Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes
Abstract
:1. Introduction
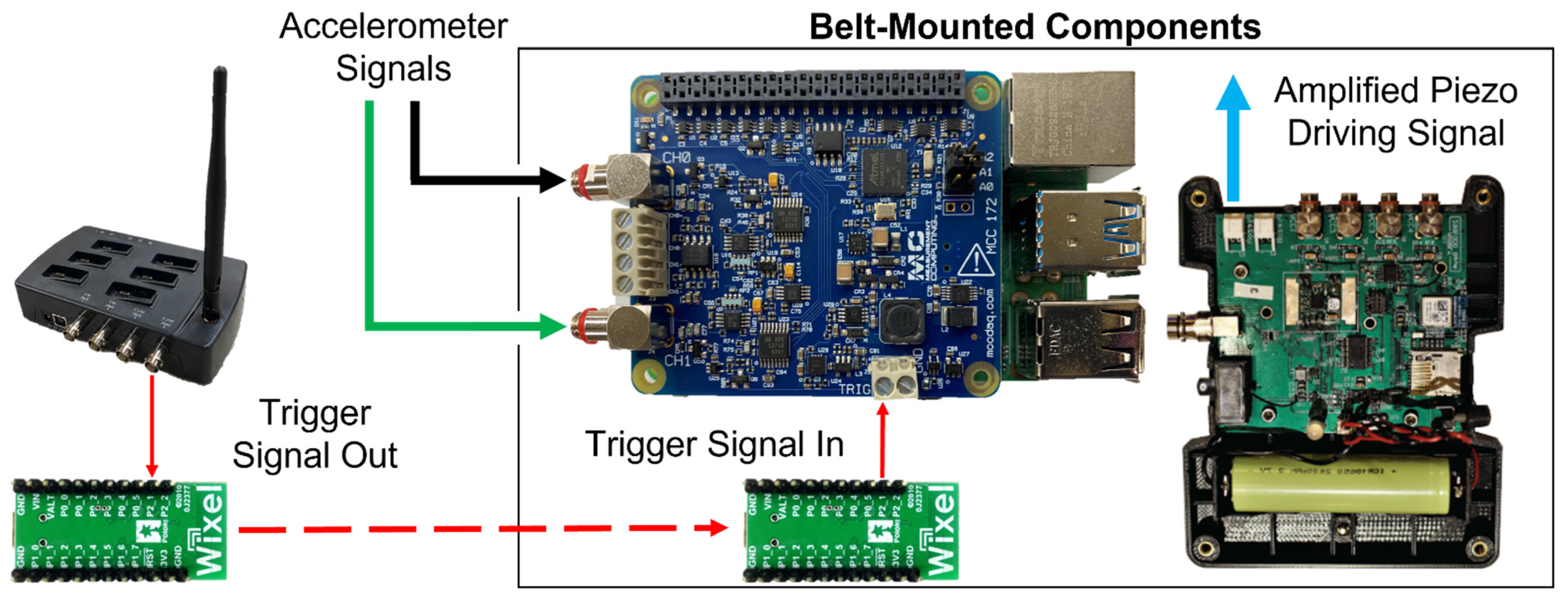
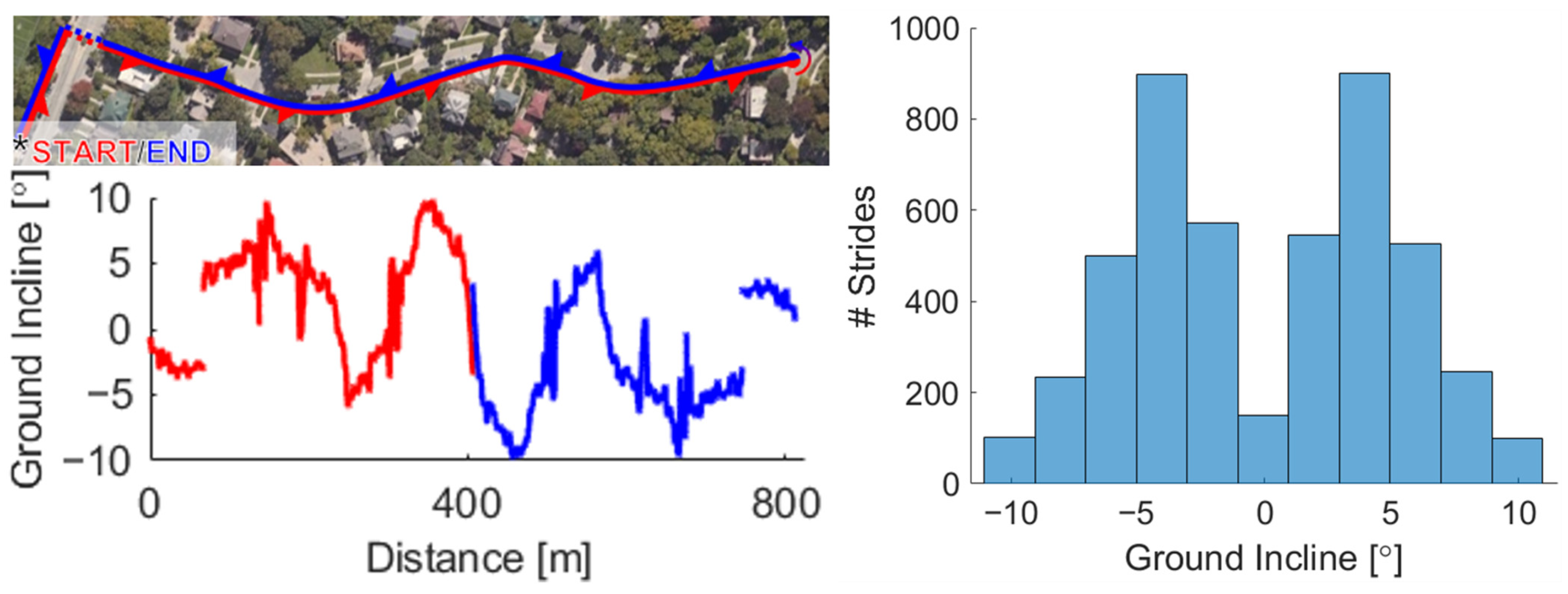
2. Materials and Methods
2.1. Data Processing
2.2. Estimation of Outcome Metrics
2.3. Statistical Analyses
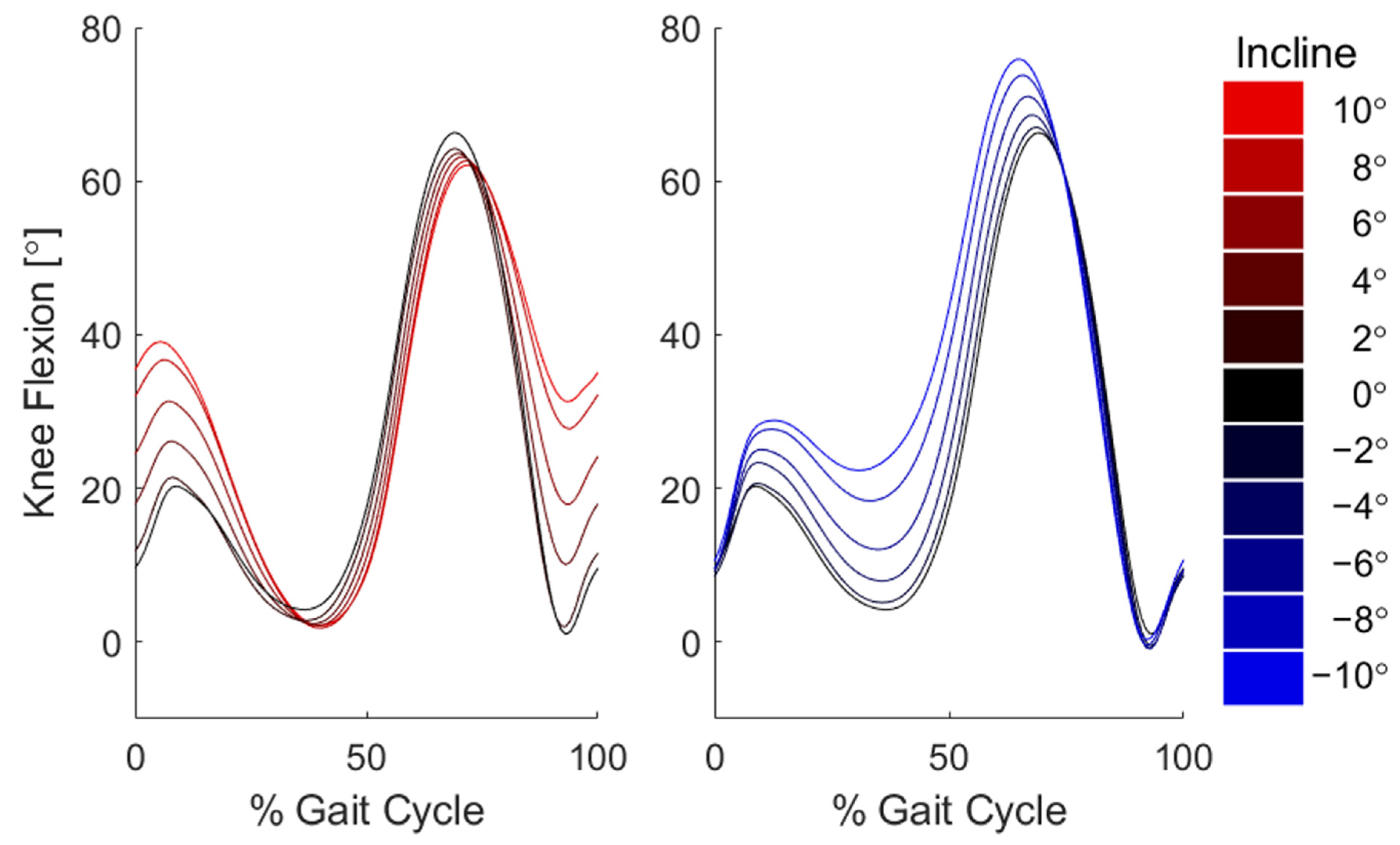
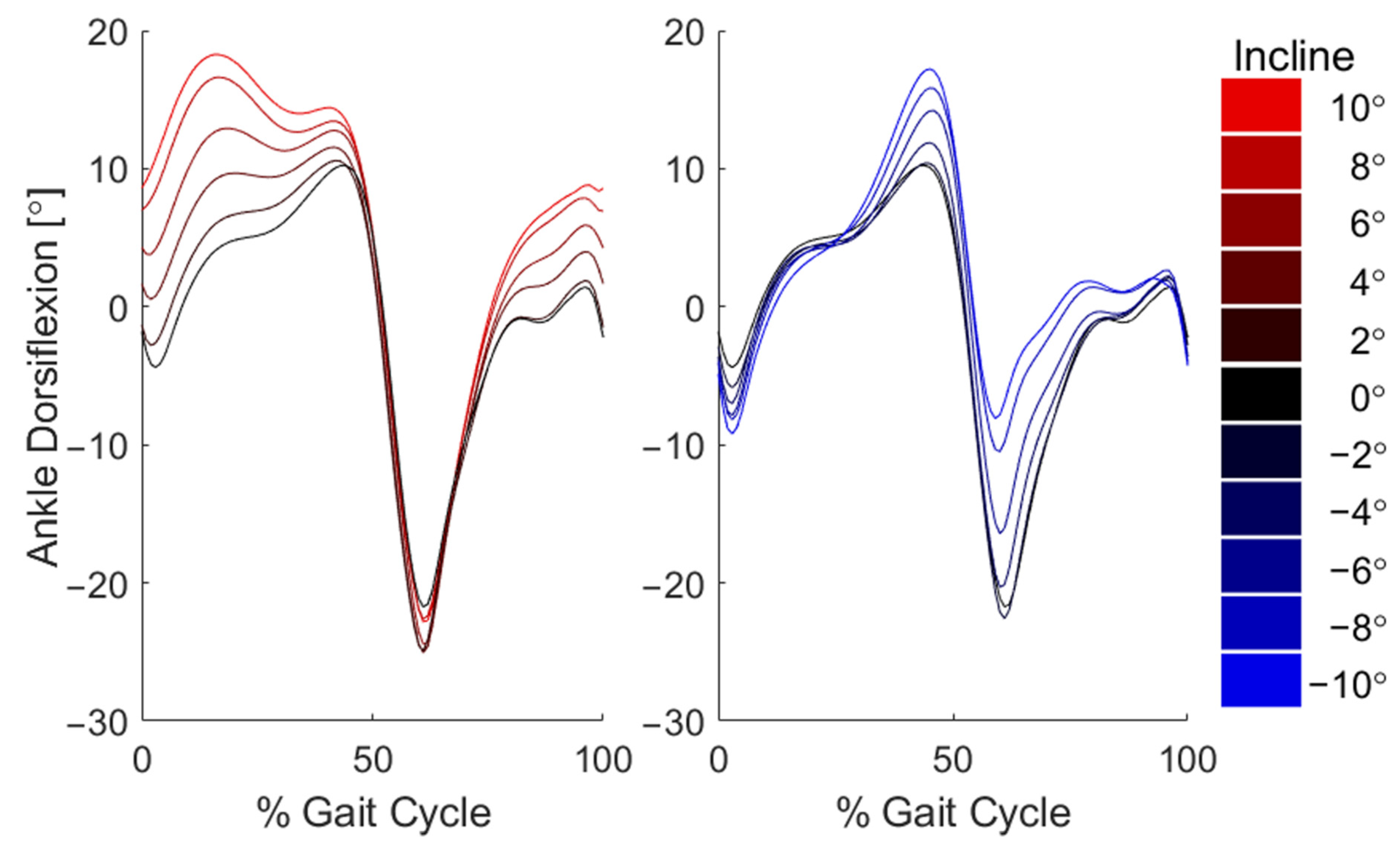
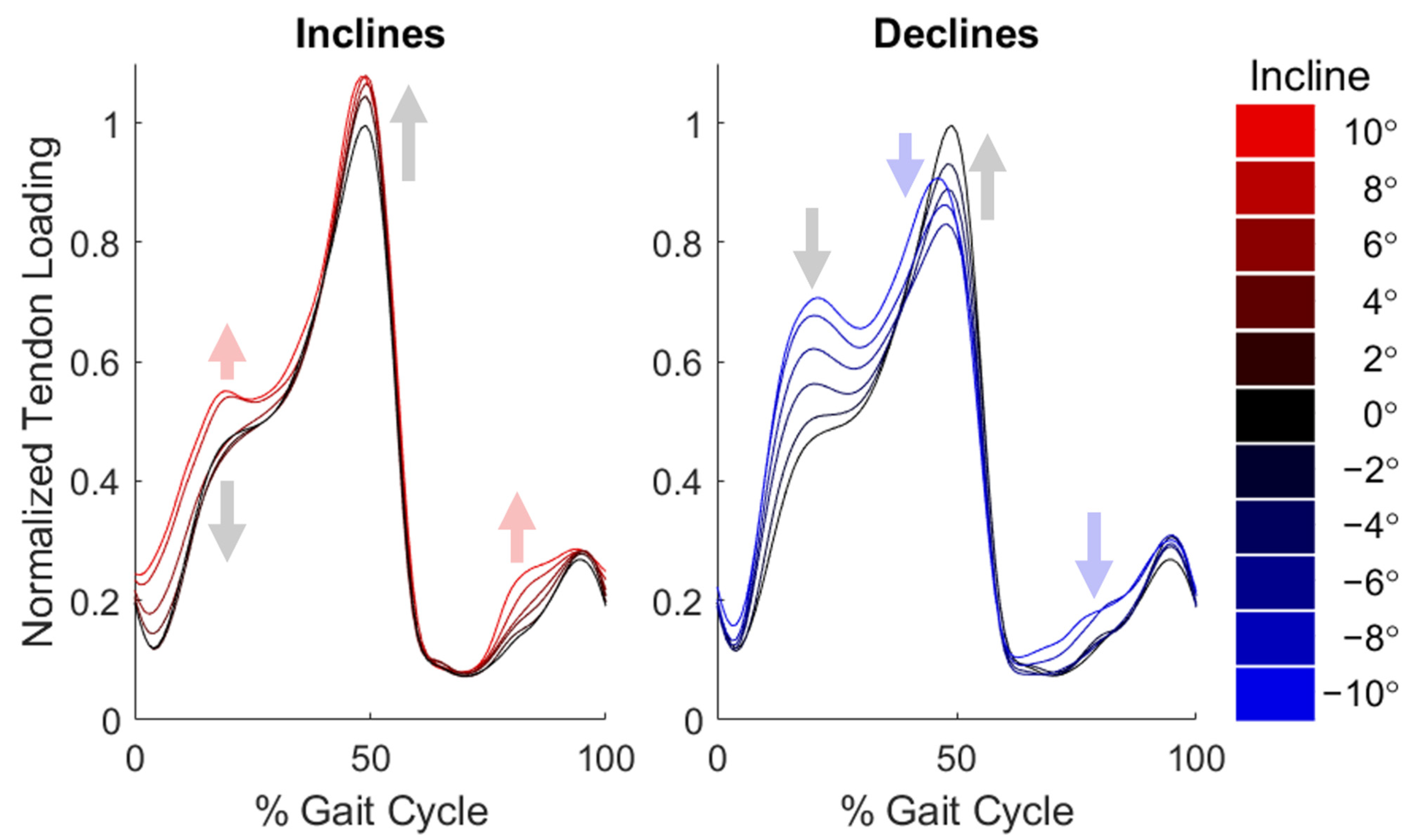
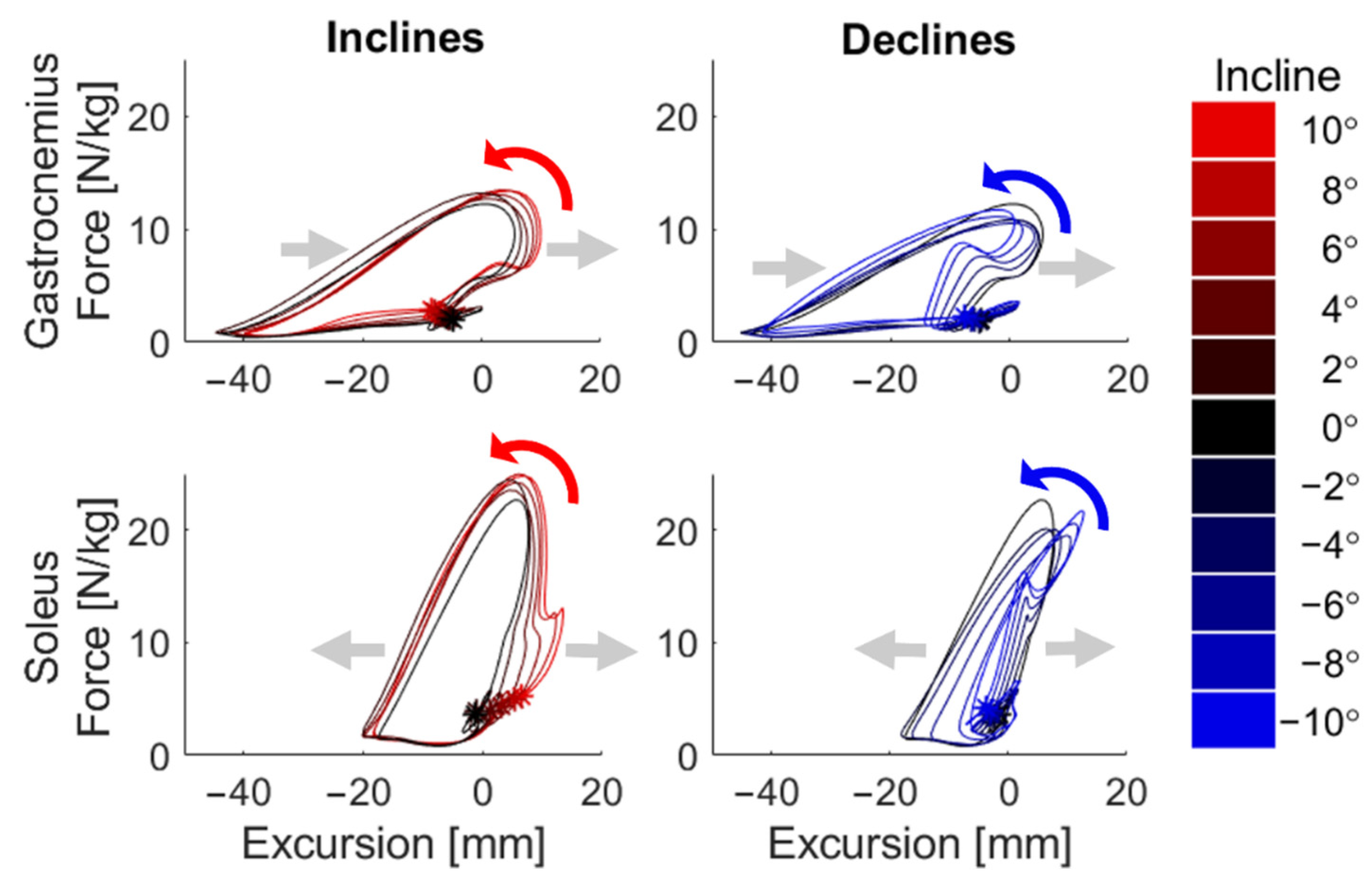
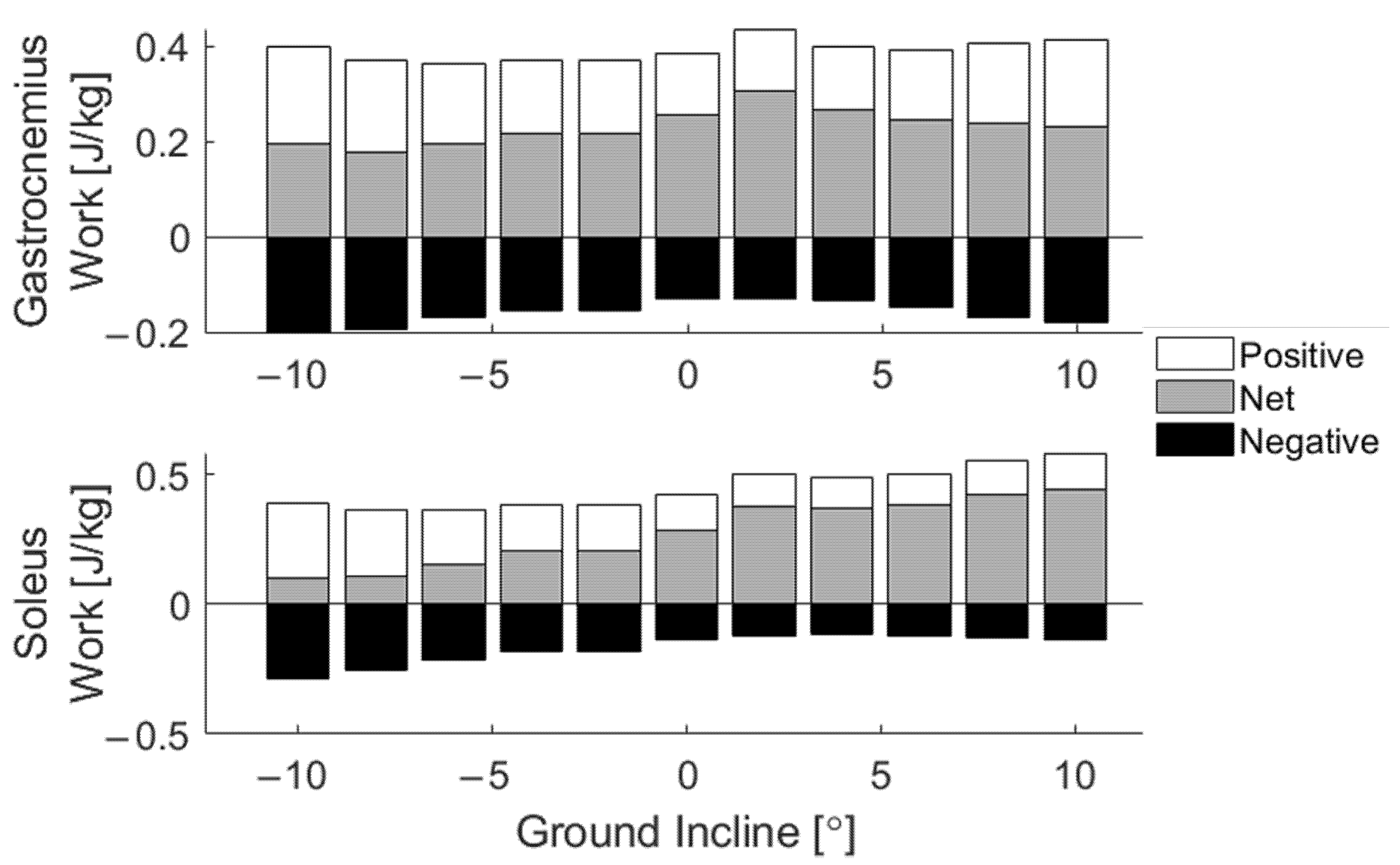
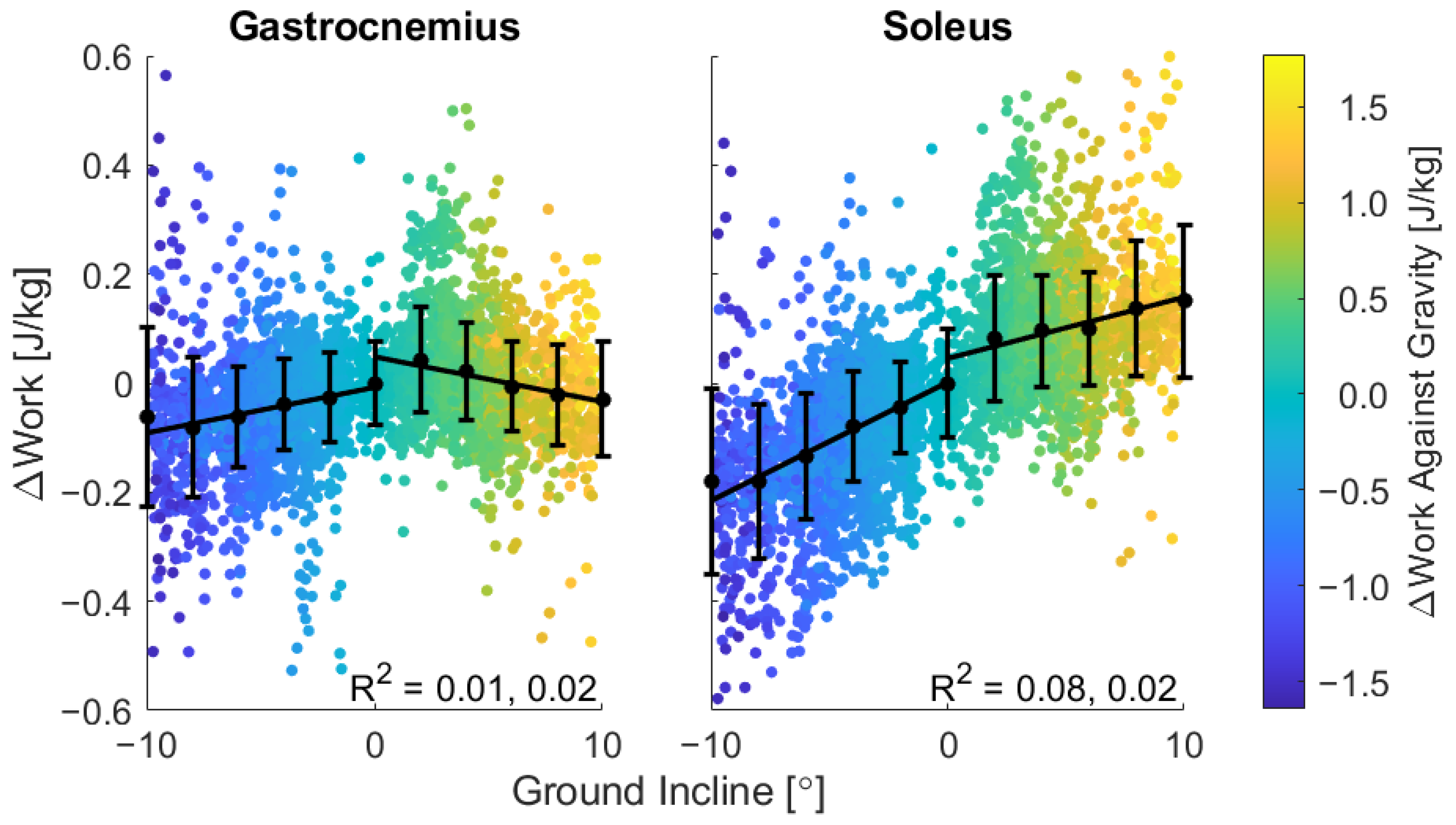
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Participant Number | Sex | Age (Years) | Height (Meters) | Weight (kg) | Body Mass Index (kg/m2) |
|---|---|---|---|---|---|
| 1 | M | 23 | 1.87 | 79.4 | 22.8 |
| 2 | F | 20 | 1.65 | 68.0 | 25.0 |
| 3 | F | 25 | 1.83 | 83.9 | 25.1 |
| 4 | M | 28 | 1.78 | 86.2 | 27.3 |
| 5 | M | 25 | 1.87 | 88.5 | 25.4 |
| 6 | M | 24 | 1.93 | 86.2 | 23.1 |
| 7 | F | 19 | 1.70 | 63.5 | 21.9 |
| 8 | F | 24 | 1.68 | 63.5 | 22.6 |
| 9 | F | 24 | 1.52 | 47.6 | 20.5 |
| 10 | M | 22 | 1.92 | 86.2 | 23.4 |
| 11 | M | 22 | 1.78 | 87.0 | 27.8 |
| Mean ± Standard Deviation | 5 F/6 M | 23.3 ± 2.5 | 1.77 ± 0.13 | 76.5 ± 13.6 | 24.1 ± 2.2 |
| Participant Number | Regression Coefficient | Intercept | R-Squared |
|---|---|---|---|
| 1 | 0.093 | −28.51 | 0.87 |
| 2 | 0.044 | −18.49 | 0.92 |
| 3 | 0.049 | −10.15 | 0.71 |
| 4 | 0.030 | −7.51 | 0.72 |
| 5 | 0.080 | −45.14 | 0.91 |
| 6 | 0.033 | −6.32 | 0.84 |
| 7 | 0.023 | −6.55 | 0.94 |
| 8 | 0.037 | −9.12 | 0.90 |
| 9 | 0.022 | −2.17 | 0.83 |
| 10 | 0.074 | −35.76 | 0.87 |
| 11 | 0.086 | −51.65 | 0.74 |
| Mean ± Standard Deviation | 0.048 ± 0.03 | −16.97 ± 17.36 | 0.85 ± 0.08 |
Appendix B



References
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Qu, F.; Liu, H.; Jiang, L.; Cui, C.; Rietdyk, S. The Relative Contributions of Sagittal, Frontal, and Transverse Joint Works to Self-Paced Incline and Decline Slope Walking. J. Biomech. 2019, 92, 35–44. [Google Scholar] [CrossRef]
- McIntosh, A.S.; Beatty, K.T.; Dwan, L.N.; Vickers, D.R. Gait Dynamics on an Inclined Walkway. J. Biomech. 2006, 39, 2491–2502. [Google Scholar] [CrossRef]
- Alexander, N.; Strutzenberger, G.; Ameshofer, L.M.; Schwameder, H. Lower Limb Joint Work and Joint Work Contribution during Downhill and Uphill Walking at Different Inclinations. J. Biomech. 2017, 61, 75–80. [Google Scholar] [CrossRef]
- Riener, R.; Rabuffetti, M.; Frigo, C. Stair Ascent and Descent at Different Inclinations. Gait Posture 2002, 15, 32–44. [Google Scholar] [CrossRef]
- Protopapadaki, A.; Drechsler, W.I.; Cramp, M.C.; Coutts, F.J.; Scott, O.M. Hip, Knee, Ankle Kinematics and Kinetics during Stair Ascent and Descent in Healthy Young Individuals. Clin. Biomech. 2007, 22, 203–210. [Google Scholar] [CrossRef]
- DeVita, P.; Helseth, J.; Hortobagyi, T. Muscles Do More Positive than Negative Work in Human Locomotion. J. Exp. Biol. 2007, 210, 3361–3373. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, T.S.; Lloyd, D.G.; Manal, K.; Besier, T.F. Neuromusculoskeletal Modeling: Estimation of Muscle Forces and Joint Moments and Movements from Measurements of Neural Command. J. Appl. Biomech. 2004, 20, 367–395. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.P.; Kinney, A.L.; Banks, S.A.; D’Lima, D.D.; Besier, T.F.; Lloyd, D.G.; Fregly, B.J. Muscle Synergies May Improve Optimization Prediction of Knee Contact Forces during Walking. J. Biomech. Eng. 2014, 136, 0210311–0210319. [Google Scholar] [CrossRef] [Green Version]
- Sartori, M.; Farina, D.; Lloyd, D.G. Hybrid Neuromusculoskeletal Modeling to Best Track Joint Moments Using a Balance between Muscle Excitations Derived from Electromyograms and Optimization. J. Biomech. 2014, 47, 3613–3621. [Google Scholar] [CrossRef]
- Crowninshield, R.D.; Brand, R.A. A Physiologically Based Criterion of Muscle Force Prediction in Locomotion. J. Biomech. 1981, 14, 793–801. [Google Scholar] [CrossRef]
- Crowninshield, R.D. Use of Optimization Techniques to Predict Muscle Forces. J. Biomech. Eng. 1978, 100, 88–92. [Google Scholar] [CrossRef]
- Dul, J.; Townsend, M.A.; Shiavi, R.; Johnson, G.E. Muscular Synergism-I. On Criteria for Load Sharing between Synergistic Muscles. J. Biomech. 1984, 17, 663–673. [Google Scholar] [CrossRef]
- Dul, J.; Johnson, G.E.; Shiavi, R.; Townsend, M.A. Muscular Synergism-II. A Minimum-Fatigue Criterion for Load Sharing between Synergistic Muscles. J. Biomech. 1984, 17, 675–684. [Google Scholar] [CrossRef]
- Collins, J.J. The Redundant Nature of Locomotor Optimization Laws. J. Biomech. 1995, 28, 251–267. [Google Scholar] [CrossRef]
- Erdemir, A.; McLean, S.; Herzog, W.; van den Bogert, A.J. Model-Based Estimation of Muscle Forces Exerted during Movements. Clin. Biomech. 2007, 22, 131–154. [Google Scholar] [CrossRef] [Green Version]
- Schepers, M.; Giuberti, M.; Bellusci, G. Xsens MVN: Consistent Tracking of Human Motion Using Inertial Sensing. Xsens Technol. 2018, 1, 8. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Hijmans, J.M.; Moeini, F.; Rajabi, R.; Ferber, R.; van der Worp, H.; Zwerver, J. Validity and Reliability of a Smartphone Motion Analysis App for Lower Limb Kinematics during Treadmill Running. Phys. Ther. Sport 2020, 43, 27–35. [Google Scholar] [CrossRef]
- Hurkmans, H.L.P.; Bussmann, J.B.J.; Benda, E.; Verhaar, J.A.N.; Stam, H.J. Accuracy and Repeatability of the Pedar Mobile System in Long-Term Vertical Force Measurements. Gait Posture 2006, 23, 118–125. [Google Scholar] [CrossRef]
- Fong, D.T.P.; Chan, Y.Y.; Hong, Y.; Yung, P.S.H.; Fung, K.Y.; Chan, K.M. Estimating the Complete Ground Reaction Forces with Pressure Insoles in Walking. J. Biomech. 2008, 41, 2597–2601. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.M.; Kobayashi, T.; Hayes, H.A.; Foreman, K.B.; Bamberg, S.J.M. Kinetic Gait Analysis Using a Low-Cost Insole. IEEE Trans. Biomed. Eng. 2013, 60, 3284–3290. [Google Scholar] [CrossRef]
- Harper, S.E.; Roembke, R.A.; Zunker, J.D.; Thelen, D.G.; Adamczyk, P.G. Wearable Tendon Kinetics. Sensors 2020, 20, 4805. [Google Scholar] [CrossRef]
- Chambers, R.; Gabbett, T.J.; Cole, M.H.; Beard, A. The Use of Wearable Microsensors to Quantify Sport-Specific Movements. Sports Med. 2015, 45, 1065–1081. [Google Scholar] [CrossRef]
- Li, R.T.; Kling, S.R.; Salata, M.J.; Cupp, S.A.; Sheehan, J.; Voos, J.E. Wearable Performance Devices in Sports Medicine. Sports Health 2016, 8, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Peppoloni, L.; Filippeschi, A.; Ruffaldi, E.; Avizzano, C.A. (WMSDs Issue) A Novel Wearable System for the Online Assessment of Risk for Biomechanical Load in Repetitive Efforts. Int. J. Ind. Ergon. 2014, 52, 1–11. [Google Scholar] [CrossRef]
- Alberto, R.; Draicchio, F.; Varrecchia, T.; Silvetti, A.; Iavicoli, S. Wearable Monitoring Devices for Biomechanical Risk Assessment at Work: Current Status and Future Challenges—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2001. [Google Scholar] [CrossRef] [Green Version]
- Conforti, I.; Mileti, I.; Del Prete, Z.; Palermo, E. Assessing Ergonomics and Biomechanical Risk in Manual Handling of Loads through a Wearable System. In Proceedings of the 2019 IEEE International Workshop on Metrology for Industry 4.0 and IoT, MetroInd 4.0 and IoT 2019—Proceedings, Naples, Italy, 4–6 June 2019. [Google Scholar] [CrossRef]
- Kwon, J.; Park, J.H.; Ku, S.; Jeong, Y.H.; Paik, N.J.; Park, Y.L. A Soft Wearable Robotic Ankle-Foot-Orthosis for Post-Stroke Patients. IEEE Robot. Autom. Lett. 2019, 4, 2547–2552. [Google Scholar] [CrossRef]
- Iosa, M.; De Sanctis, M.; Summa, A.; Bergamini, E.; Morelli, D.; Vannozzi, G. Usefulness of Magnetoinertial Wearable Devices in Neurorehabilitation of Children with Cerebral Palsy. Appl. Bionics Biomech. 2018, 2018, 5405680. [Google Scholar] [CrossRef] [Green Version]
- Havens, K.L.; Cohen, S.C.; Pratt, K.A.; Sigward, S.M. Accelerations from Wearable Accelerometers Reflect Knee Loading during Running after Anterior Cruciate Ligament Reconstruction. Clin. Biomech. 2018, 58, 57–61. [Google Scholar] [CrossRef]
- Hullfish, T.J.; Qu, F.; Stoeckl, B.D.; Gebhard, P.M.; Mauck, R.L.; Baxter, J.R. Measuring Clinically Relevant Knee Motion with a Self-Calibrated Wearable Sensor. J. Biomech. 2019, 89, 105–109. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait Analysis Using Wearable Sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Stetter, B.J.; Ringhof, S.; Krafft, F.C.; Sell, S.; Stein, T. Estimation of Knee Joint Forces in Sport Movements Using Wearable Sensors and Machine Learning. Sensors 2019, 19, 3690. [Google Scholar] [CrossRef] [Green Version]
- Taborri, J.; Keogh, J.; Kos, A.; Santuz, A.; Umek, A.; Urbanczyk, C.; van der Kruk, E.; Rossi, S. Sport Biomechanics Applications Using Inertial, Force, and EMG Sensors: A Literature Overview. Appl. Bionics Biomech. 2020, 2020, 2041549. [Google Scholar] [CrossRef]
- Gurchiek, R.D.; Cheney, N.; McGinnis, R.S. Estimating Biomechanical Time-Series with Wearable Sensors: A Systematic Review of Machine Learning Techniques. Sensors 2019, 19, 5227. [Google Scholar] [CrossRef] [Green Version]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, J.R.; Grabowski, A.M. The Contributions of Ankle, Knee and Hip Joint Work to Individual Leg Work Change during Uphill and Downhill Walking over a Range of Speeds. R. Soc. Open Sci. 2018, 5, 180550. [Google Scholar] [CrossRef] [Green Version]
- Franz, J.R.; Lyddon, N.E.; Kram, R. Mechanical Work Performed by the Individual Legs during Uphill and Downhill Walking. J. Biomech. 2012, 45, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Lay, A.N.; Hass, C.J.; Gregor, R.J. The Effects of Sloped Surfaces on Locomotion: A Kinematic and Kinetic Analysis. J. Biomech. 2006, 39, 1621–1628. [Google Scholar] [CrossRef]
- Josephson, R.K. Mechanical Power Output from Striated Muscle during Cyclic Contraction. J. Exp. Biol. 1985, 114, 493–512. [Google Scholar] [CrossRef]
- Biewener, A.A.; Roberts, T.J. Muscle and Tendon Contributions to Force, Work, and Elastic Energy Savings: A Comparative Perspective. Exerc. Sport Sci. Rev. 2000, 28, 99–107. [Google Scholar]
- Cruz, M.A.A.D.; Legaspi, K.M.C.; Marcelino, R.M.D.; Rosete, J.R.; Sangalang, D.A.R.; Suarez, C.G.; Roxas, E.A.; Serrano, K.K.D.; Dela Cruz, A.R. Joint Gait Kinematic and Kinetic Analysis Using Inertial Measurement Units and Plantar Pressure Sensor System. In Proceedings of the 2019 IEEE 11th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management, HNICEM 2019, Laoag, Philippines, 29 November–1 December 2019. [Google Scholar] [CrossRef]
- Fong, D.T.-P.; Chan, Y.-Y. The Use of Wearable Inertial Motion Sensors in Human Lower Limb Biomechanics Studies: A Systematic Review. Sensors 2010, 10, 11556–11565. [Google Scholar] [CrossRef] [Green Version]
- Brodie, M.; Walmsley, A.; Page, W. Fusion Motion Capture: A Prototype System Using Inertial Measurement Units and GPS for the Biomechanical Analysis of Ski Racing. Sport. Technol. 2008, 1, 17–28. [Google Scholar] [CrossRef]
- Ancillao, A.; Tedesco, S.; Barton, J.; O’flynn, B. Indirect Measurement of Ground Reaction Forces and Moments by Means of Wearable Inertial Sensors: A Systematic Review. Sensors 2018, 18, 2564. [Google Scholar] [CrossRef] [Green Version]
- Abdelhady, M.; Van Den Bogert, A.J.; Simon, D. A High-Fidelity Wearable System for Measuring Lower-Limb Kinetics and Kinematics. IEEE Sens. J. 2019, 19, 12482–12493. [Google Scholar] [CrossRef]
- Mundt, M.; Koeppe, A.; David, S.; Witter, T.; Bamer, F.; Potthast, W.; Markert, B. Estimation of Gait Mechanics Based on Simulated and Measured IMU Data Using an Artificial Neural Network. Front. Bioeng. Biotechnol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Lee, M.; Park, S. Estimation of Three-Dimensional Lower Limb Kinetics Data during Walking Using Machine Learning from a Single Imu Attached to the Sacrum. Sensors 2020, 20, 6277. [Google Scholar] [CrossRef]
- Lim, H.; Kim, B.; Park, S. Prediction of Lower Limb Kinetics and Kinematics during Walking by a Single IMU on the Lower Back Using Machine Learning. Sensors 2020, 20, 130. [Google Scholar] [CrossRef] [Green Version]
- Karatsidis, A.; Bellusci, G.; Schepers, H.M.; de Zee, M.; Andersen, M.S.; Veltink, P.H. Estimation of Ground Reaction Forces and Moments during Gait Using Only Inertial Motion Capture. Sensors 2017, 17, 75. [Google Scholar] [CrossRef] [Green Version]
- Latella, C.; Kuppuswamy, N.; Romano, F.; Traversaro, S.; Nori, F. Whole-Body Human Inverse Dynamics with Distributed Micro-Accelerometers, Gyros and Force Sensing. Sensors 2016, 16, 727. [Google Scholar] [CrossRef] [Green Version]
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Muscle, Ligament, and Joint-Contact Forces at the Knee during Walking. Med. Sci. Sports Exerc. 2005, 37, 1948–1956. [Google Scholar] [CrossRef] [Green Version]
- Cao, E.; Inoue, Y.; Liu, T.; Shibata, K. Analysis of Muscle Forces in Lower Limbs based on Wearable Sensors. In Proceedings of the 2010 IEEE International Conference on Information and Automation, ICIA 2010, Harbin, China, 20–23 June 2010. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, M.; Lee, K.; Koo, S. Ground Reaction Force Estimation Using an Insole-Type Pressure Mat and Joint Kinematics during Walking. J. Biomech. 2014, 47, 2693–2699. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, M.; van der Straaten, R.; Timmermans, A.; Jonkers, I. Estimating Joint Loading Using Inertial Measurement Units and Ground Reaction Forces. In Proceedings of the 2018 European Orthopaedic Research Society, EORS 2018, Galway, Ireland, 25–28 September 2018. [Google Scholar]
- Ebrahimi, A.; Martin, J.A.; Schmitz, D.G. Shear Wave Tensiometry Reveals an Age-Related Deficit in Triceps Surae Work Across a Broad Range of Walking Speeds. Front. Sports Act. Living 2020, 2, 69. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kuchler, R.L.; Pomeroy, R.L.; Loegering, I.F.; Martin, J.A.; Thelen, D.G. Normative Achilles and Patellar Tendon Shear Wave Speeds and Loading Patterns during Walking in Typically Developing Children. Gait Posture 2021, 88, 185–191. [Google Scholar] [CrossRef]
- Abid, M. Walking Gait Features Extraction and Characterization. Ph.D. Thesis, École Centrale de Nantes, Nantes, France, 2018. [Google Scholar]
- Boekesteijn, R.J.; Smolders, J.M.H.; Busch, V.J.J.F.; Geurts, A.C.H.; Smulders, K. Independent and Sensitive Gait Parameters for Objective Evaluation in Knee and Hip Osteoarthritis Using Wearable Sensors. BMC Musculoskelet. Disord. 2021, 22, 242. [Google Scholar] [CrossRef]
- Stanev, D.; Filip, K.; Bitzas, D.; Zouras, S.; Giarmatzis, G.; Tsaopoulos, D.; Moustakas, K. Real-Time Musculoskeletal Kinematics and Dynamics Analysis Using Marker-and Imu-Based Solutions in Rehabilitation. Sensors 2021, 21, 1804. [Google Scholar] [CrossRef]
- Martin, J.A.; Brandon, S.C.E.; Keuler, E.M.; Hermus, J.R.; Ehlers, A.C.; Segalman, D.J.; Allen, M.S.; Thelen, D.G. Gauging Force by Tapping Tendons. Nat. Commun. 2018, 9, 1592. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, A.; Loegering, I.F.; Martin, J.A.; Pomeroy, R.L.; Roth, J.D.; Thelen, D.G. Achilles Tendon Loading Is Lower in Older Adults than Young Adults across a Broad Range of Walking Speeds. Exp. Gerontol. 2020, 137, 110966. [Google Scholar] [CrossRef]
- MTw Awinda. Available online: https://www.xsens.com/products/mtw-awinda (accessed on 1 June 2021).
- Wixel Programmable USB Wireless Module (Fully Assembled). Available online: https://www.pololu.com/product/1336 (accessed on 1 June 2021).
- MCC 172. Available online: https://www.mccdaq.com/DAQ-HAT/MCC-172.aspx (accessed on 1 June 2021).
- Sinclair, J.; Hobbs, S.J.; Protheroe, L.; Edmundson, C.J.; Greenhalgh, A. Determination of Gait Events Using an Externally Mounted Shank Accelerometer. J. Appl. Biomech. 2013, 29, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Jasiewicz, J.M.; Allum, J.H.J.; Middleton, J.W.; Barriskill, A.; Condie, P.; Purcell, B.; Li, R.C.T. Gait Event Detection Using Linear Accelerometers or Angular Velocity Transducers in Able-Bodied and Spinal-Cord Injured Individuals. Gait Posture 2006, 24, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Keuler, E.M.; Loegering, I.F.; Martin, J.A.; Roth, J.D.; Thelen, D.G. Shear Wave Predictions of Achilles Tendon Loading during Human Walking. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cronin, N.J.; Avela, J.; Finni, T.; Peltonen, J. Differences in Contractile Behaviour between the Soleus and Medial Gastrocnemius Muscles during Human Walking. J. Exp. Biol. 2013, 216, 909–914. [Google Scholar] [CrossRef] [Green Version]
- Brennan, S.F.; Cresswell, A.G.; Farris, D.J.; Lichtwark, G.A. The Effect of Muscle-Tendon Unit vs. Fascicle Analyses on Vastus Lateralis Force-Generating Capacity during Constant Power Output Cycling with Variable Cadence. J. Appl. Physiol. 2018, 124, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.R.; MacIntosh, B.R. Estimates of Achilles Tendon Moment Arm Length at Different Ankle Joint Angles: Effect of Passive Moment. J. Appl. Biomech. 2018, 9, 220–225. [Google Scholar] [CrossRef]
- Maganaris, C.N. In Vivo Measurement-Based Estimations of the Moment Arm in the Human Tibialis Anterior Muscle-Tendon Unit. J. Biomech. 2000, 33, 375–379. [Google Scholar] [CrossRef]
- Buford, J.; Ivey, J.; Malone, J.D.; Patterson, R.M.; Peare, G.L.; Nguyen, D.K.; Stewart, A.A. Muscle Balance at the Knee—Moment Arms for the Normal Knee and the ACL-Minus Knee. IEEE Trans. Rehabil. Eng. 1997, 5, 367–379. [Google Scholar] [CrossRef]
- Visser, J.J.; Hoogkamer, J.E.; Bobbert, M.F.; Huijing, P.A. Length and Moment Arm of Human Leg Muscles as a Function of Knee and Hip-Joint Angles. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 453–460. [Google Scholar] [CrossRef]
- Handsfield, G.G.; Meyer, C.H.; Hart, J.M.; Abel, M.F.; Blemker, S.S. Relationships of 35 Lower Limb Muscles to Height and Body Mass Quantified Using MRI. J. Biomech. 2014, 47, 631–638. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. Interactions between the Human Gastrocnemius Muscle and the Achilles Tendon during Incline, Level and Decline Locomotion. J. Exp. Biol. 2006, 209, 4379–4388. [Google Scholar] [CrossRef] [Green Version]
- Zelik, K.E.; Franz, J.R. It’s Positive to Be Negative: Achilles Tendon Work Loops during Human Locomotion. PLoS ONE 2017, 12, e0179976. [Google Scholar] [CrossRef] [Green Version]
- MODEL: 352C23. Available online: http://www.pcb.com/products?m=352C23 (accessed on 15 December 2021).
- Céspedes, I.; Huang, Y.; Ophir, J.; Spratt, S. Methods for Estimation of Subsample Time Delays of Digitized Echo Signals. Ultrason. Imaging 1995, 17, 142–171. [Google Scholar] [CrossRef]
- Cronin, N.J.; Finni, T. Treadmill versus Overground and Barefoot versus Shod Comparisons of Triceps Surae Fascicle Behaviour in Human Walking and Running. Gait Posture 2013, 38, 528–533. [Google Scholar] [CrossRef]
- Palermi, S.; Massa, B.; Vecchiato, M.; Mazza, F.; De Blasiis, P.; Romano, A.M.; Di Salvatore, M.G.; Della Valle, E.; Tarantino, D.; Ruosi, C.; et al. Indirect Structural Muscle Injuries of Lower Limb: Rehabilitation and Therapeutic Exercise. J. Funct. Morphol. Kinesiol. 2021, 6, 75. [Google Scholar] [CrossRef]
- Hauret, K.G.; Jones, B.H.; Bullock, S.H.; Canham-Chervak, M.; Canada, S. Musculoskeletal Injuries: Description of an under-Recognized Injury Problem among Military Personnel. Am. J. Prev. Med. 2010, 38, S61–S70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, S.E.; Schmitz, D.G.; Adamczyk, P.G.; Thelen, D.G. Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes. Sensors 2022, 22, 1589. https://doi.org/10.3390/s22041589
Harper SE, Schmitz DG, Adamczyk PG, Thelen DG. Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes. Sensors. 2022; 22(4):1589. https://doi.org/10.3390/s22041589
Chicago/Turabian StyleHarper, Sara E., Dylan G. Schmitz, Peter G. Adamczyk, and Darryl G. Thelen. 2022. "Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes" Sensors 22, no. 4: 1589. https://doi.org/10.3390/s22041589
APA StyleHarper, S. E., Schmitz, D. G., Adamczyk, P. G., & Thelen, D. G. (2022). Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes. Sensors, 22(4), 1589. https://doi.org/10.3390/s22041589







