Bio-Interface on Freestanding Nanosheet of Microelectromechanical System Optical Interferometric Immunosensor for Label-Free Attomolar Prostate Cancer Marker Detection
Abstract
:1. Introduction
2. Materials and Methods
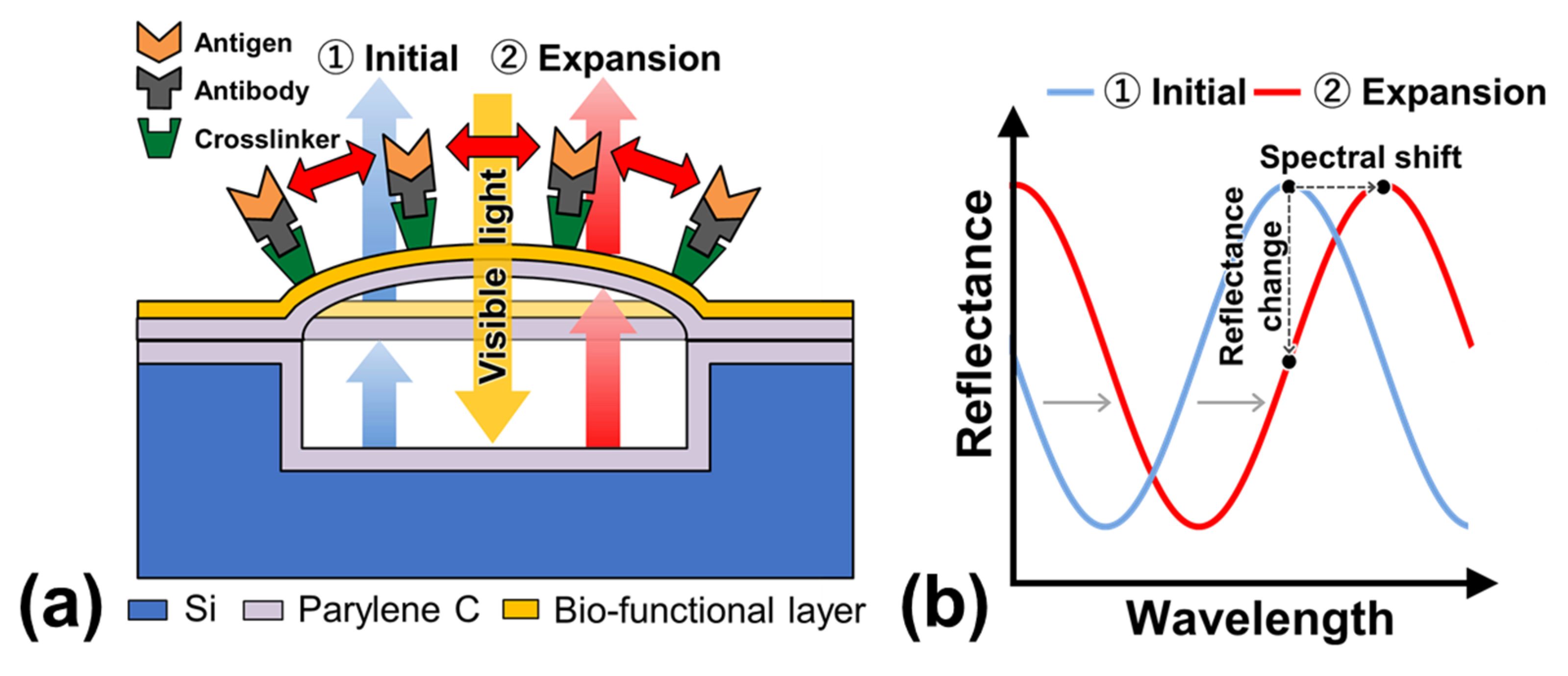
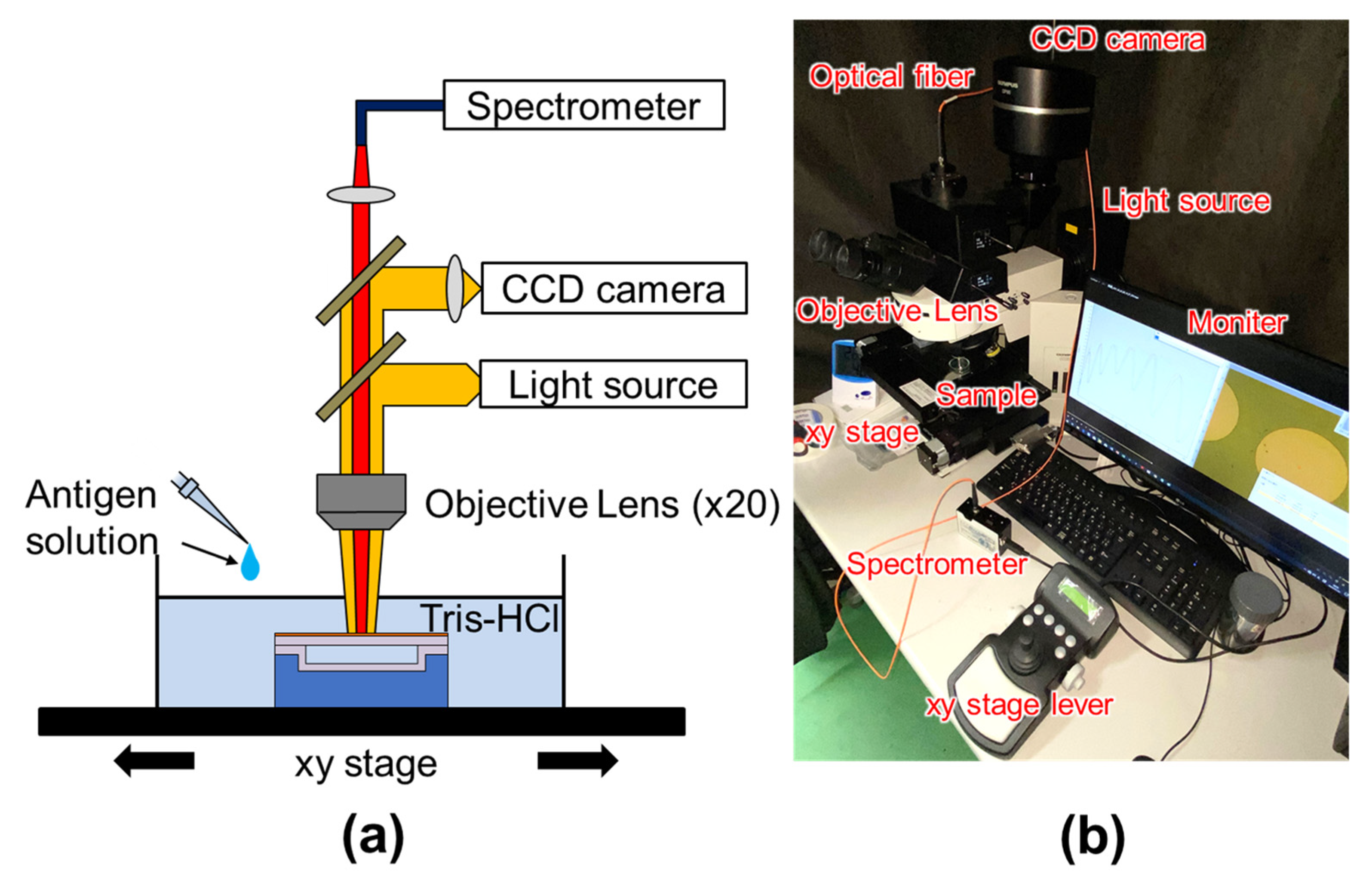
2.1. Structure and Operation of MEMS Optical Interferometric Surface-Stress Immunosensor
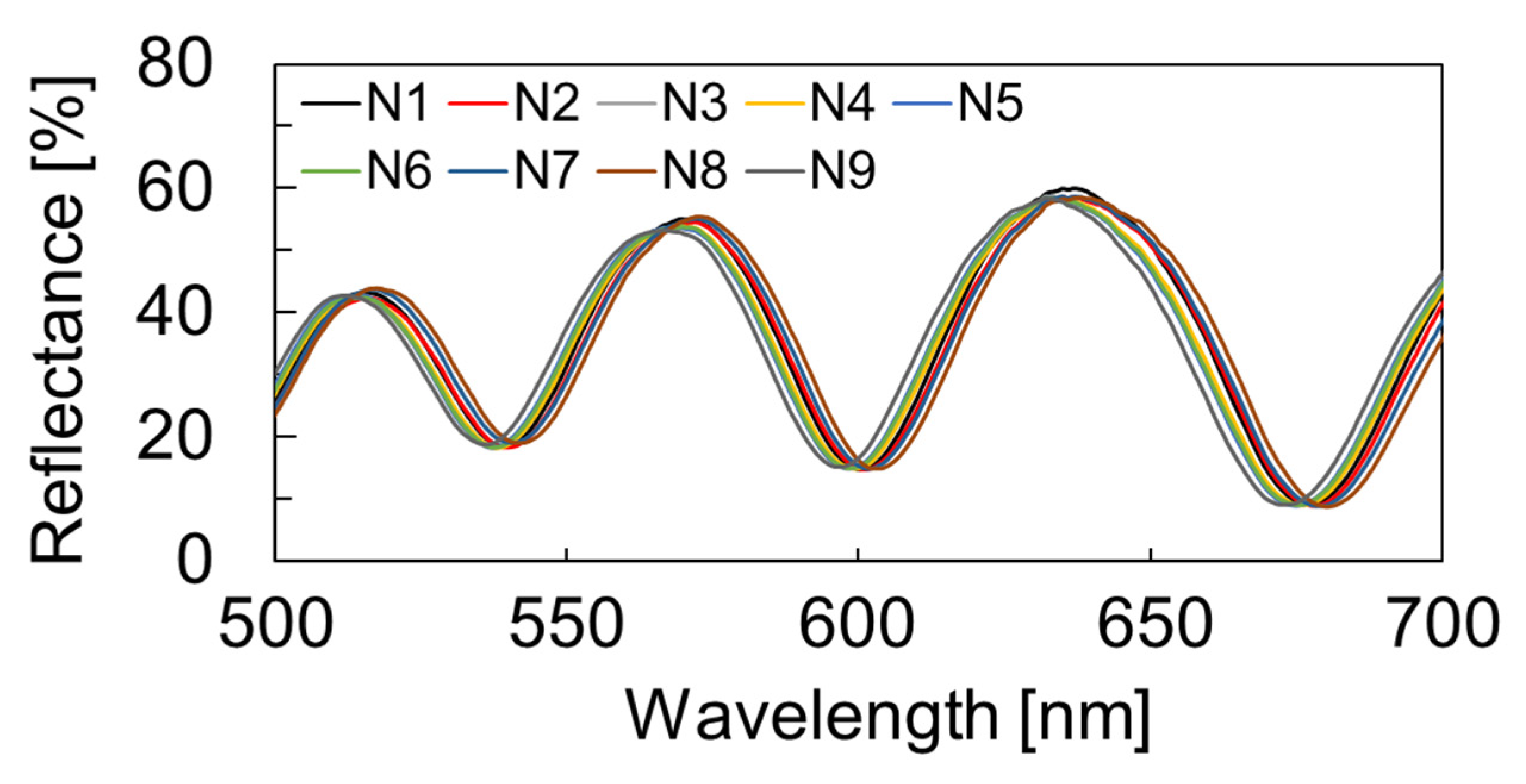
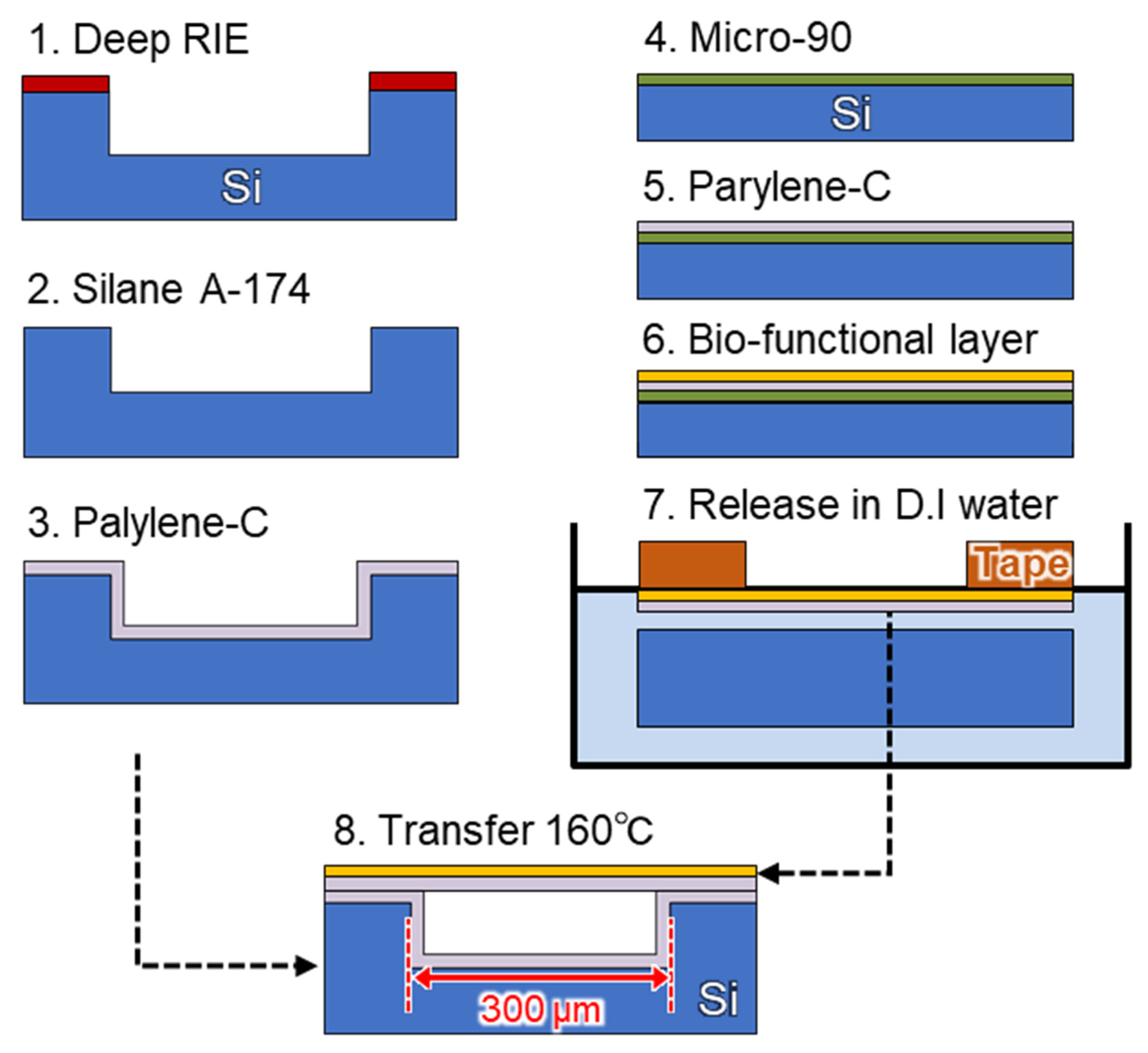
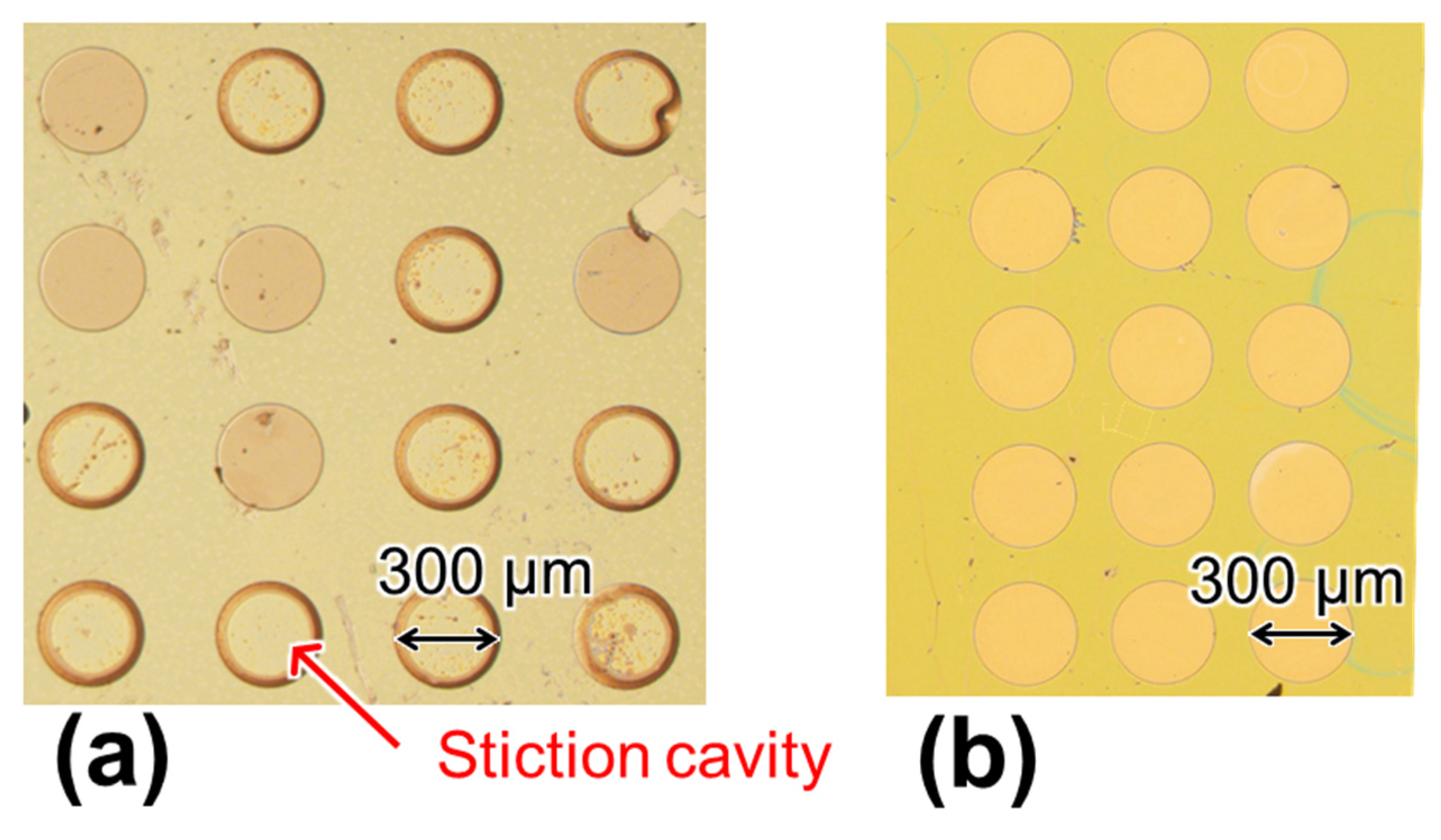
2.2. Cavity Formation by Nanosheet Transfer
2.3. Molecular Modification Using Oxidized Poly Methyl Methacrylate (PMMA)
2.4. Molecular Modification Using Amino-Functionalized Membrane
2.5. Zeta Potential Measurement
2.6. Label-Free PSA Detection Using Optical Interferometry
3. Results and Discussion
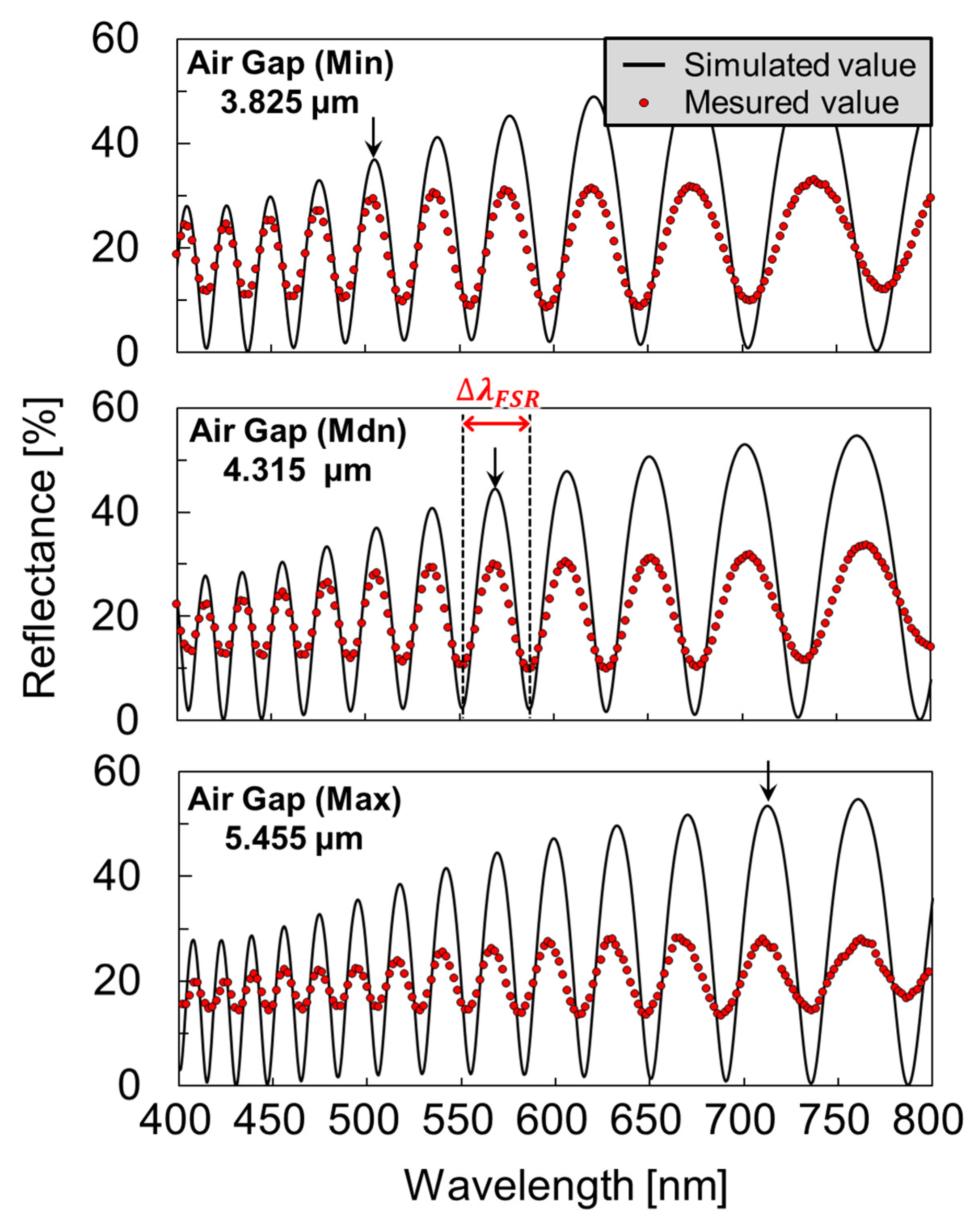
3.1. Deformed Shape Evaluation of Molecular Modified Membrane
3.2. Surface Condition Evaluation by Zeta Potential Measurement
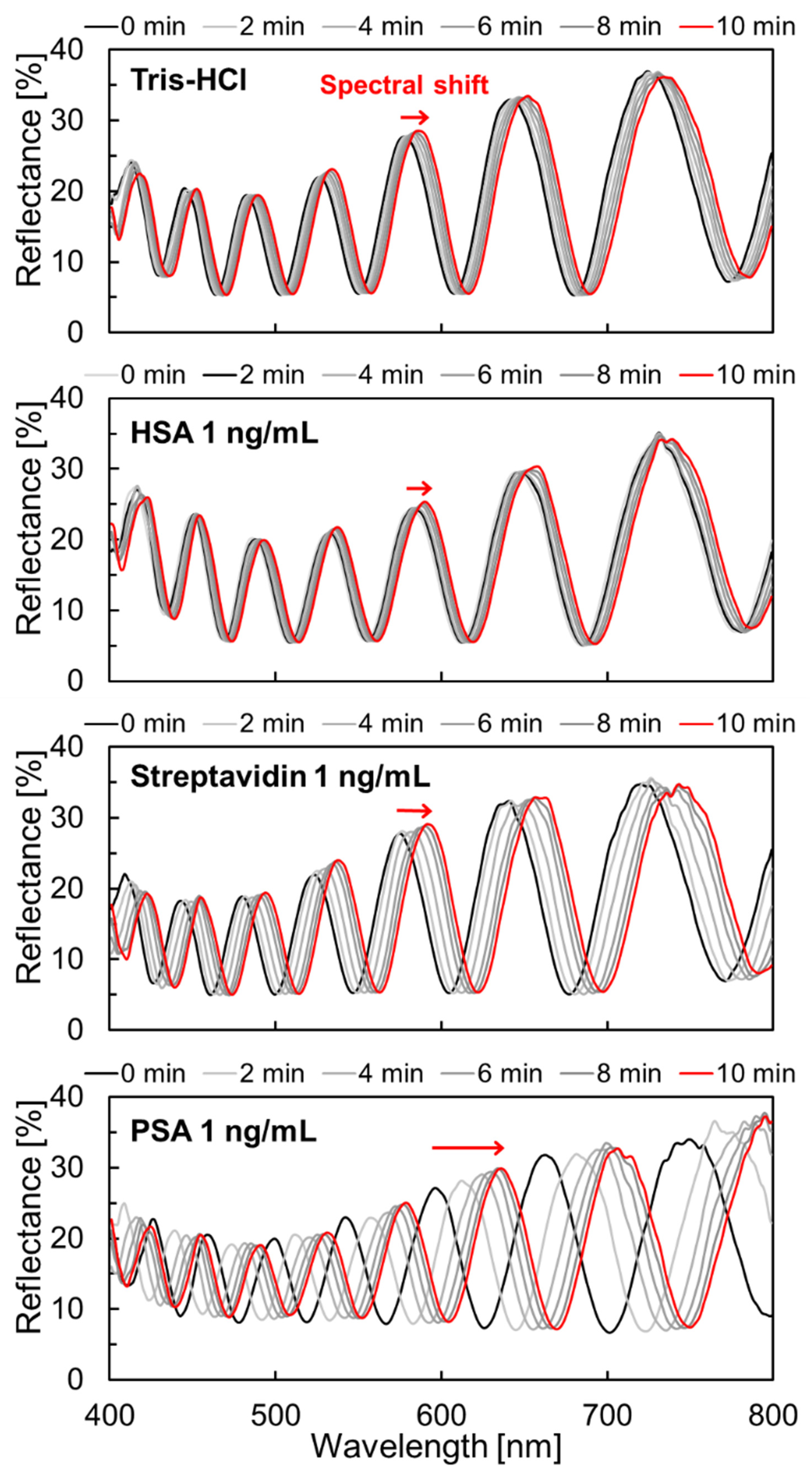
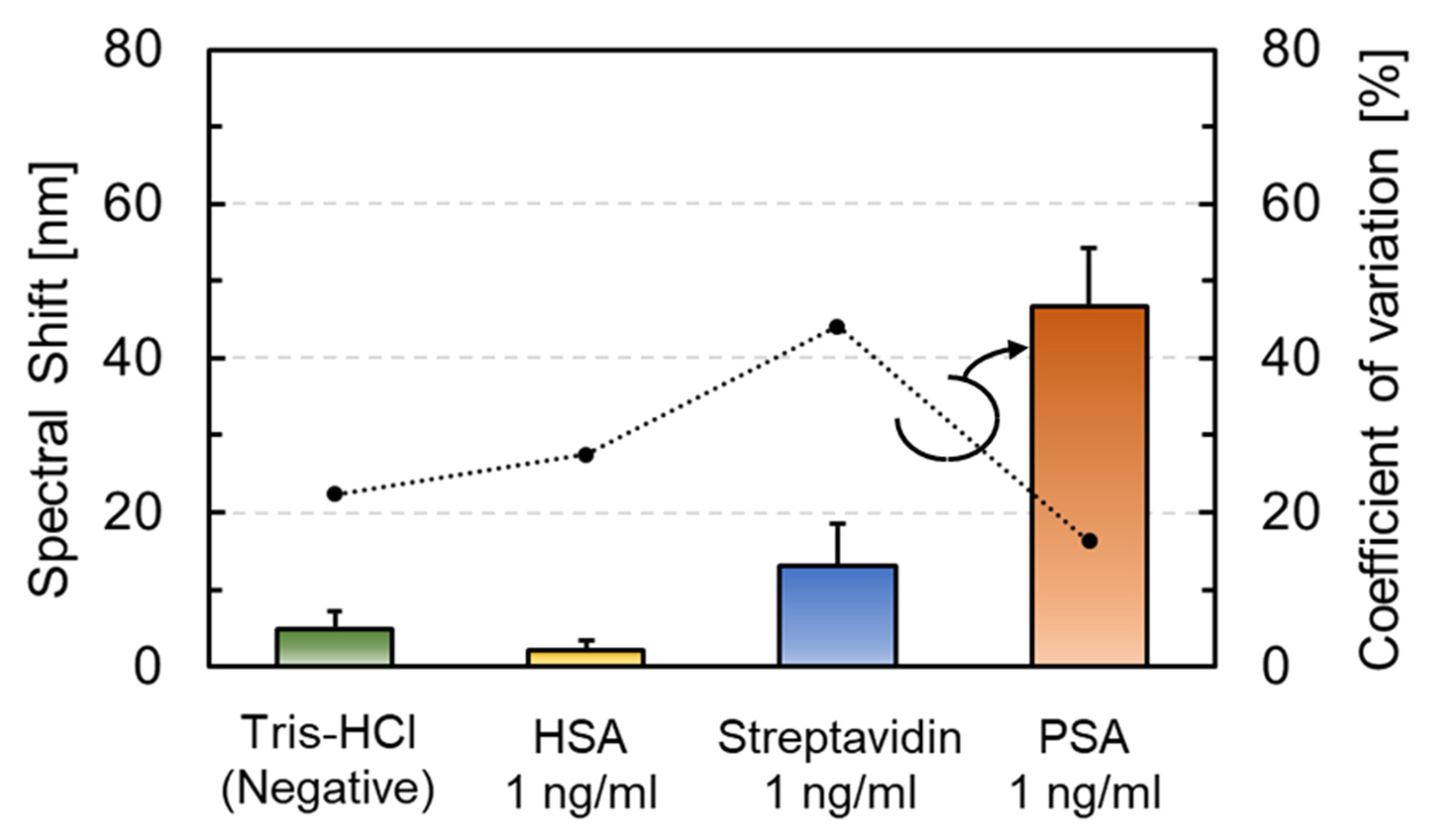
3.3. Molecular Selectivity and Label-Free PSA Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IARC. WORLD CANCER REPORT: Cancer Research for Cancer Development. Available online: https://digitallibrary.in.one.un.org/TempPdfFiles/5932_1.pdf (accessed on 5 January 2022).
- World Health Organization. World Health Statistics 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/342703/9789240027053-eng.pdf (accessed on 5 January 2022).
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S. Disease proteomics. Nature 2003, 422, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J. What are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Iwai, S.; Araki, S.; Sakakihara, S.; Iino, R.; Noji, H. Large-scale femtoliter droplet array for digital counting of single biomolecules. Lab Chip 2012, 12, 4986–4991. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC-Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Xu, G.; Abbott, J.; Qin, L.; Yeung, K.Y.M.; Song, Y.; Yoon, H.; Kong, J.; Ham, D. Electrophoretic and field-effect graphene for all-electrical DNA array technology. Nat. Commun. 2014, 5, 4866. [Google Scholar] [CrossRef] [Green Version]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curreli, M.; Zhang, R.; Ishikawa, F.N.; Chang, H.K.; Cote, R.J.; Zhou, C.; Thompson, M.E. Real-time, label-free detection of biological entities using nanowire-based FETs. IEEE Trans. Nanotechnol. 2008, 7, 651–667. [Google Scholar] [CrossRef]
- Lin, S.P.; Pan, C.Y.; Tseng, K.C.; Lin, M.C.; Chen, C.D.; Tsai, C.C.; Yu, S.H.; Sun, Y.C.; Lin, T.W.; Chen, Y.T. A reversible surface functionalized nanowire transistor to study protein-protein interactions. Nano Today 2009, 4, 235–243. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Sarangadharan, I.; Regmi, A.; Chen, Y.W.; Hsu, C.P.; Chang, W.H.; Lee, G.Y.; Chyi, J.I.; Chen, C.C.; Shiesh, S.C.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Lloret, N.; Frederiksen, R.S.; Møller, T.C.; Rieben, N.I.; Upadhyay, S.; De Vico, L.; Jensen, J.H.; Nygård, J.; Martinez, K.L. Effects of buffer composition and dilution on nanowire field-effect biosensors. Nanotechnology 2013, 24, 035501. [Google Scholar] [CrossRef]
- Waggoner, P.S.; Craighead, H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A.; Mohamed, A.M.A.; Kahraman, R. A novel classification of prostate specific antigen (PSA) biosensors based on transducing elements. Talanta 2017, 168, 52–61. [Google Scholar] [CrossRef]
- Vančura, C.; Li, Y.; Lichtenberg, J.; Kirstein, K.U.; Hierlemann, A.; Josse, F. Liquid-phase chemical and biochemical detection using fully integrated magnetically actuated complementary metal oxide semiconductor resonant cantilever sensor systems. Anal. Chem. 2007, 79, 1646–1654. [Google Scholar] [CrossRef]
- Lee, S.M.; Hwang, K.S.; Yoon, H.J.; Yoon, D.S.; Kim, S.K.; Lee, Y.S.; Kim, T.S. Sensitivity enhancement of a dynamic mode microcantilever by stress inducer and mass inducer to detect PSA at low picogram levels. Lab Chip 2009, 9, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Datar, R.H.; Hansen, K.M.; Thundat, T.; Cote, R.J.; Majumdar, A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nat. Biotechnol. 2001, 19, 856–860. [Google Scholar] [CrossRef]
- Yue, M.; Stachowiak, J.C.; Lin, H.; Datar, R.; Cote, R.; Majumdar, A. Label-free protein recognition two-dimensional array using nanomechanical sensors. Nano Lett. 2008, 8, 520–524. [Google Scholar] [CrossRef]
- Buchapudi, K.R.; Huang, X.; Yang, X.; Ji, H.F.; Thundat, T. Microcantilever biosensors for chemicals and bioorganisms. Analyst 2011, 136, 1539–1556. [Google Scholar] [CrossRef]
- Takahashi, K.; Oyama, H.; Misawa, N.; Okumura, K.; Ishida, M.; Sawada, K. Surface stress sensor using MEMS-based Fabry-Perot interferometer for label-free biosensing. Sens. Actuators B Chem. 2013, 188, 393–399. [Google Scholar] [CrossRef]
- Maruyama, S.; Hizawa, T.; Takahashi, K.; Sawada, K. Optical-interferometry-based CMOS-MEMS sensor transduced by stress-induced nanomechanical deflection. Sensors 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Fujie, T.; Teramoto, R.; Takahashi, I.; Sato, N.; Takeoka, S.; Sawada, K. Elastomer-based MEMS optical interferometric transducers for highly sensitive surface stress sensing for biomolecular detection. MRS Commun. 2019, 9, 381–389. [Google Scholar] [CrossRef]
- Choi, Y.J.; Takahashi, T.; Taki, M.; Sawada, K.; Takahashi, K. Label-free attomolar protein detection using a MEMS optical interferometric surface-stress immunosensor with a freestanding PMMA/parylene-C nanosheet. Biosens. Bioelectron. 2021, 172, 112778. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Takahashi, T.; Choi, Y.J.; Sawada, K.; Takahashi, K. A ppm ethanol sensor based on fabry–perot interferometric surface stress transducer at room temperature. Sensors 2020, 20, 6868. [Google Scholar] [CrossRef]
- Rowinska, M.; Kelleher, S.M.; Soberon, F.; Ricco, A.J.; Daniels, S. Fabrication and characterisation of spin coated oxidised PMMA to provide a robust surface for on-chip assays. J. Mater. Chem. B 2015, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Walt, D.R.; Agayn, V.I. The chemistry of enzyme and protein immobilization with glutaraldehyde. TrAC-Trends Anal. Chem. 1994, 13, 425–430. [Google Scholar] [CrossRef]
- Jeon, B.J.; Kim, M.H.; Pyun, J.C. Application of a functionalized parylene film as a linker layer of SPR biosensor. Sens. Actuators B Chem. 2011, 154, 89–95. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Kim, K.; Pyun, J.C.; Sung, G.Y. Parylene-coated polytetrafluoroethylene-membrane-based portable urea sensor for real-time monitoring of urea in peritoneal dialysate. Sensors 2019, 19, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midmore, B.R.; Hunter, R.J. The effect of electrolyte concentration and co-ion type on the ζ-potential of polystyrene latices. J. Colloid Interface Sci. 1988, 122, 521–529. [Google Scholar] [CrossRef]
- Yousif, E.; Bakir, E.; Salimon, J.; Salih, N. Evaluation of Schiff bases of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer for poly(methyl methacrylate). J. Saudi Chem. Soc. 2012, 16, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Fortin, J.B.; Lu, T.M. Ultraviolet radiation induced degradation of poly-para-xylylene (parylene) thin films. Thin Solid Films 2001, 397, 223–228. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Murray, A.F.; Curtis, J.C. Effects of parylene-C photooxidation on serum-assisted glial and neuronal patterning. J. Biomed. Mater. Res.-Part A 2010, 94, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Konno, T.; Takai, M.; Ishihara, K. Photoinduced phospholipid polymer grafting on Parylene film: Advanced lubrication and antibiofouling properties. Colloids Surf. B Biointerfaces 2007, 54, 67–73. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Huang, C.W.; Liu, H.Y.; Huang, M.C.; Sun, T.P.; Chen, W.C.; Wu, C.Y.; Ding, S.T.; Chen, H.Y. Enhanced bone morphogenic property of parylene-C. Biomater. Sci. 2016, 4, 1754–1760. [Google Scholar] [CrossRef]
- Butterworth, M.D.; Corradi, R.; Johal, J.; Lascelles, S.F.; Maeda, S.; Armes, S.P. Zeta Potential Measurements on Conducting Polymer-Inorganic Oxide Nanocomposite Particles. J. Colloid Interface Sci. 1995, 174, 510–517. [Google Scholar] [CrossRef]
- Schultz, N.; Metreveli, G.; Franzreb, M.; Frimmel, F.H.; Syldatk, C. Zeta potential measurement as a diagnostic tool in enzyme immobilisation. Colloids Surf. B Biointerfaces 2008, 66, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Morykwas, M.J.; Thornton, J.W.; Bartlett, R.H. Zeta potential of synthetic and biological skin substitutes: Effects on initial adherence. Plast. Reconstr. Surg. 1987, 79, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Buijs, J.; Van Den Berg, P.A.W.; Lichtenbelt, J.W.T.; Norde, W.; Lyklema, J. Adsorption dynamics of IgG and its F(ab′)2 and Fc fragments studied by reflectometry. J. Colloid Interface Sci. 1996, 178, 594–605. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Garcia-Gancedo, L.; Flewitt, A.J.; Ashley, G.M.; Luo, J.; Lu, J.R. Interfacial recognition of human prostate-specific antigen by immobilized monoclonal antibody: Effects of solution conditions and surface chemistry. J. R. Soc. Interface 2012, 9, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Jachimska, B.; Wasilewska, M.; Adamczyk, Z. Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir 2008, 24, 6867–6872. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and theoretical interpretation of points of zero charge/potential of BSA protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Papsidero, L.D.; Kuriyama, M.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Prostate antigen: A new potential marker for prostatic cancer. Prostate 1981, 2, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Mayampurath, A.; Yu, C.Y.; Tang, H.; Mechref, Y. Glycoproteomics: Identifying the glycosylation of prostate specific antigen at normal and high isoelectric points by LC-MS/MS. J. Proteome Res. 2014, 13, 5570–5580. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; Von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Gast, A.P.; Robertson, C.R.; Wang, S.W.; Yatcilla, M.T. Two-dimensional streptavidin crystals: Macropatterns and micro-organization. Biomol. Eng. 1999, 16, 21–27. [Google Scholar] [CrossRef]
- Liu, H.; Bachand, G.D.; Kim, H.; Hayden, C.C.; Abate, E.A.; Sasaki, D.Y. Lipid nanotube formation from streptavidin-membrane binding. Langmuir 2008, 24, 3686–3689. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ah, C.S.; Yu, H.Y.; Yang, J.H.; Baek, I.B.; Ahn, C.G.; Park, C.W.; Jun, M.S.; Lee, S. Ultrasensitive, label-free, and real-time immunodetection using silicon field-effect transistors. Appl. Phys. Lett. 2007, 91, 11–14. [Google Scholar] [CrossRef]




| Device Type | Operating Environment | LOD for PSA | Ref. |
|---|---|---|---|
| FET | in liquid (low ionic strength) | 1 fg/mL | [57] |
| Microcantilever (Dynamic method) | in liquid | 10 ng/mL | [22] |
| air (25 °C) | 1 pg/mL | [23] | |
| Microcantilever (Static method) | in liquid | 0.2 ng/mL | [24] |
| in liquid | 0.2 ng/mL | [25] | |
| This study | in liquid | 100 ag/mL | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, T.; Kanamori, R.; Choi, Y.-J.; Taki, M.; Noda, T.; Sawada, K.; Takahashi, K. Bio-Interface on Freestanding Nanosheet of Microelectromechanical System Optical Interferometric Immunosensor for Label-Free Attomolar Prostate Cancer Marker Detection. Sensors 2022, 22, 1356. https://doi.org/10.3390/s22041356
Maeda T, Kanamori R, Choi Y-J, Taki M, Noda T, Sawada K, Takahashi K. Bio-Interface on Freestanding Nanosheet of Microelectromechanical System Optical Interferometric Immunosensor for Label-Free Attomolar Prostate Cancer Marker Detection. Sensors. 2022; 22(4):1356. https://doi.org/10.3390/s22041356
Chicago/Turabian StyleMaeda, Tomoya, Ryoto Kanamori, Yong-Joon Choi, Miki Taki, Toshihiko Noda, Kazuaki Sawada, and Kazuhiro Takahashi. 2022. "Bio-Interface on Freestanding Nanosheet of Microelectromechanical System Optical Interferometric Immunosensor for Label-Free Attomolar Prostate Cancer Marker Detection" Sensors 22, no. 4: 1356. https://doi.org/10.3390/s22041356
APA StyleMaeda, T., Kanamori, R., Choi, Y.-J., Taki, M., Noda, T., Sawada, K., & Takahashi, K. (2022). Bio-Interface on Freestanding Nanosheet of Microelectromechanical System Optical Interferometric Immunosensor for Label-Free Attomolar Prostate Cancer Marker Detection. Sensors, 22(4), 1356. https://doi.org/10.3390/s22041356







