1. Introduction
Worldwide, about 10% of all children are born prematurely, which results in 15 million premature infants each year. Even though advanced medical treatment in combination with technical development have already led to a 50% reduction in child mortality from 1990 to 2019 [
1,
2], the World Health Organisation (WHO) estimated 2.4 million deaths in 2019, which means on average about 6700 neonates per day worldwide [
3]. About one million of these infants die due to complications related to preterm birth, making prematurity the second-leading cause of death in children under 5 years in 2012 [
4].
Despite the high mortality, only data of 2.5% of global neonatal deaths are based on reliable vital registration systems, while the rest originates from estimations and surveys [
5]. Prematurity is an often occurring phenomenon with rising rates. In 2012, the WHO reported that 5–18% of all infants were born preterm. The mortality of these patients strongly decreases with higher gestational age, higher birth weight, and more expensive care. In a neonatal intensive care unit (NICU), the special care especially focuses on keeping the newborns’ body core temperature within a range, which maximizes their chances of survival and is beneficial regarding morbidity and growth [
5]. This support is vital, because the thermoregulation of a preterm neonate cannot yet handle large deviations of body temperature, which could lead to a deterioration of the health state due to hypothermia or even death from associated severe infections [
6]. Regardless of the core temperature, changes in the central-peripheral temperature difference can indicate abnormal clinical states, such as an unspecific sign of a sepsis [
7].
Preterm infants receive medical care inside an incubator in a NICU where several vital signs such as heart rate (HR), respiration rate, oxygen saturation and temperature are monitored. The continuous surveillance of the cardio-respiratory system enables the determination of a patient’s health status and an early detection of a deterioration of this state. Furthermore, monitoring a newborn’s thermal condition can also help to detect signs of abnormal clinical states such as cold or heat stress as well as infections by closely tracking not only the central but also the peripheral temperature [
6,
8]. While adhesive electrodes are used for the measurement of cardio-respiratory activities, the thermoregulation monitoring is usually conducted using adhering thermistors, whose resistance varies with temperature. Despite benefits such as low price and easy handling, the removal of the probes can cause skin irritations to the still immature skin and induce wounds, which can lead to an infection [
9]. Additionally, wired probes require cables, which not only complicate transport and interaction like kangaroo-care, but also cause psychological stress for the parents [
10]. To overcome the disadvantages of wired patient-monitoring for neonates, contact-free camera surveillance may be a promising technology and is welcomed by clinical staff [
11].
The research interest in the field of camera-based vital signs measurement has continuously increased in recent years. While RGB cameras can be used for Region-of-Interest (ROI) detection and monitoring of vital signs such as HR using photoplethysmography imaging (PPGI) and respiration activity, infrared thermography (IRT) devices can measure the surface temperature of objects and, therefore, are able to derive the thermal state of a patient [
12]. Since continuous vital sign monitoring requires real-time data processing, classical approaches from the field of medical imaging may not be applicable due to the high computing power required. In contrast to this, deep learning (DL)-based approaches have shown to enable real-time image processing for clinical surveillance [
13,
14]. To determine the HR or skin temperature distribution of the neonatal body, segmentation or keypoint detection algorithms can be used, featuring contact-less supervision of all desired and visually accessible body parts [
12,
15]. These algorithms could further facilitate contactless alarm systems by classifying the extracted vital signs for pathology detection. However, for the training and validation steps of such DL-based algorithms, large datasets with various disease progressions are required, which are not publicly available at this moment. Therefore, the literature in this field is still in an early stage.
So far, there have been several studies about neonatal pathology detection which used vital signs recorded from cable-based sensors in combination with DL or machine learning (ML). In 2019, Ansari et al. [
16] published a study about neonatal seizure detection from electroencephalography (EEG) data using deep convolutional neural networks (CNNs). In the same year, Ihlen et al. [
17] applied ML on neonatal movement data for the early prediction of cerebral palsy. One year later, Turova et al. [
18] applied ML models for the prediction of cerebral hemorrhage in infants from clinical data. In addition, in recent studies DL was used to support vital signs monitoring and pathology classification from images [
19]. In 2019, Villarroel et al. [
20] implemented a multitask DL algorithm to segment skin areas and estimate vital signs in a NICU. In 2021, Huang et al. [
14] trained spatio-temporal neural networks for non-contact neonatal HR monitoring. Furthermore, Nagy et al. and Khanam et al. [
19,
21] both proposed an algorithm for the measurement of pulse and breathing rate. Simultaneously, Ervural et al. [
22] classified neonatal diseases using thermographic data in combination with CNNs. Although these DL-based approaches showed promising results, the models were trained on relatively small neonatal image datasets. Thus, any statement on generalization of the models is difficult. To overcome the issue of missing large neonatal image datasets, one solution could be the generation of artificial data using a neonatal phantom which can simulate vital signs such as cardiac activity and thermoregulatory processes and enables the configuration of pathological states. In a previous work, we introduced a neonatal phantom, which is able to simulate a PPG signal and thermoregulatory processes on the skin surface in individually controllable areas [
23].
In this work, we present a low-cost, camera-based monitoring approach which is able to measure vital signs simulated by such a neonatal phantom inside an incubator in order to detect pathology patterns. A pre-trained DL-based keypoint detector was used to extract ROIs on the skin surface of the phantom and monitor vital signs such as HR and thermoregulation in real-time. During live monitoring, pathological vital signs patterns were set for cardiac activity and temperature distribution, which were independently measured and classified with the camera-based system. A simultaneous simulation of vital signs using the neonatal phantom and the camera-based measurement of the signals with a deep learning-supported detection of pathological patterns have so far not been reported in the literature, and are considered a novel contribution.
3. Setup Implementation
The presented neonatal phantom was placed inside a closed incubator of type Thermocare Vita (Weyer, Germany), which has an infrared (IR) inspection window ClearIR-4-P (IRISS, USA) inserted into the hood to allow thermal radiation to pass. The setup should be low-cost, highly accurate, and at the same time compact enough to be placed on top of the incubator.
3.1. Hardware Setup
A
Jetson Xavier NX (NVIDIA, USA) was used as image processing unit for the setup. This embedded GPU is a very powerful yet small system-on-module, which allows running deep neural networks and to process data from multiple camera sensors in real-time. The included 6 Carmel ARM CPUs, 384 CUDA cores, and 48 tensor cores enable a high performance for image processing. The Jetson module was used for image acquisition and processing, as well as ROI detection (cf.
Section 3.3).
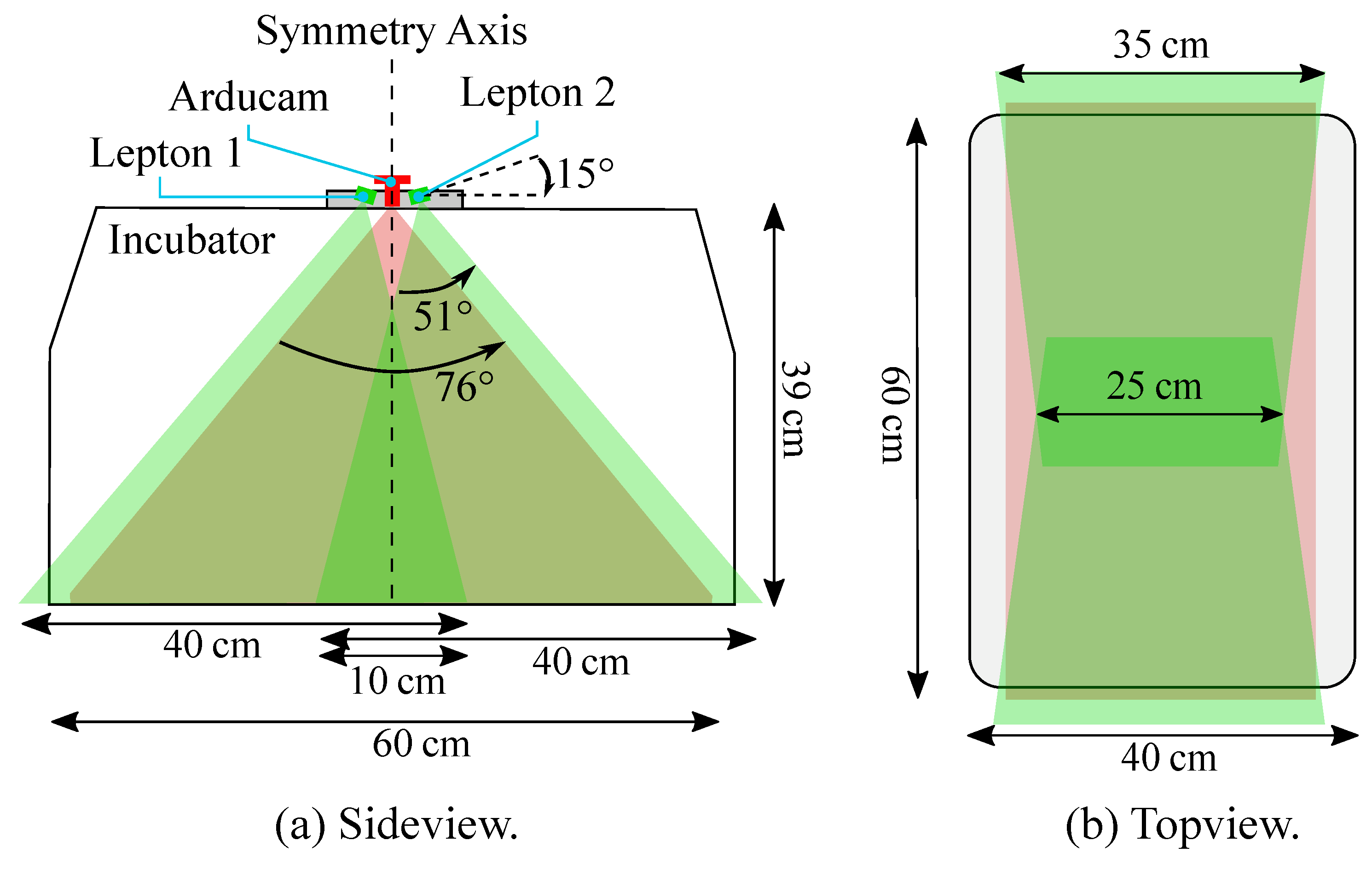
The hardware setup was implemented using a low-cost multi-modal recording system consisting of two infrared thermography devices and one RGB camera. For thermal imaging, we integrated two long wave infrared (LWIR) cameras of type Lepton 3.5 (FLIR Systems, USA) into the hardware setup. These low-power, uncooled, FPA microthermal imaging modules are easily integratable into small camera system designs. With respect to the distance of the cameras due to the geometry of the incubator, one Lepton was not sufficient to overview the whole mattress area. Therefore, two thermal sensors were arranged in a line, but slightly tilted. The absolute temperature accuracy is given as and the module includes an on-board shutter for live calibration. Each Lepton has a horizontal field-of-view (HFOV) of 57, which in combination allows to guarantee a large enough FOV.
Further, we used one RGB camera module for ROI determination and PPGI extraction. The
Arducam MINI High HQ Camera Module (Arducam, China) uses the 12.3 MP SONY IMX477 sensor with an optical format of 1/2.3
and a M12 mount lens, enabling video streaming with up to 4K at 30 fps [
30]. The Arducam uses the camera serial interface (CSI) specification for connection and can be connected directly to the Jetson module. The most relevant properties of both cameras are listed in
Table 3.
While a Full-HD resolution was applied for the RGB camera using a frame rate of 30 fps, the IRT device had a slower frame rate of approx. 9 fps (maximum frame rate), which was, however, sufficient for measuring the inert temperature deviations. Since the camera setup is supposed to overview the entire mattress of the incubator, considerations regarding area coverage of the Leptons (in green) and the Arducam (in red) were done as depicted in
Figure 3a.
The Lepton cameras were tilted by
against the horizontal line, and with their wider viewing angle parallel to the longer axis of the incubator, in order to capture as much of the mattress area as possible. With this arrangement and the usage of two Leptons, the whole scene could be captured, apart from some small cutouts in the overlapping area (cf.
Figure 3b). The Arducam was centered in between the Leptons and had a large HFOV, providing an overview of the whole mattress.
A modular housing, holding all three cameras and the Jetson Xavier NX, was designed using the 3D CAD software Fusion 360 (Autodesk, USA) and 3D-printed using an Ultimaker 3 printer (Ultimaker, The Netherlands).
3.2. Thermal Calibration
Despite their thermal sensitivity of 0.05 K, the Leptons currently have a nominal absolute temperature accuracy of only ±
in the high gain mode (range of −10 to 140
). Considering the small size and the low price of this thermal imaging sensor, the accuracy is relatively high. Nevertheless, it should be mentioned that it is about as high as the thermal dynamics, which will be simulated during this work. Therefore, a thermal calibration was performed to improve the absolute measurement accuracy. In fact, in combination with the IR window the Leptons were found to overestimate low temperatures and underestimate higher temperatures, especially after applying the Lepton’s calibration shutter. Thus, a linear temperature calibration according to (
1) was chosen to convert the measured surface temperature
to the true surface temperature
. Apart from the slope correction
, an offset
is assumed.
In order to determine both the slope correction
and the offset
of the temperature calibration, a heatable black body was developed and used as an active thermal reference. Therefore, a heating foil (Thermo, Germany) and a more than
emittive, black coated aluminum foil (Advanced Coating, Germany) were integrated into a 3D-printed ABS housing, resisting temperatures of at least
. Both foils are separated by a thin layer of ABS, which is supposed to cause a more homogeneous temperature distribution in the black foil. To obtain the reference temperature, a Weyer skin temperature probe (Weyer, Germany) was placed on the black foil. These adhesive thermistors are clinically used as the gold standard for neonatal skin temperature measurements and have a range of
–
. The maximum error of the probes were specified as ±
[
32]. The active thermal reference body is shown in
Figure 4.
3.3. Software Implementation
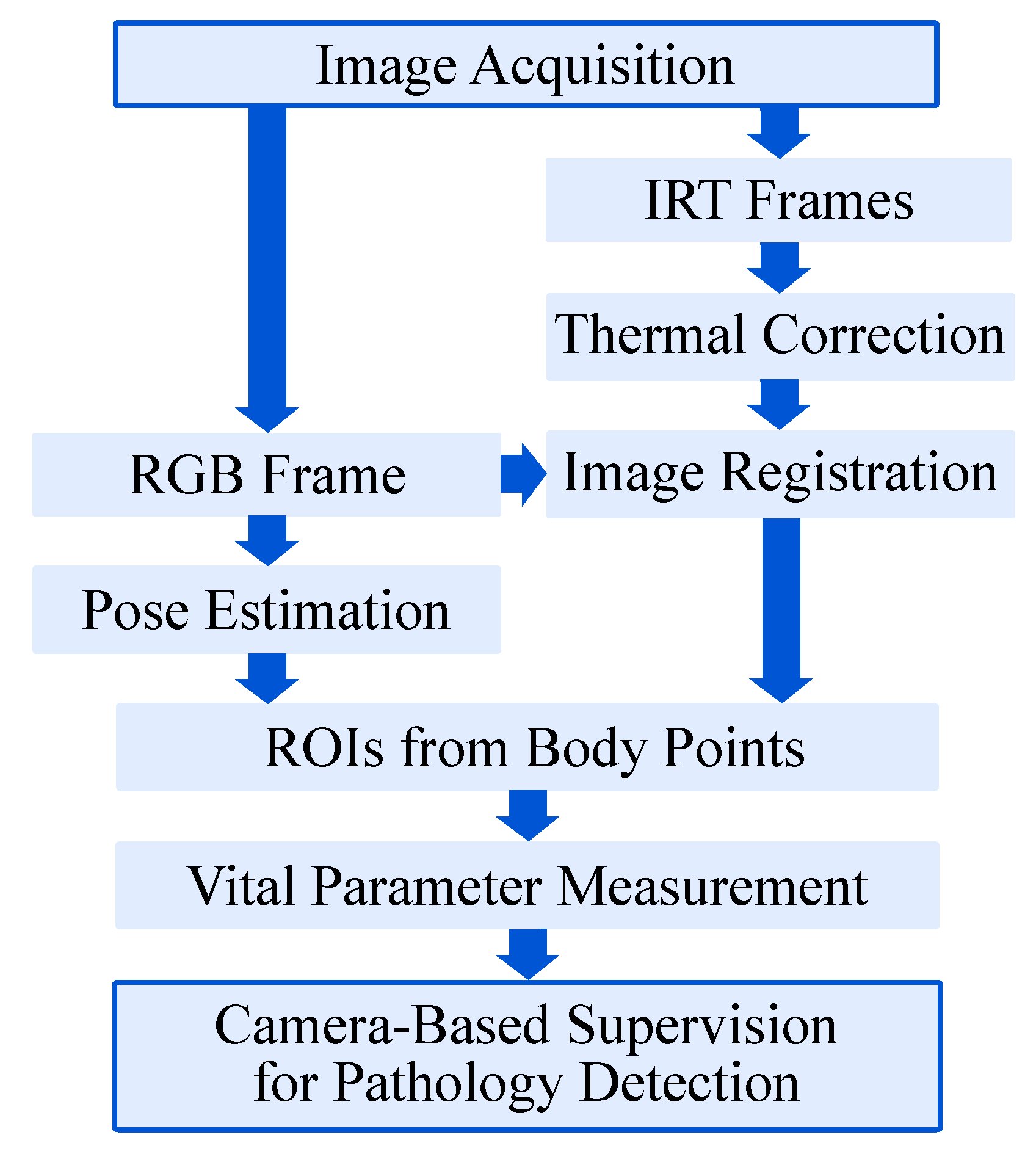
Since image data from several cameras need to be processed simultaneously, the software routine of the setup was designed as a multithreaded process in Python 3. First, the frames were acquired from the multi-modal camera devices. Subsequently, the gain-offset correction for the IRT frames was performed as presented in
Figure 5.
The infrared images and the RGB frame were then registered and transformed, allowing both image modalities to have the same Point-of-View (POV). Afterwards, the RGB frame was used to determine the ROIs using a body pose estimation (BPE) algorithm. Before obtaining the desired vital parameters, the thermal correction on the transformed infrared images was done. Finally, the PPGI signal for HR detection and the temperature values were extracted from the RGB and the IR image, respectively. The single steps of the algorithm are explained in more detail in the following section.
Regarding the image registration, a manual approach was chosen by selecting ten points on the IRT image and the corresponding feature points on the RGB image. Using the Ransac algorithm [
33] for optimal matching, these feature were taken to determine a 3 × 3 homography matrix
H, which can rotate, translate and distort the Lepton images to match the RGB image. As the Leptons have a fixed position in their housing, the manual labeling process described above was only done once. The registration process is shown in
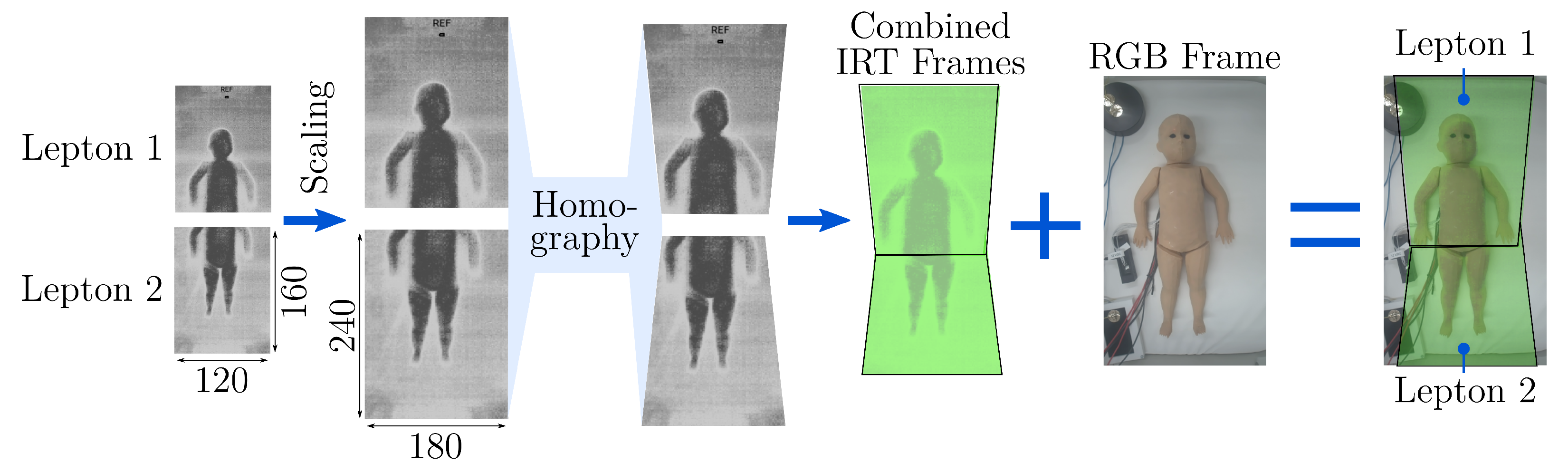
Figure 6. After rescaling of both Lepton frames, they were transformed using the homography matrices
and
to create the combined IRT image. The overlapping region was filled with temperature data from Lepton 1 that mainly records the upper body.
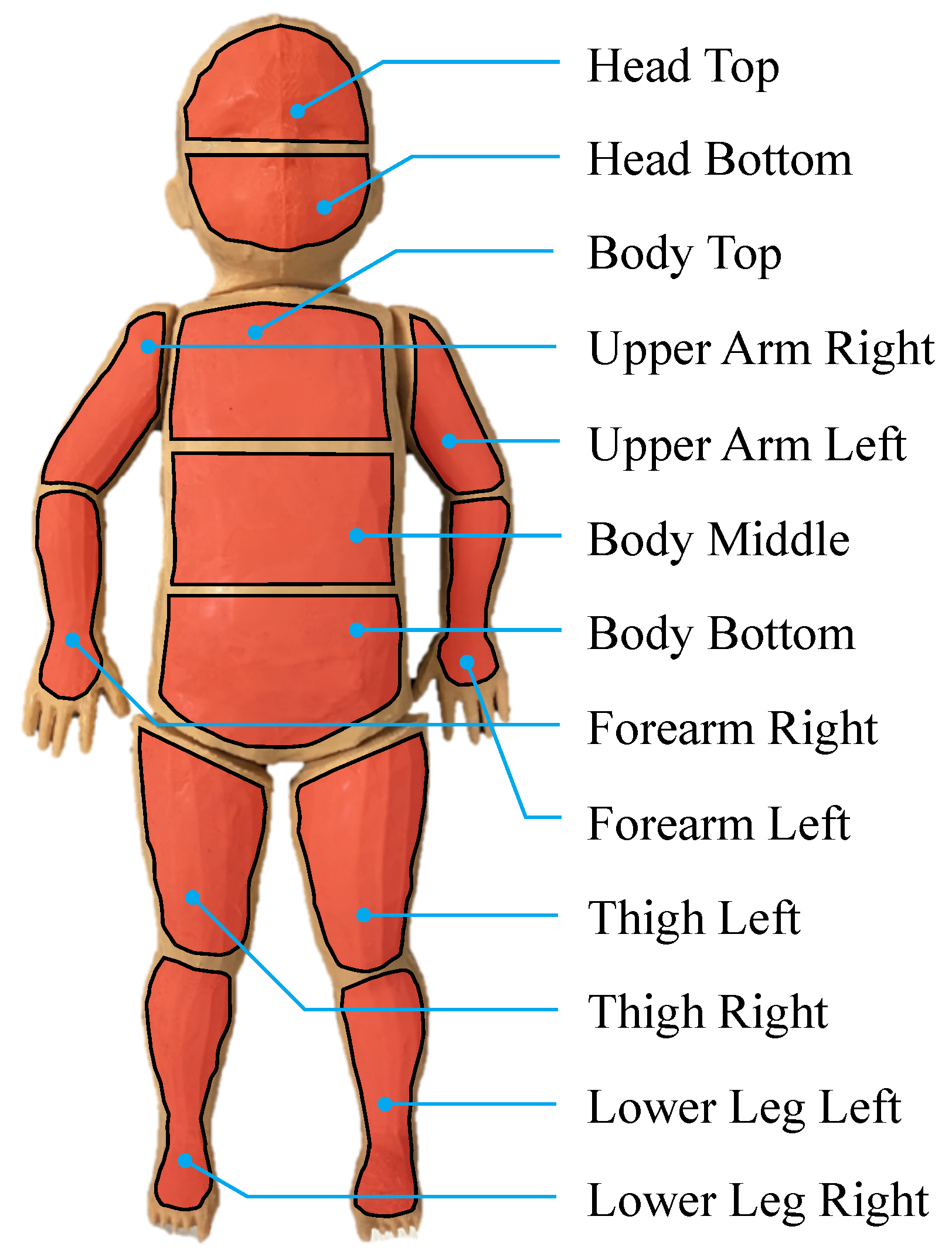
In order to determine ROIs on hands, feet, the forehead and the chest, we used a state-of-the-art DL-based BPE algorithm. The BPE was executed on the images captured by the RGB camera rather than the IRT camera, because existing open-source models are pre-trained in RGB. During this work, the NVIDIA project
trt_pose was used, which enables a BPE on embedded NVIDIA Jetson modules [
34]. No further transfer-learning step using additional data was conducted, but only the pre-trained version has been used. The network architecture is based on the
CMU-Pose real-time multi-person 2D pose estimator of Cao et al. [
15]. In contrast to
CMU-Pose, the NVIDIA approach works on feature maps extracted from an image by a ResNet-18 feature extractor instead of the 10 first layers of VGG19, as suggested by Xiao et al. [
35].
CMU-Pose is a two-branch multi-stage CNN, with one branch for learning Part Affinity Fields (PAFs) and one for learning Confidence Maps (CMAPs). A CMAP describes the area, where the respective body part can be found. The PAFs represent a 2D vector field which shows the association between two body points. For example, the body part could be the left forearm, connecting the keypoints in the left elbow and the left wrist. Subsequent stages in the CNN receive the CMAPs and PAFs as well as the initial features
to refine the solution. In contrast to
CMU-Pose, the
trt_pose approach optimized the architecture by preventing the feature exchange between the CMAP and PAF branches, which decreased the computational effort. The matching of keypoints with individuals is performed using a greedy graph matching algorithm, where every association gets a weight and the association with the largest weight was selected [
15]. An overview of the keypoints delivered from the pose estimation and the desired six ROIs for temperature and HR measurements is illustrated in
Figure 7.
With the detected ROIs, the skin temperature distribution of the phantom was obtained from the infrared image. Since red LEDs were integrated into the neonatal phantom, the extraction of the heart rate was performed by taking the mean intensity of the red channel inside a head ROI for each RGB frame. A find_peaks function was applied to detect maxima in the signal. Since small peaks caused by noise or the smaller, local maxima of the simulated LED brightness would lead to a corrupted HR, the maxima were compared to the minimum values to select only major peaks. The time distance between the detected peaks was evaluated and irrelevant HRs above 300 bpm and below 36 bpm were filtered out, as they were considered to originate from noise. The use of as mean of the time distance array, which contained only valid measurements from the last 10 s, enabled the calculation of the estimated heart rate .
5. Discussion
5.1. Thermal Validation
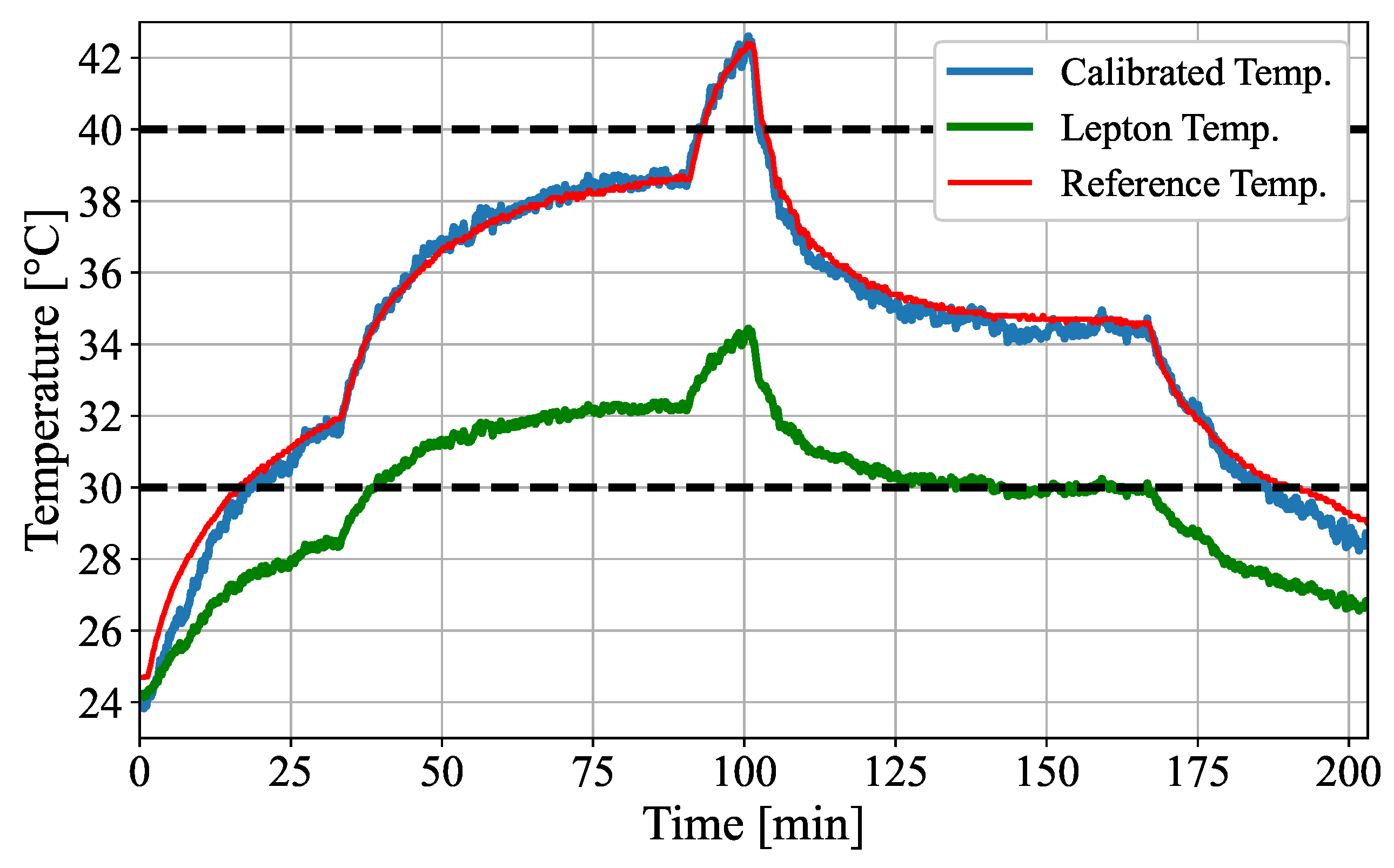
The absolute temperature accuracy for the Leptons was originally stated as ±
in the data sheet [
31] within the relevant temperature range of 30–
. The measured maximum errors of the Leptons of up to
(cf.
Figure 8) even exceeded the nominal error, most likely because of the absorption of the IR window, which favors underestimation. As demonstrated in this paper (cf.
Table 4), proper calibrations of the Leptons with an active thermal reference can lead to an accuracy improvement of at least 79% (
to
).
Normally, one would expect that the error is smallest in the measurement that was used to determine calibration factors. However, the calibrated temperature of the validation measurement was found to have a higher accuracy. In contrast to the validation measurement, the temperature data used to determine the calibration showed a thermal hysteresis for the thermal camera. The Leptons measured different temperatures, although the active reference body had the same temperature. One reason could be the different thermal behavior during heating or cooling of the reference body. However, since this behavior was not seen in the validation measurement, a quantitative analysis should verify this in the future.
Additionally, the custom-made active reference body and its usage impose a potential error during the measurements. The reference did not have an absolute homogeneous temperature distribution, especially during heat-up, and the skin temperature probe was isolated and contacted by thermal contact fluid. In future studies, a passive reference inside the FOV could be used to perform a Live-Offset compensation. In this case, not only a one-time calibration was conducted to compensate the infrared window, but an offset compensation was performed continuously during the measurements. Finally, research should be conducted concerning the temperature difference between the core and the skin temperature.
5.2. Pathology Detection
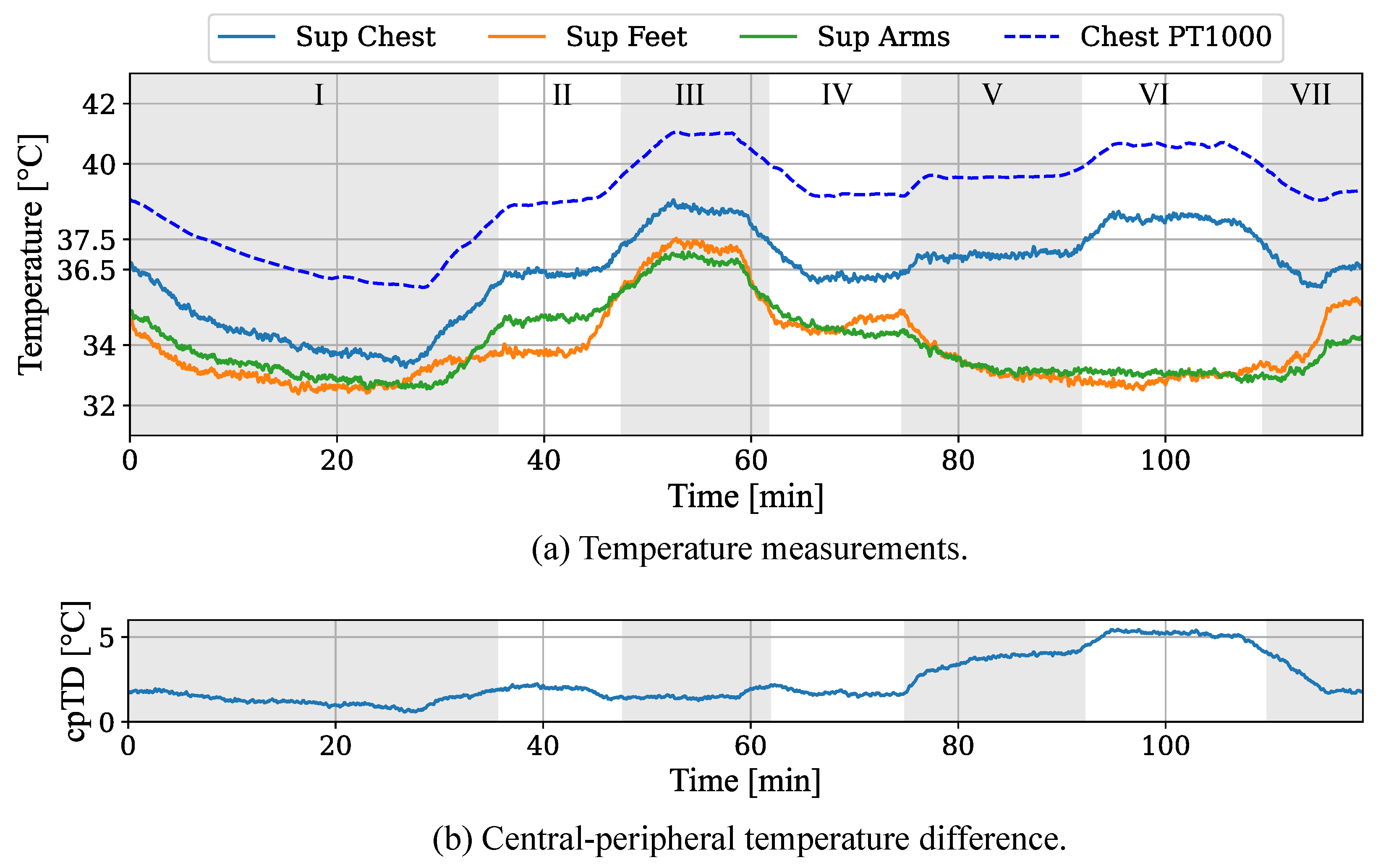
The results of the temperature measurements demonstrated the feasibility to simulate thermoregulatory processes and the capability of a camera-based detection of pathological conditions. While the camera-based temperatures measured from the skin showed an accurate relative behavior for the chest area, the absolute values were underestimated, with a mean temperature difference of approx. . This can be explained with the position of the Pt1000 sensor, which was located inside the neonatal phantom next to the heating elements, whereas the Lepton recorded lower temperatures from the outer skin. Further, the emissivity of the colored silicone could influence the remote temperature measurement. In future work, the integration of the Pt1000 sensors into the silicone layer needs to be investigated. Additionally, a profound emissivity analysis of the silicone will be conducted.
The analysis of the HR simulation of the neonatal phantom and the monitoring using the implemented setup demonstrated that the developed algorithms enabled the real-time extraction of a PPGI signal. Several pathological HR levels between 60 and 230 bpm were simulated. The analysis revealed a MAE of 0.89 bpm during the phases described in
Section 4.2. The resulted high accuracy can be explained by the reduction of potential external impacts for the PPGI measurement. However, the simulation of a physiological HRV led to errors of up to 4.62 bpm. In this case, the sliding window approach with a window length of 30 s prevented a higher accuracy.
Unlike real infants, the phantom was lying still inside the incubator without any movement, which describes one of the biggest problems in camera-based vital signs measurement. In future work, a movement simulation will be added to the neonatal phantom using actuators. Further, the dependencies of PPGI measurements on ambient conditions will be analyzed.
6. Conclusions
During this work, a neonatal phantom was used to simulate various stages of hypothermia and hyperthermia. In addition, cardiac-related pathologies, such as tachycardia and bradycardia, were simulated via LED modulation. The ability of the neonatal phantom to create a temperature distribution and a PPG signal in a controlled manner enabled the development and the validation of a contactless, camera-based monitoring system to measure the simulated vital signs and classify the pathologies. The accuracy of the temperature measurements was improved by at least 79% with a maximum absolute error of less than by using an active thermal reference body, compensating the spectral absorption of the HR window. A DL-based pre-trained human pose estimator was used to determine the ROIs of the hands, the feet, the chest and the head, allowing to semantically match the measured skin temperatures with the areas of the body. Further, the PPGI signal was extracted, allowing to supervise pathological HR conditions.
Even though the presented system achieved promising measurement accuracies and provided a reliable detection of pathologic states, challenges remain, which will be addressed in future work. Since the error for temperature measurement is still relatively high with a maximum error of , it would be feasible to add a passive thermal reference for live offset compensation or use a data-driven approach for temperature correction. Furthermore, the functionality of PPGI recordings could be extended by adding another camera with an IR filter for measuring the oxygen saturation, which can be simulated by the neonatal phantom already. An additional series of measurements should analyze the emissivity of the phantom’s skin to evaluate the impact of the ambient temperature, as well as measurements involving a more realistic temperature and humidity inside the incubator. Finally, the integration of motors into the neonatal phantom to enable the simulation of motion artifacts will be addressed in the future.