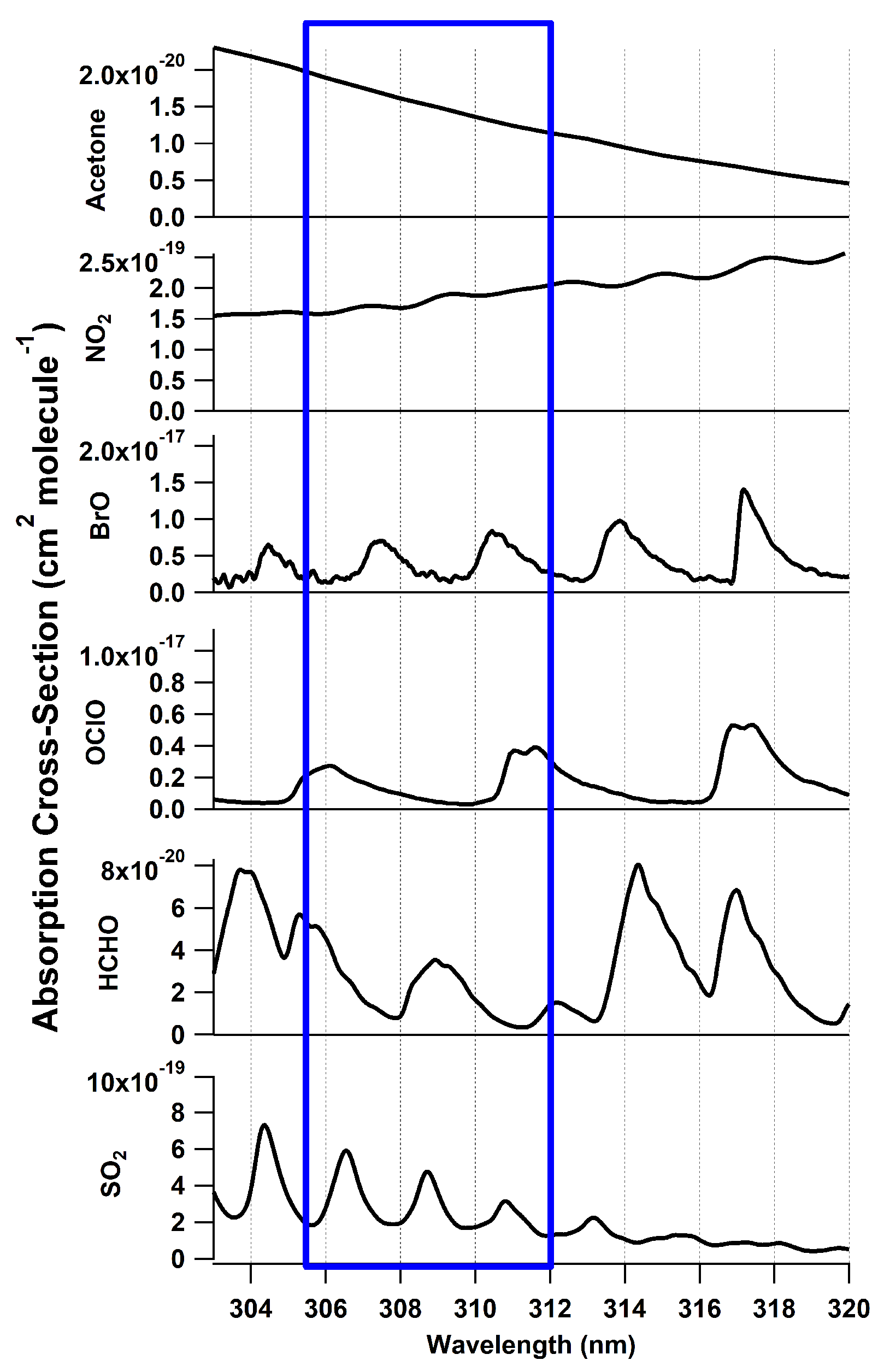



| Species | * (cm3 molecule−1) | LOD (ppbv) | Reference |
|---|---|---|---|
| SO2 | 3.97 × 10−19 | 0.75 | Rufus et al. [45] |
| NO2 | 2.2 × 10−20 | 13.5 | Vandaele et al. [42] |
| HCHO | 2.73 × 10−20 | 10.9 | Meller and Moortgat [46] |
| OClO ** | 3.6 × 10−18 | 0.09 | Dong et al. [29] |
| BrO ** | 6.6 × 10−18 | 0.05 | Wilmouth et al. [43] |
| ClO ** | 3.7 × 10−19 | 1.2 | Sander and Friedl [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalman, R.; Bhardwaj, N.; Flowerday, C.E.; Hansen, J.C. Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS). Sensors 2022, 22, 2626. https://doi.org/10.3390/s22072626
Thalman R, Bhardwaj N, Flowerday CE, Hansen JC. Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS). Sensors. 2022; 22(7):2626. https://doi.org/10.3390/s22072626
Chicago/Turabian StyleThalman, Ryan, Nitish Bhardwaj, Callum E. Flowerday, and Jaron C. Hansen. 2022. "Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS)" Sensors 22, no. 7: 2626. https://doi.org/10.3390/s22072626
APA StyleThalman, R., Bhardwaj, N., Flowerday, C. E., & Hansen, J. C. (2022). Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS). Sensors, 22(7), 2626. https://doi.org/10.3390/s22072626