Denoising of Fluorescence Image on the Surface of Quantum Dot/Nanoporous Silicon Biosensors
Abstract
:1. Introduction
2. Theoretical Analysis
2.1. Detection of Noise Types in Fluorescence Emitted by Quantum Dots in Porous Silicon
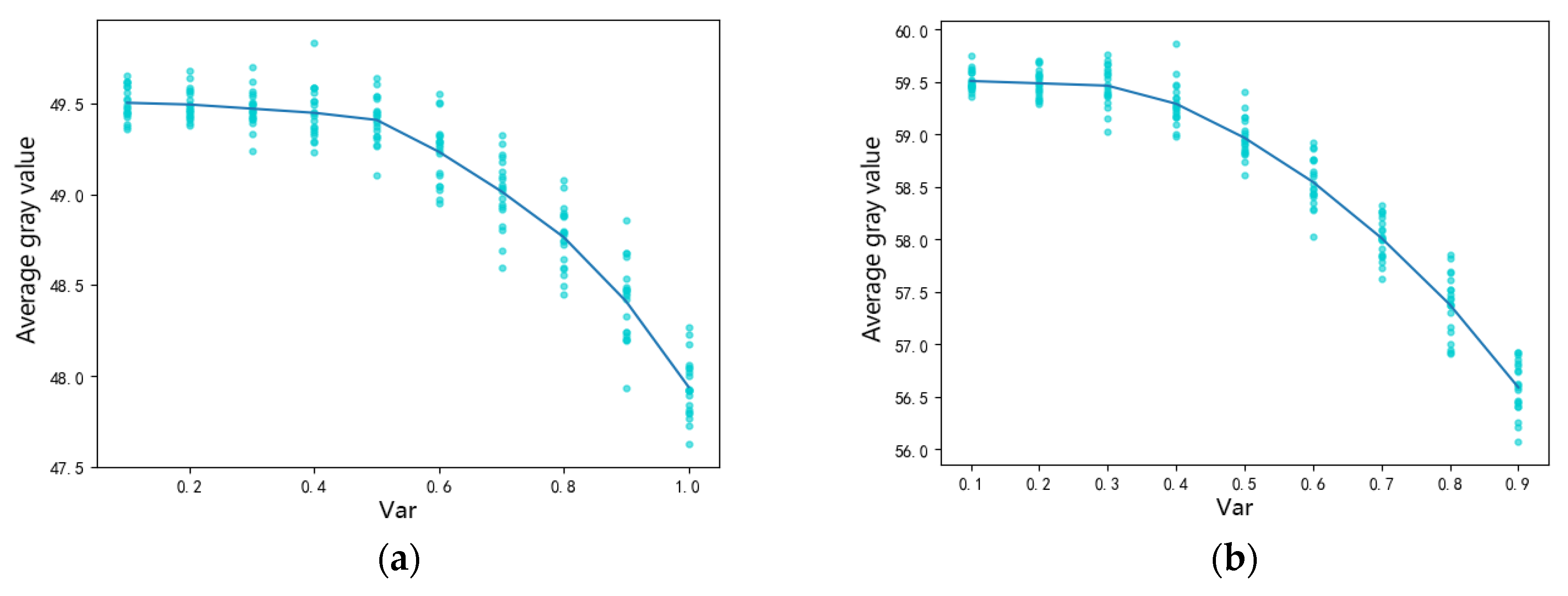
2.2. Influence of Gamma Multiplicative Noise on the Gray Value of Quantum Dot Fluorescence Images in PSi
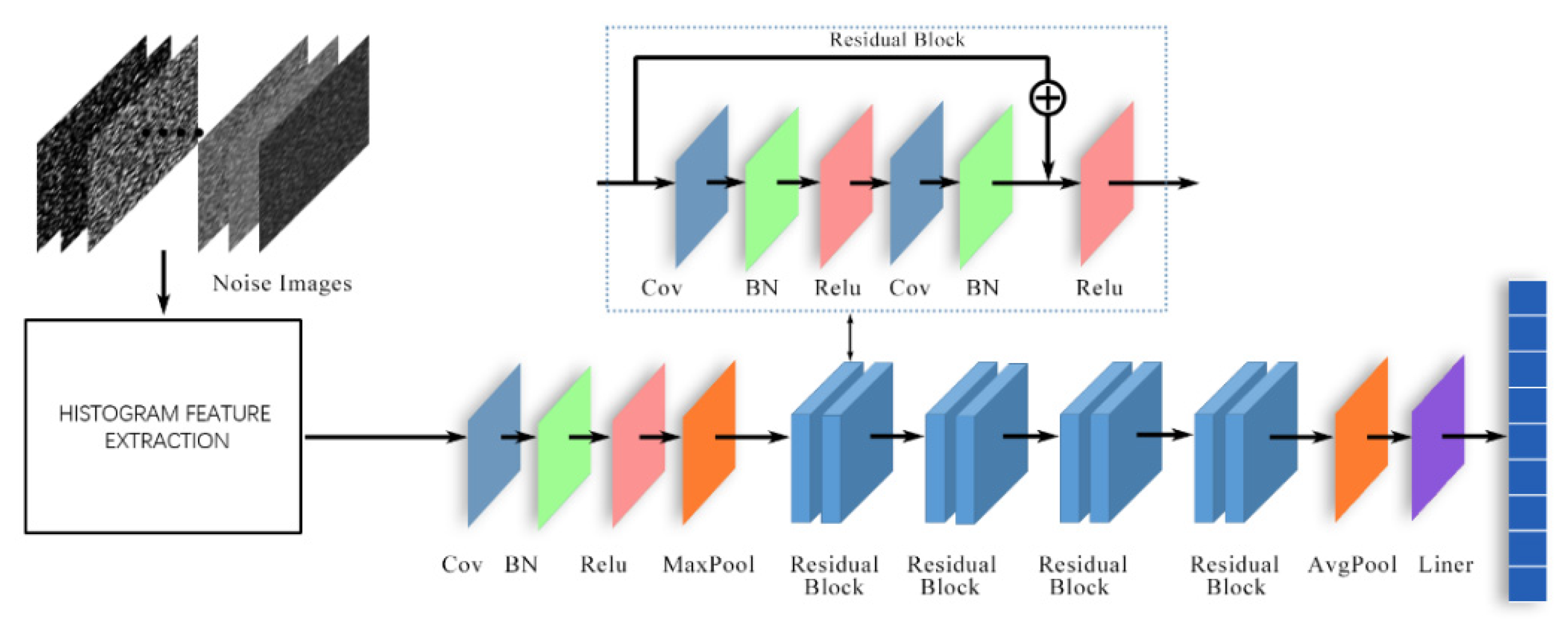
3. Proposed Denoising Algorithm
3.1. Related Research
3.2. Proposed Denoising Algorithm
4. Results and Discussion
4.1. Simulation Image Denoising
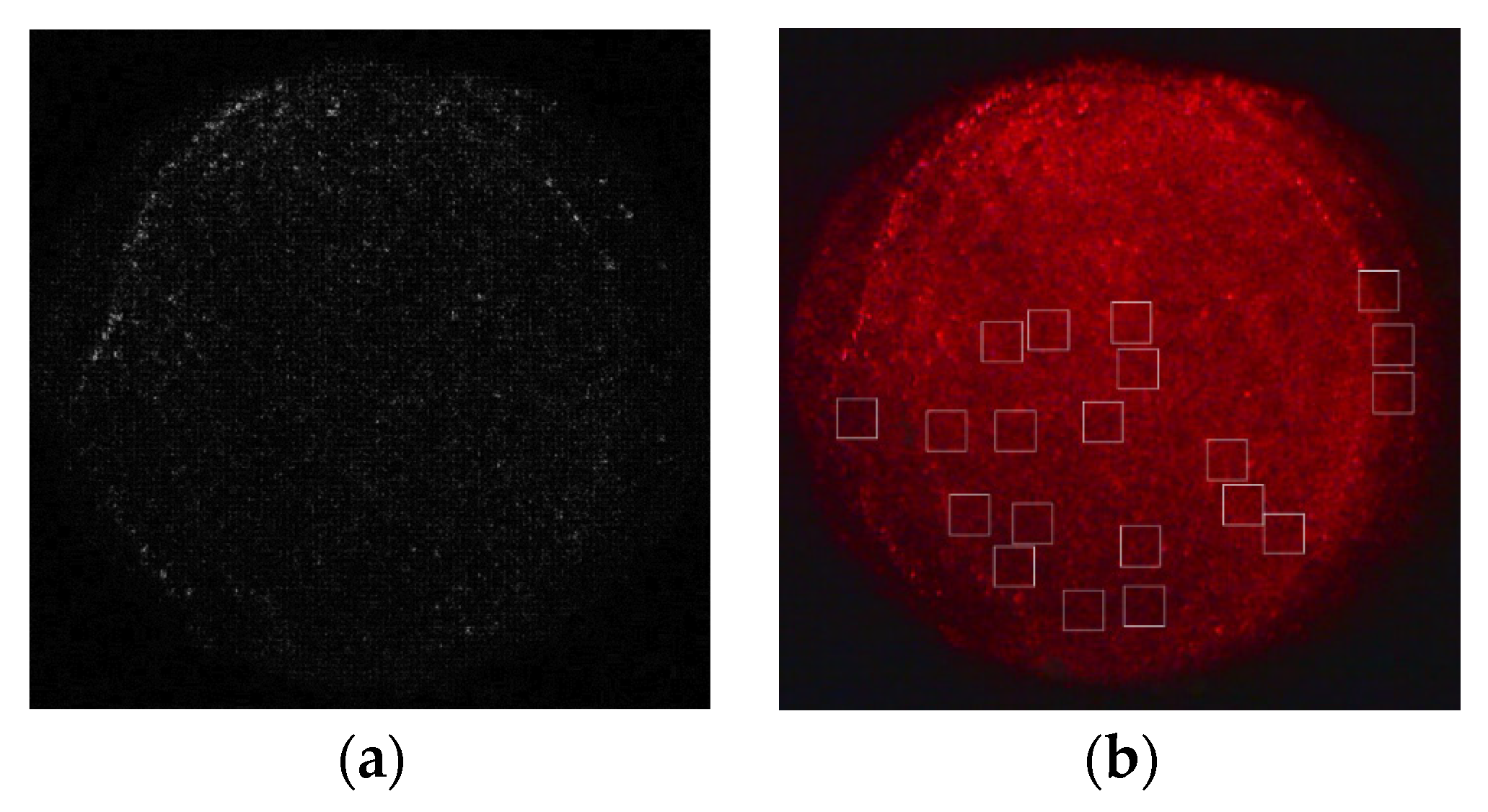
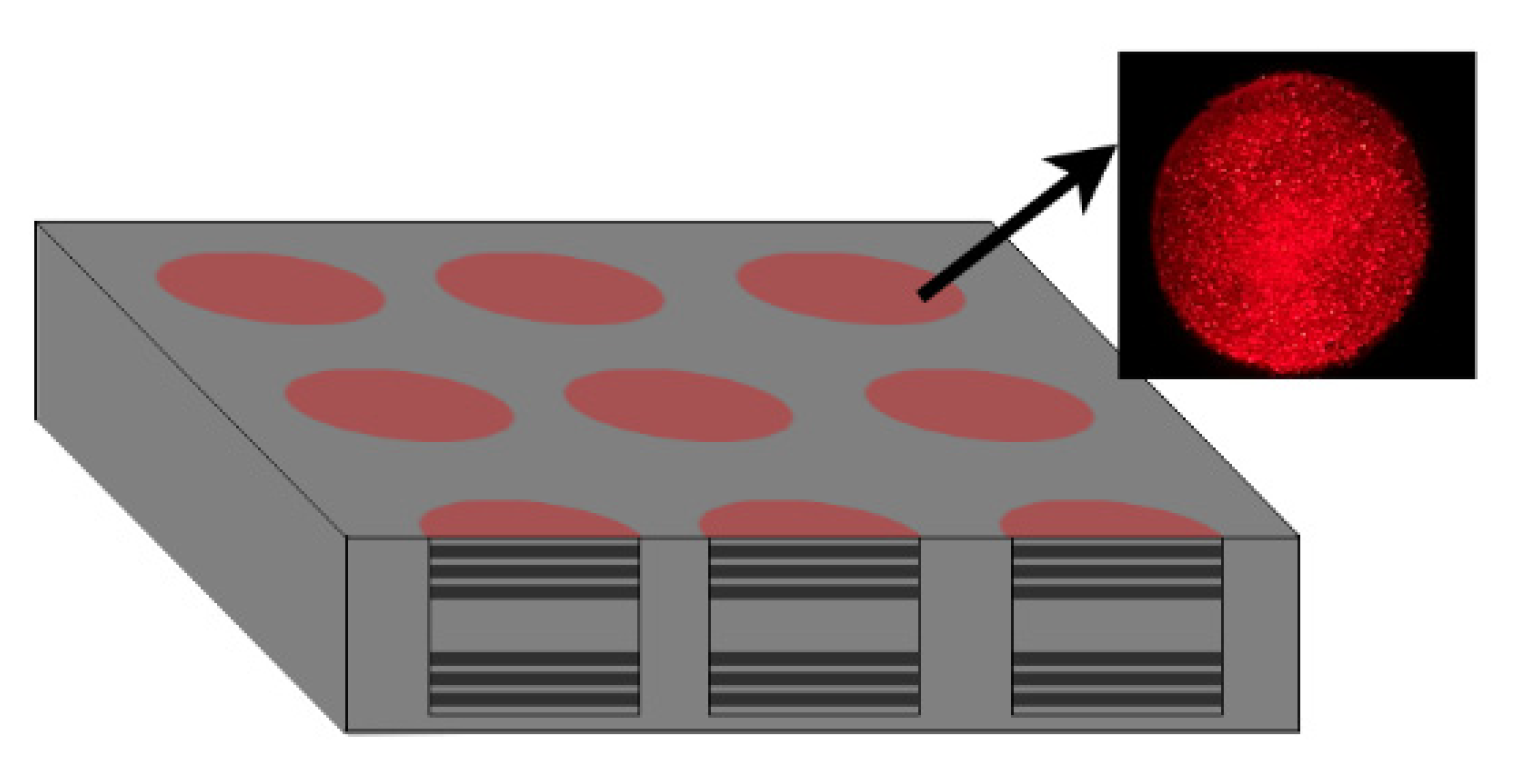
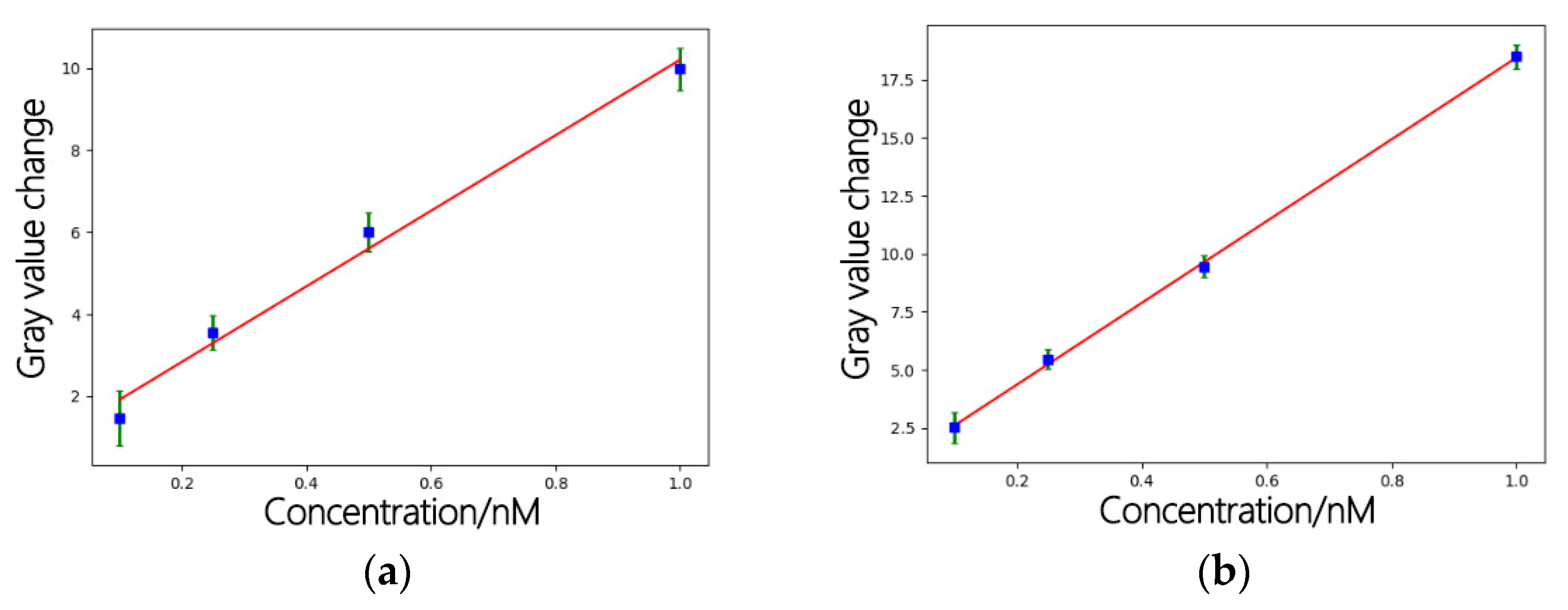
4.2. Detection Using a Quantum Dot/Porous Silicon Optical Biosensor Based on Digital Fluorescence Images
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Jane, A.; Dronov, R.; Hodges, A.; Voelcker, N.H. Porous silicon biosensors on the advance. Trends Biotechnol. 2009, 27, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Saha, H.; Basu, S. Effect of porosity on the performance of surface modified porous silicon hydrogen sensors. Sens. Actuators B Chem. 2010, 147, 145–151. [Google Scholar] [CrossRef]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative Monitoring of Gene Expression Patterns with a Complementary DNA Microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, P.S.; Gokarna, A.; Kulkarni, M.; Karve, M.S.; Bhoraskar, S.V. Porous silicon as an entrapping matrix for the immobilization of urease. Sens. Actuators B 2005, 107, 258–263. [Google Scholar] [CrossRef]
- Schechter, I.; Benchorin, M.; Kux, A. Gas Sensing Properties of Porous Silicon. Anal. Chem. 1995, 67, 3727–3732. [Google Scholar] [CrossRef]
- Cho, B.; Lee, B.Y.; Kim, H.C.; Woo, H.G.; Sohn, H. Fabrication of Human IgG Sensors Based on Porous Silicon Interferometer Containing Bragg Structures. J. Nanosci. Nanotechnol. 2012, 12, 4159–4162. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Lü, X.; Liu, Y.; Ning, X.; Mo, J.; Wang, J. Spectrometer-free biological detection method using porous silicon microcavity devices. Opt. Express 2015, 23, 24626–24633. [Google Scholar] [CrossRef]
- Zhiqing, G.; Zhenhong, J.; Jie, Y.; Nikola, K.; Chuanxi, L. Image Processing of Porous Silicon Microarray in Refractive Index Change Detection. Sensors 2017, 17, 1335. [Google Scholar]
- Li, C.; Jia, Z.; Li, P.; Wen, H.; Lv, G.; Huang, X. Parallel Detection of Refractive Index Changes in a Porous Silicon Microarray Based on Digital Images. Sensors 2017, 17, 750. [Google Scholar] [CrossRef]
- Rossi, A.M.; Wang, L.; Reipa, V.; Murphy, T.E. Porous silicon biosensor for detection of viruses. Biosens. Bioelectron. 2007, 23, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Z.; Lv, G.; Wen, H.; Li, P.; Zhang, H.; Wang, J. Detection of Echinococcus granulosus antigen by a quantum dot/porous silicon optical biosensor. Biomed. Opt. Express 2017, 8, 3458–3469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jia, Z.; Lv, X.; Huang, X. Biological Detection Based on the Transmitted Light Image From a Porous Silicon Microcavity. IEEE Sens. J. 2020, 20, 12184–12189. [Google Scholar] [CrossRef]
- Ren, R.; Guo, Z.; Jia, Z.; Yang, J.; Kasabov, N.K.; Li, C. Speckle Noise Removal in Image-based Detection of Refractive Index Changes in Porous Silicon Microarrays. Sci. Rep. 2019, 9, 15001. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, M.; Jia, Z.; Zhang, H.; Lv, C. Detection using a quantum dots/porous silicon optical biosensor based on digital fluorescence images. Sens. Actuators B Chem. 2020, 315, 128108. [Google Scholar] [CrossRef]
- Pawan, P.; Manoj, G.; Sumit, S.; Nagawat, A.K. Image De-noising by Various Filters for Different Noise. Int. J. Comput. Appl. 2010, 9, 45–50. [Google Scholar]
- Rakhshanfar, M.; Amer, M.A. Estimation of Gaussian, Poissonian-Gaussian, and Processed Visual Noise and its Level Function. IEEE Trans. Image Processing 2016, 25, 4172–4185. [Google Scholar] [CrossRef]
- Xiong, B.; Yin, Z. A Universal Denoising Framework With a New Impulse Detector and Non-local Means. IEEE Trans. Image Processing 2012, 21, 1663–1675. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G. ImageNet Classification with Deep Convolutional Neural Networks. Adv. Neural Inf. Processing Syst. 2012, 25, 1097–1105. [Google Scholar] [CrossRef]
- Santosa, B. Multiclass Classification with Cross Entropy-Support Vector Machines. Procedia Comput. Sci. 2015, 72, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Khaw, H.Y.; Soon, F.C.; Chuah, J.H.; Chow, C.O. Image noise types recognition using convolutional neural network with principal components analysis. IET Image Processing 2017, 11, 1238–1245. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Kuan, D.T.; Sawchuk, A.A.; Strand, T.C.; Chavel, P. Adaptive Noise Smoothing Filter for Images with Signal-Dependent Noise. IEEE Trans. Pattern Anal. Mach. Intell. 1985, PAMI-7, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Frost, V.S.; Stiles, J.A.; Shanmugan, K.S.; Holtzman, J.C. A Model for Radar Images and Its Application to Adaptive Digital Filtering of Multiplicative Noise. IEEE Trans. Pattern Anal. Mach. Intell. 1982, 4, 157–166. [Google Scholar] [CrossRef]
- Lee, J.S.; Pottier, E. Polarimetric Radar Imaging: From Basics to Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Ma, X.; Wu, P. Multitemporal SAR Image Despeckling Based on a Scattering Covariance Matrix of Image Patch. Sensors 2019, 19, 3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, J.M.; Perona, P. Scale-Space and Edge Detection Using Anisotropic Diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 629–639. [Google Scholar]
- Yu, Y.; Acton, S.T. Speckle reducing anisotropic diffusion. IEEE Trans. Image Processing A Publ. IEEE Signal Processing Soc. 2002, 11, 1260. [Google Scholar]
- Maggioni, M.; Katkovnik, V.; Egiazarian, K.; Foi, A. Nonlocal Transform-Domain Filter for Volumetric Data Denoising and Reconstruction. Image Processing IEEE Trans. 2013, 22, 119–133. [Google Scholar] [CrossRef]
- Yu, H.; Gao, J.; Li, A. Probability-based non-local means filter for speckle noise suppression in optical coherence tomography images. Opt. Lett. 2016, 41, 994. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Yin, J.; Han, X.; Hao, W. An Adaptive Denoising and Detection Approach for Underwater Sonar Image. Remote Sens. 2019, 11, 396. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, J.G.; Zhang, B.; Hong, W.; Wu, Y. Adaptive Total Variation Regularization Based SAR Image Despeckling and Despeckling Evaluation Index. IEEE Trans. Geosci. Remote Sens. 2015, 53, 2765–2774. [Google Scholar] [CrossRef] [Green Version]
- Deledalle, C.A.; Denis, L.; Tupin, F. Iterative Weighted Maximum Likelihood Denoising With Probabilistic Patch-Based Weights. IEEE Trans. Image Processing A Publ. IEEE Signal Processing Soc. 2009, 18, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Fu, S.; Zhang, C.; Zhai, L. Speckle noise reduction of multi-frame optical coherence tomography data using multi-linear principal component analysis. Opt. Express 2018, 26, 11804. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahmad, M.O.; Swamy, M.N.S. An efficient denoising framework using weighted overlapping group sparsity. Inf. Sci. 2018, 454, 292–311. [Google Scholar] [CrossRef]
- Huang, Y.M.; Moisan, L.; Ng, M.K.; Zeng, T. Multiplicative Noise Removal via a Learned Dictionary. IEEE Trans. Image Processing 2012, 21, 4534–4543. [Google Scholar] [CrossRef]
- Awad, A.S. Fusion of External and Internal Prior Information for the Removal of Gaussian Noise in Images. J. Imaging 2020, 6, 103. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Zhang, L. FFDNet: Toward a Fast and Flexible Solution for CNN based Image Denoising. IEEE Trans. Image Processing 2017, 27, 4608–4622. [Google Scholar] [CrossRef] [Green Version]
- Buades, A.; Coll, B.; Morel, J.M. A non-local algorithm for image denoising. In Proceedings of the 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Diego, CA, USA, 20–25 June 2005. [Google Scholar]
- Chen, Y.; He, T. Image denoising via an adaptive weighted anisotropic diffusion. Multidimens. Syst. Signal Processing 2021, 32, 651–669. [Google Scholar] [CrossRef]
- Ahmed, F.; Das, S. Removal of High-Density Salt-and-Pepper Noise in Images With an Iterative Adaptive Fuzzy Filter Using Alpha-Trimmed Mean. IEEE Trans. Fuzzy Syst. 2014, 22, 1352–1358. [Google Scholar] [CrossRef]
- Zhou, W.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process 2004, 13, 600–612. [Google Scholar]
- Sun, M.; Zhang, S.; Wang, J.; Jia, Z.; Lv, X.; Huang, X. Enhanced Biosensor Based on Assembled Porous Silicon Microcavities Using CdSe/ZnS Quantum Dots. IEEE Photonics J. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Bai, L.; Gao, Y.; Jia, Z.; Lv, X.; Huang, X. Detection of Pesticide Residues Based on a Porous Silicon Optical Biosensor With a Quantum Dot Fluorescence Label. IEEE Sens. J. 2021, 21, 21441–21449. [Google Scholar] [CrossRef]
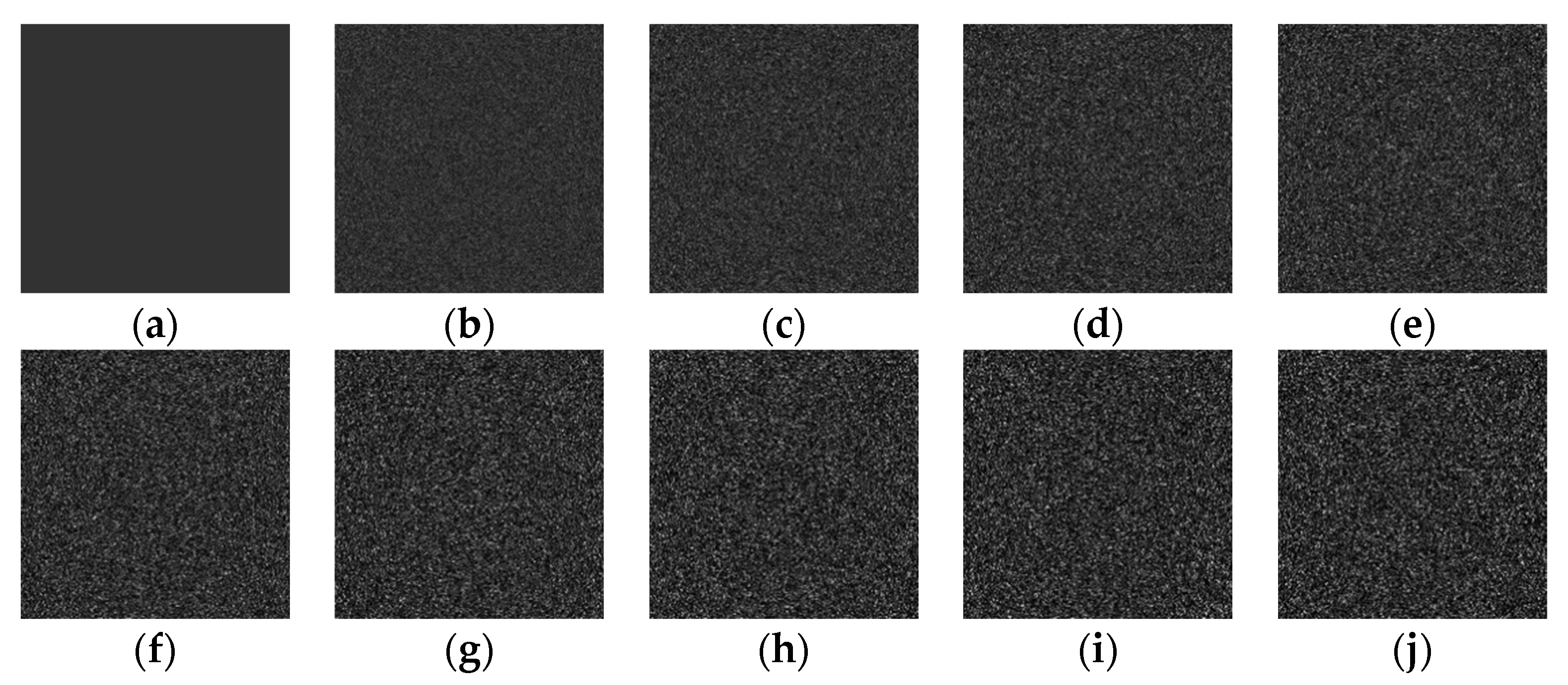
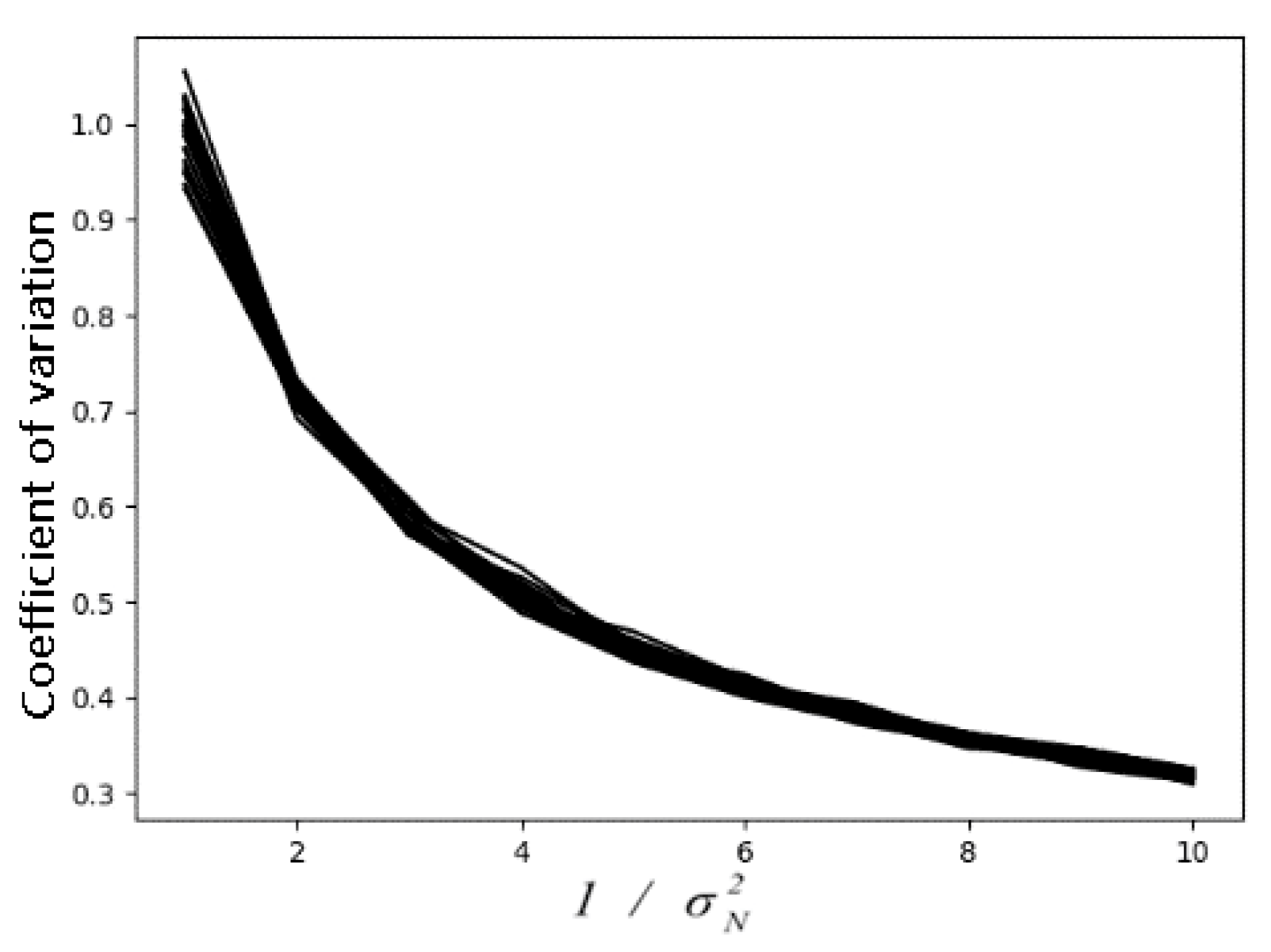
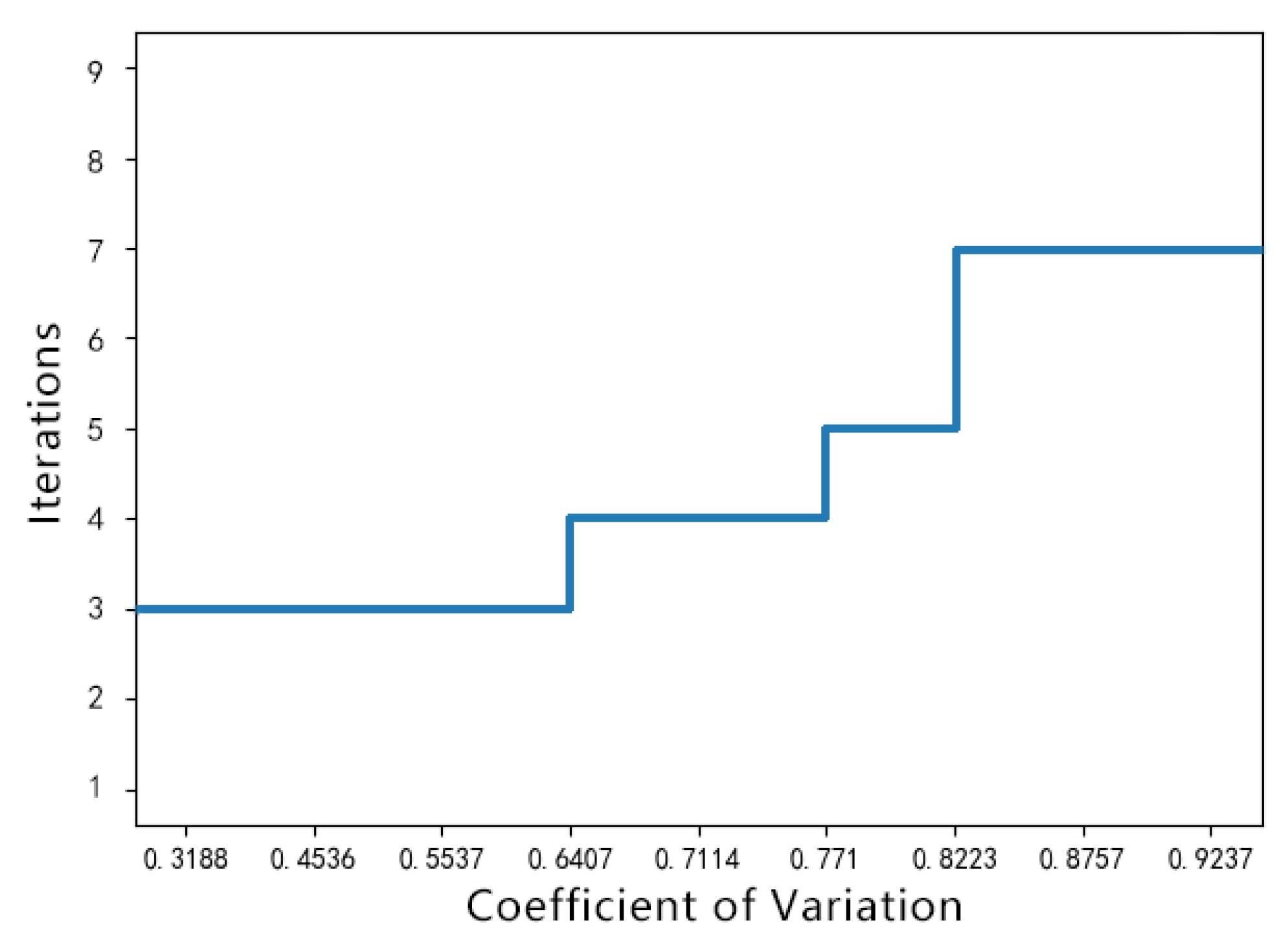

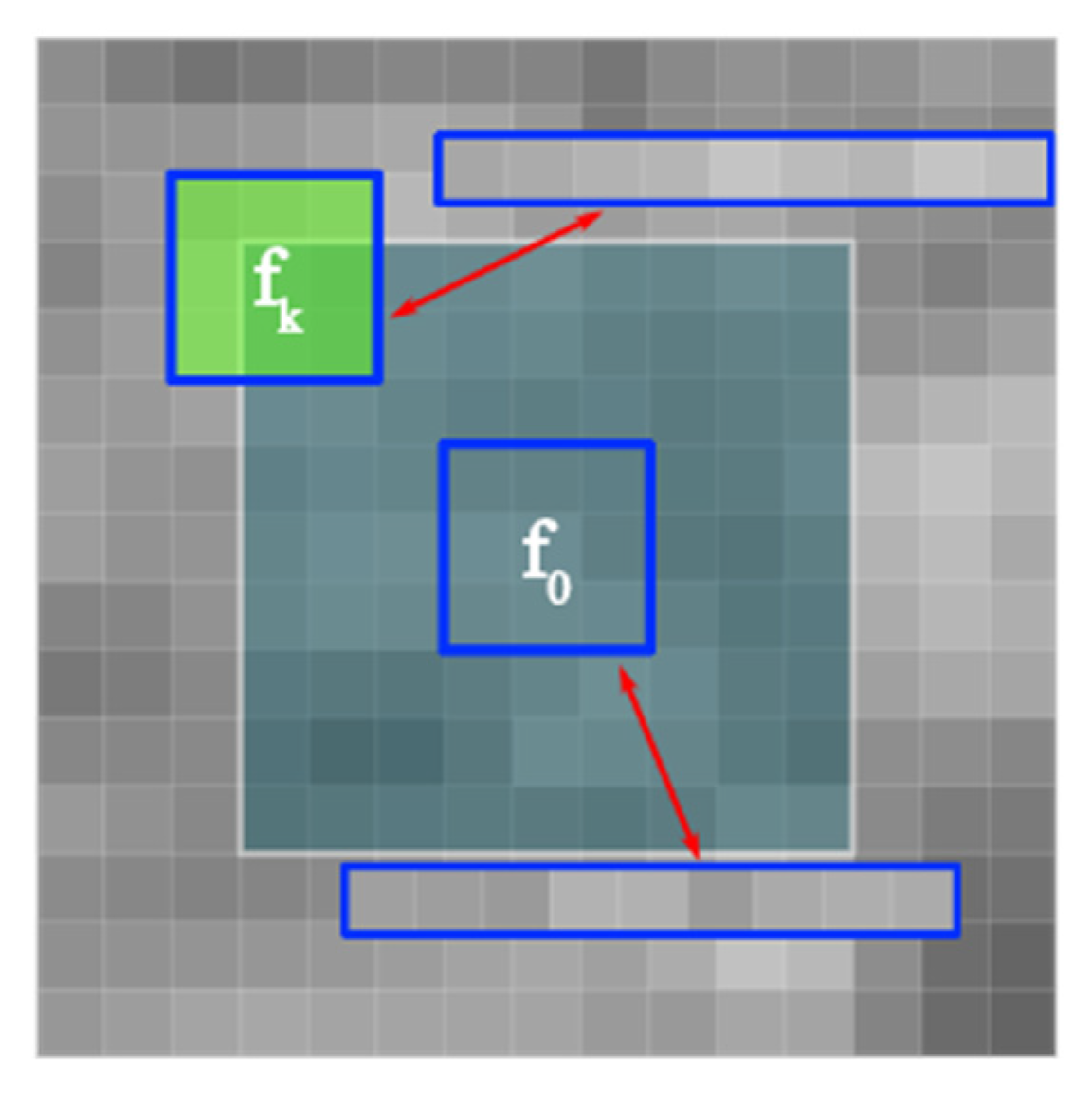
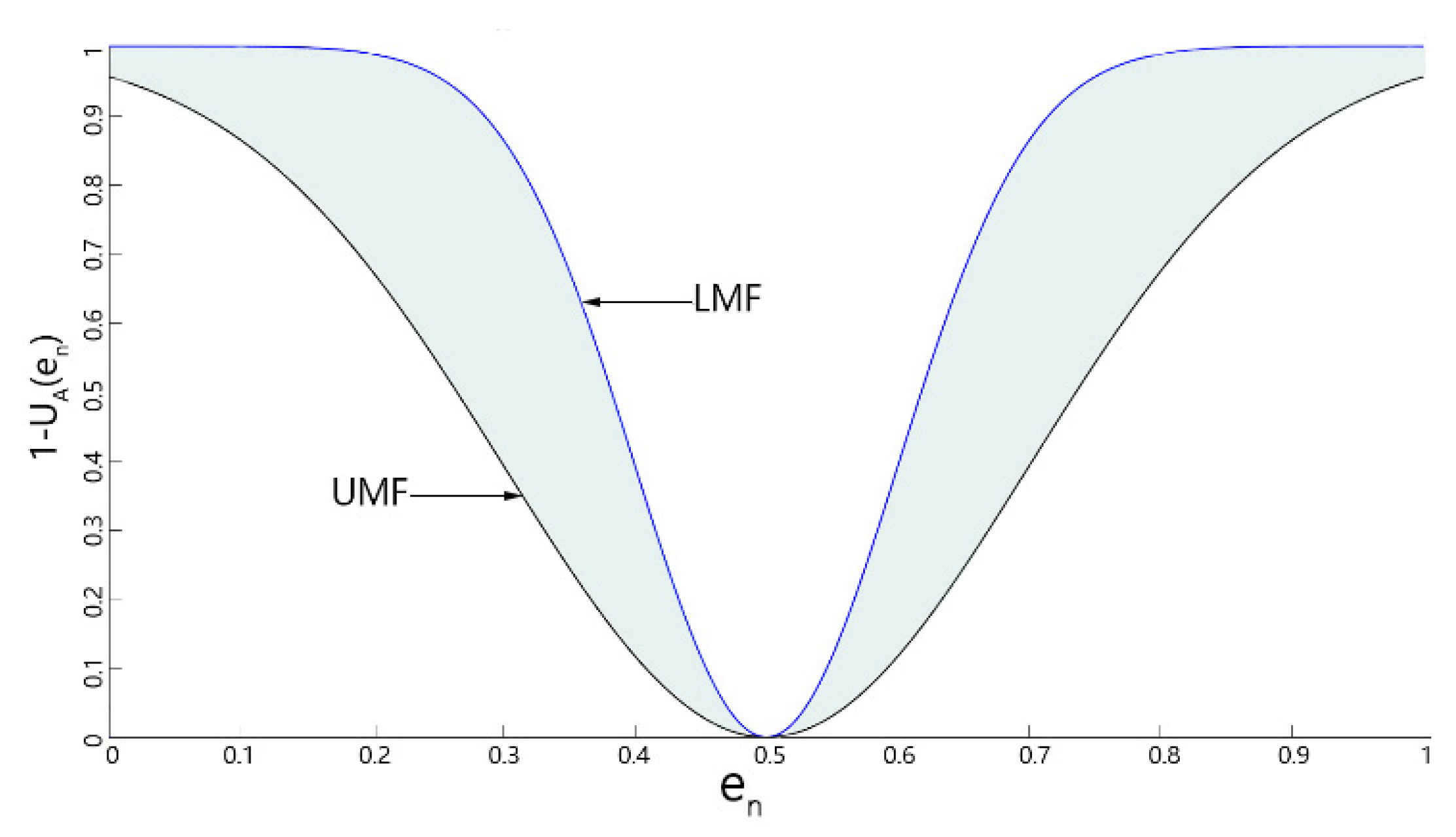

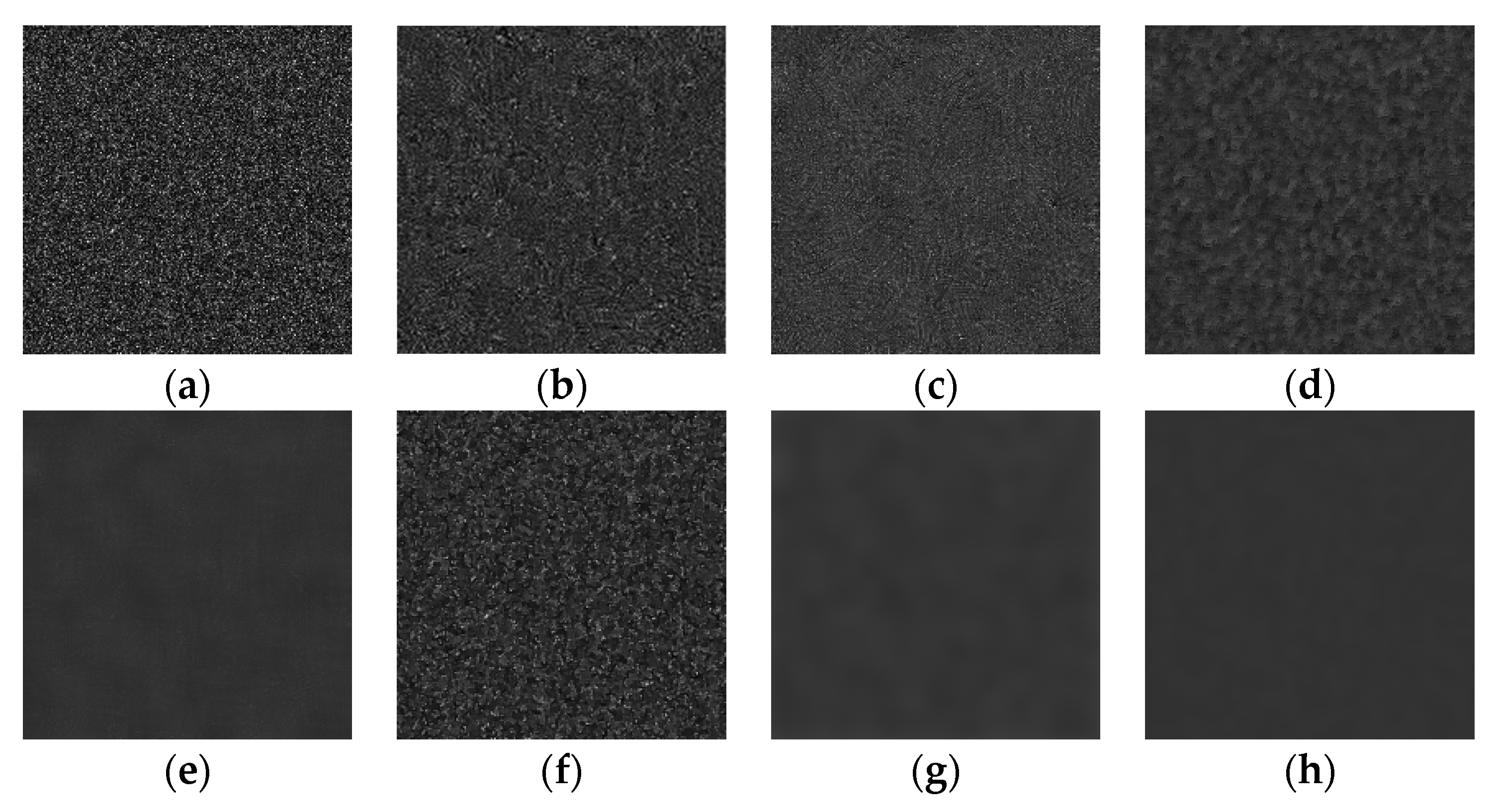



| Exp | Gamma | Rayleigh | Gamma+sp | Gaussian | Gauss+exp | Gauss+sp | Uniform | Poisson | S&P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample1 | 1.055 × 10−3 | 9.034 × 10−1 | 7.662 × 10−2 | 1.719 × 10−3 | 2.361 × 10−4 | 1.742 × 10−4 | 1.094 × 10−2 | 1.104 × 10−4 | 2.809 × 10−3 | 2.904 × 10−3 |
| Sample2 | 9.049 × 10−5 | 9.672 × 10−1 | 1.849 × 10−2 | 6.203 × 10−5 | 3.576 × 10−4 | 6.414 × 10−6 | 5.235 × 10−5 | 5.415 × 10−5 | 4.456 × 10−4 | 1.315 × 10−2 |
| Sample3 | 2.786 × 10−7 | 9.992 × 10−1 | 6.837 × 10−4 | 1.183 × 10−6 | 3.118 × 10−6 | 6.845 × 10−8 | 6.332 × 10−8 | 4.855 × 10−7 | 6.203 × 10−6 | 1.567 × 10−5 |
| Sample4 | 2.791 × 10−6 | 9.985 × 10−1 | 1.226 × 10−3 | 1.968 × 10−5 | 2.983 × 10−5 | 4.828 × 10−7 | 1.149 × 10−6 | 2.719 × 10−6 | 3.363 × 10−5 | 1.288 × 10−4 |
| Sample5 | 8.112 × 10−6 | 7.762 × 10−1 | 2.234 × 10−1 | 1.128 × 10−6 | 1.568 × 10−5 | 4.972 × 10−8 | 1.063 × 10−6 | 3.119 × 10−5 | 5.099 × 10−5 | 1.534 × 10−4 |
| Sample6 | 4.535 × 10−7 | 9.942 × 10−1 | 5.722 × 10−3 | 4.006 × 10−6 | 1.786 × 10−5 | 4.157 × 10−8 | 6.264 × 10−6 | 4.195 × 10−6 | 7.960 × 10−6 | 2.220 × 10−5 |
| Sample7 | 2.187 × 10−5 | 9.891 × 10−1 | 8.575 × 10−3 | 1.897 × 10−5 | 1.893 × 10−3 | 8.717 × 10−7 | 4.295 × 10−6 | 3.578 × 10−4 | 1.955 × 10−5 | 4.806 × 10−6 |
| Sample8 | 3.763 × 10−5 | 9.793 × 10−1 | 1.170 × 10−2 | 7.331 × 10−5 | 3.871 × 10−5 | 6.360 × 10−7 | 1.562 × 10−3 | 6.296 × 10−7 | 3.483 × 10−4 | 6.830 × 10−3 |
| Algorithm Flow |
|---|
| Step 1: The Laplace operator is used to obtain the mean region of the PSi image, and M blocks with the smallest gradient sum are used to calculate their coefficient of variation and take the mean value to estimate the coefficient of variation of the whole image, to determine the gray compression iteration number. Step 2: The input quantum dot PSi image is filtered by nonlocal means. The gray compression coefficient of each pixel is obtained by dividing the original image and the filtered image. The image is preprocessed using the GVC method. Step 3: Determine the search window with each pixel as the center, calculate the cosine distance between each pixel neighborhood and the center pixel neighborhood in the search window, determine the diffusion threshold T of anisotropic diffusion and the diffusion coefficient of each pixel, and solve the differential equation. Assess the probability that a pixel is a noise by the type-2 membership function and remove the noise with the anisotropic diffusion algorithm. |
| Var | Index | Unprocessed | PNLM | Refined Lee | BM3D | PPB | ADAPTIVE TV | FFDNET | Proposed |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | AGL | 49.65 | 49.61 | 49.64 | 49.07 | 51.95 | 48.7 | 51.04 | 49.97 |
| RMSE | 15.833 | 0.918 | 4.289 | 3.162 | 3.487 | 1.423 | 1.709 | 0.279 | |
| SSIM | 0.206 | 0.997 | 0.82 | 0.897 | 0.961 | 0.832 | 0.998 | 0.996 | |
| 0.2 | AGL | 49.57 | 49.43 | 49.52 | 48.74 | 53.74 | 48.43 | 51.25 | 49.88 |
| RMSE | 22.490 | 1.273 | 6.035 | 6.872 | 5.846 | 5.171 | 1.987 | 0.506 | |
| SSIM | 0.116 | 0.993 | 0.702 | 0.619 | 0.776 | 0.434 | 0.997 | 0.996 | |
| 0.3 | AGL | 49.44 | 49.14 | 49.42 | 48.27 | 55.0 | 47.15 | 51.48 | 49.92 |
| RMSE | 27.38 | 1.777 | 7.39 | 11.206 | 9.683 | 11.489 | 2.237 | 0.589 | |
| SSIM | 0.082 | 0.985 | 0.616 | 0.381 | 0.508 | 0.254 | 0.996 | 0.996 | |
| 0.4 | AGL | 49.42 | 48.96 | 49.42 | 48.26 | 56.29 | 46.43 | 51.97 | 49.96 |
| RMSE | 31.672 | 2.263 | 8.517 | 16.265 | 14.073 | 16.411 | 2.753 | 0.723 | |
| SSIM | 0.063 | 0.969 | 0.55 | 0.23 | 0.315 | 0.176 | 0.995 | 0.995 | |
| 0.5 | AGL | 49.27 | 48.62 | 49.29 | 48.44 | 56.7 | 45.95 | 52.32 | 50.01 |
| RMSE | 35.061 | 2.753 | 9.388 | 22.22 | 17.792 | 20.235 | 2.952 | 0.737 | |
| SSIM | 0.052 | 0.946 | 0.5 | 0.136 | 0.206 | 0.206 | 0.995 | 0.995 | |
| 0.6 | AGL | 49.18 | 48.26 | 49.14 | 48.55 | 56.76 | 45.62 | 52.66 | 50.01 |
| RMSE | 37.931 | 3.405 | 10.184 | 26.81 | 20.981 | 23.283 | 3.342 | 0.796 | |
| SSIM | 0.045 | 0.913 | 0.465 | 0.098 | 0.155 | 0.137 | 0.994 | 0.994 | |
| 0.7 | AGL | 49.11 | 48.02 | 49.16 | 48.66 | 57.35 | 45.30 | 53.14 | 50.00 |
| RMSE | 40.401 | 4.152 | 10.906 | 30.298 | 24.405 | 26.147 | 4.01 | 0.954 | |
| SSIM | 0.04 | 0.874 | 0.433 | 0.079 | 0.117 | 0.109 | 0.992 | 0.994 | |
| 0.8 | AGL | 48.44 | 47.21 | 48.69 | 48.18 | 57.19 | 44.31 | 53.08 | 49.93 |
| RMSE | 42.447 | 5.075 | 11.645 | 33.282 | 27.188 | 28.463 | 4.029 | 0.998 | |
| SSIM | 0.037 | 0.825 | 0.403 | 0.064 | 0.094 | 0.093 | 0.992 | 0.994 | |
| 0.9 | AGL | 48.36 | 46.90 | 48.71 | 48.16 | 57.2 | 44.06 | 53.63 | 49.94 |
| RMSE | 44.71 | 6.13 | 12.437 | 35.76 | 29.681 | 31.013 | 4.501 | 1.096 | |
| SSIM | 0.034 | 0.764 | 0.377 | 0.056 | 0.078 | 0.08 | 0.989 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Sun, M.; Jia, Z.; Yang, J.; Kasabov, N.K. Denoising of Fluorescence Image on the Surface of Quantum Dot/Nanoporous Silicon Biosensors. Sensors 2022, 22, 1366. https://doi.org/10.3390/s22041366
Liu Y, Sun M, Jia Z, Yang J, Kasabov NK. Denoising of Fluorescence Image on the Surface of Quantum Dot/Nanoporous Silicon Biosensors. Sensors. 2022; 22(4):1366. https://doi.org/10.3390/s22041366
Chicago/Turabian StyleLiu, Yong, Miao Sun, Zhenhong Jia, Jie Yang, and Nikola K. Kasabov. 2022. "Denoising of Fluorescence Image on the Surface of Quantum Dot/Nanoporous Silicon Biosensors" Sensors 22, no. 4: 1366. https://doi.org/10.3390/s22041366
APA StyleLiu, Y., Sun, M., Jia, Z., Yang, J., & Kasabov, N. K. (2022). Denoising of Fluorescence Image on the Surface of Quantum Dot/Nanoporous Silicon Biosensors. Sensors, 22(4), 1366. https://doi.org/10.3390/s22041366








