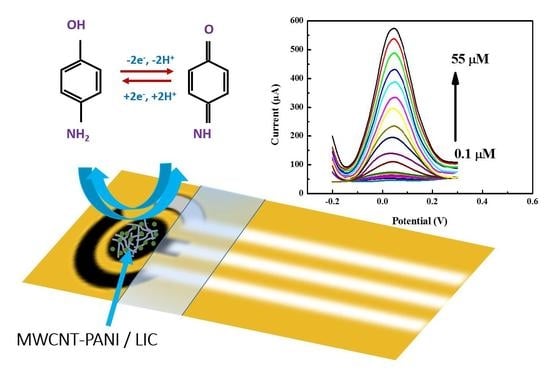
Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser Induced Graphene Electrodes Modified with MWCNT-PANI
Abstract
:1. Introduction
2. Materials and Methods
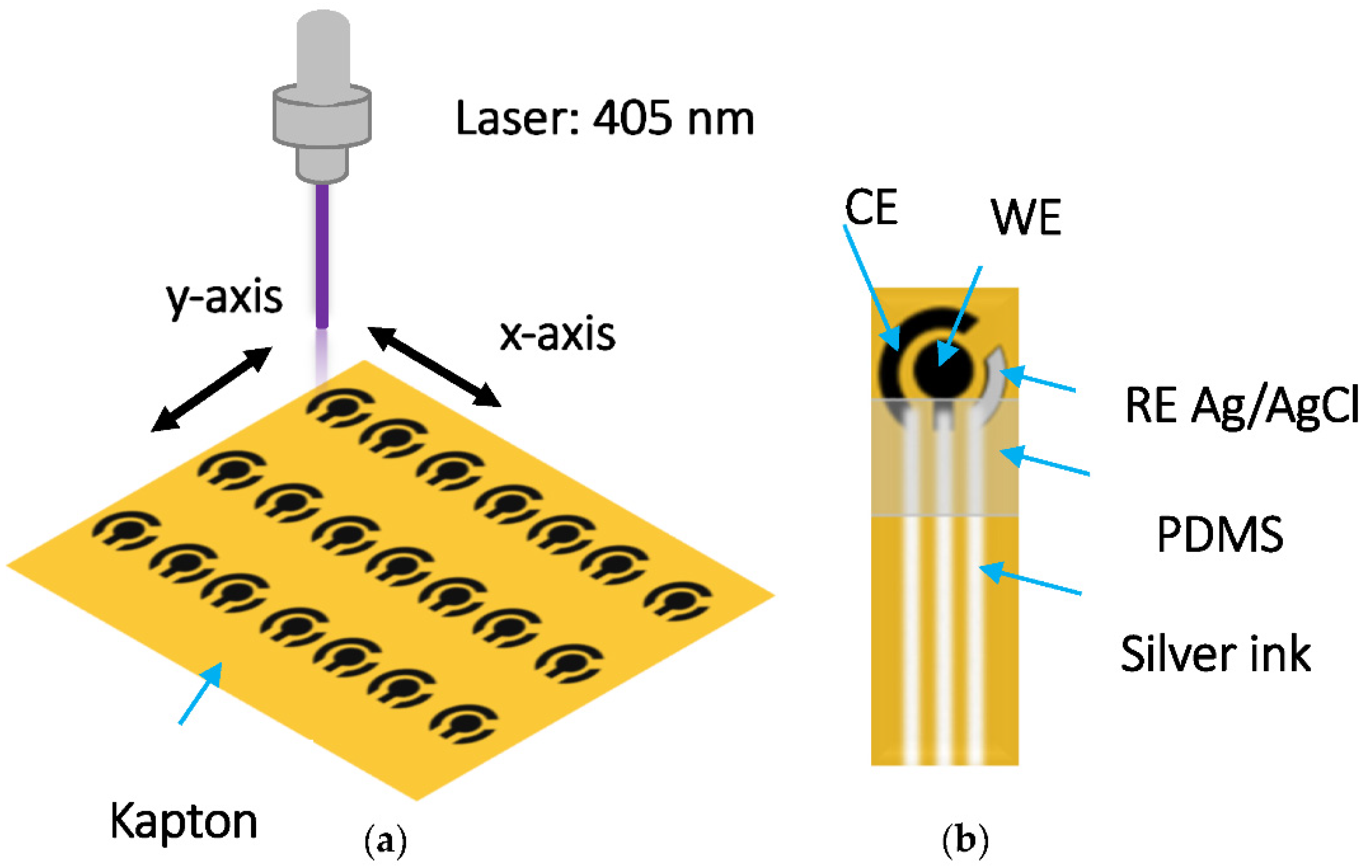
2.1. Fabrication of Laser-Induced Carbon Electrodes
2.2. Elaboration of PANI and MWCNT-PANI Composite
2.3. Preparation of PANI/LIC Electrodes and MWCNT-PANI/LIC Electrodes
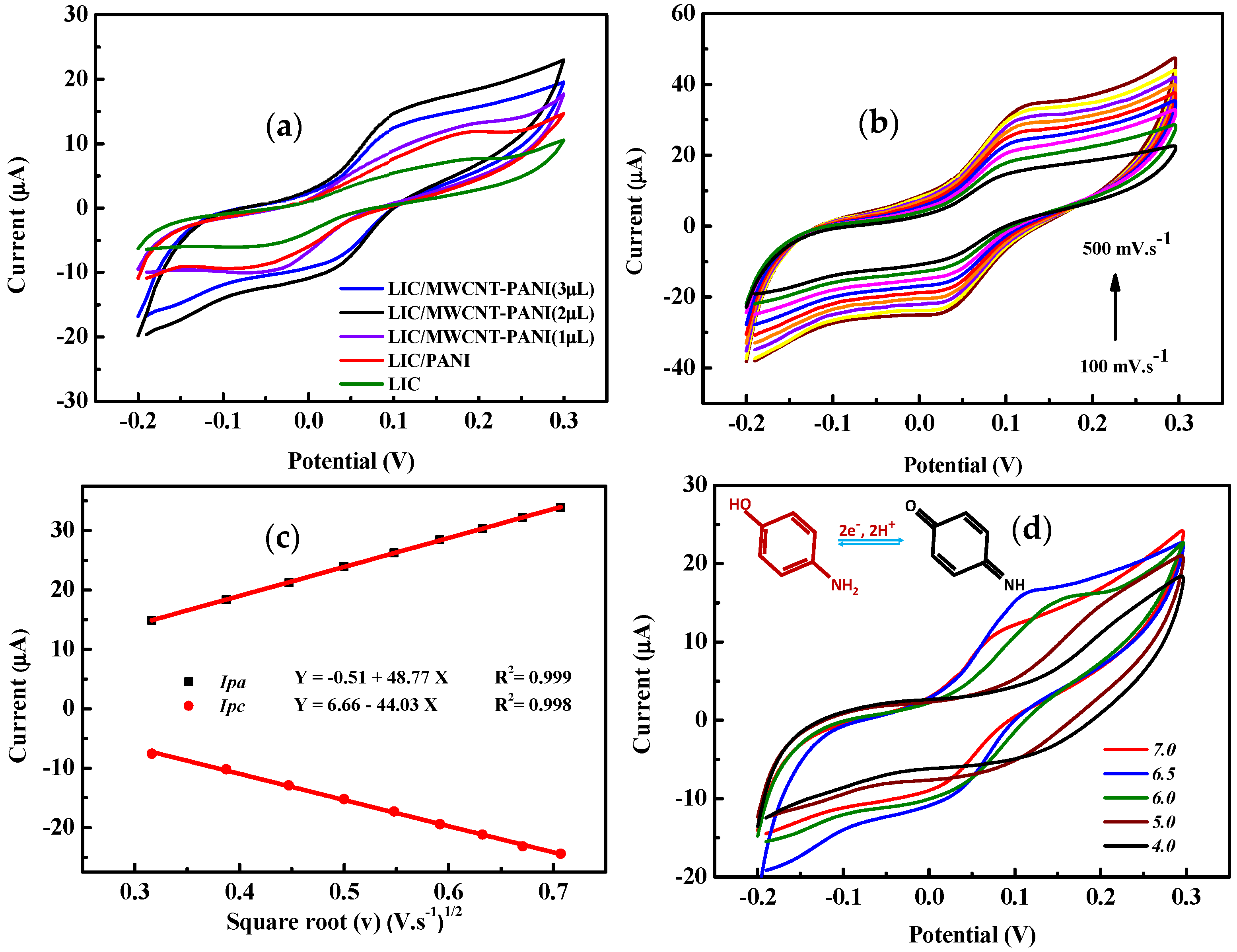
3. Results
3.1. Optical Performances of the Pristine Polyimide (PI) Sheet
3.2. Characterization of PANI and MWCNT-PANI Composite
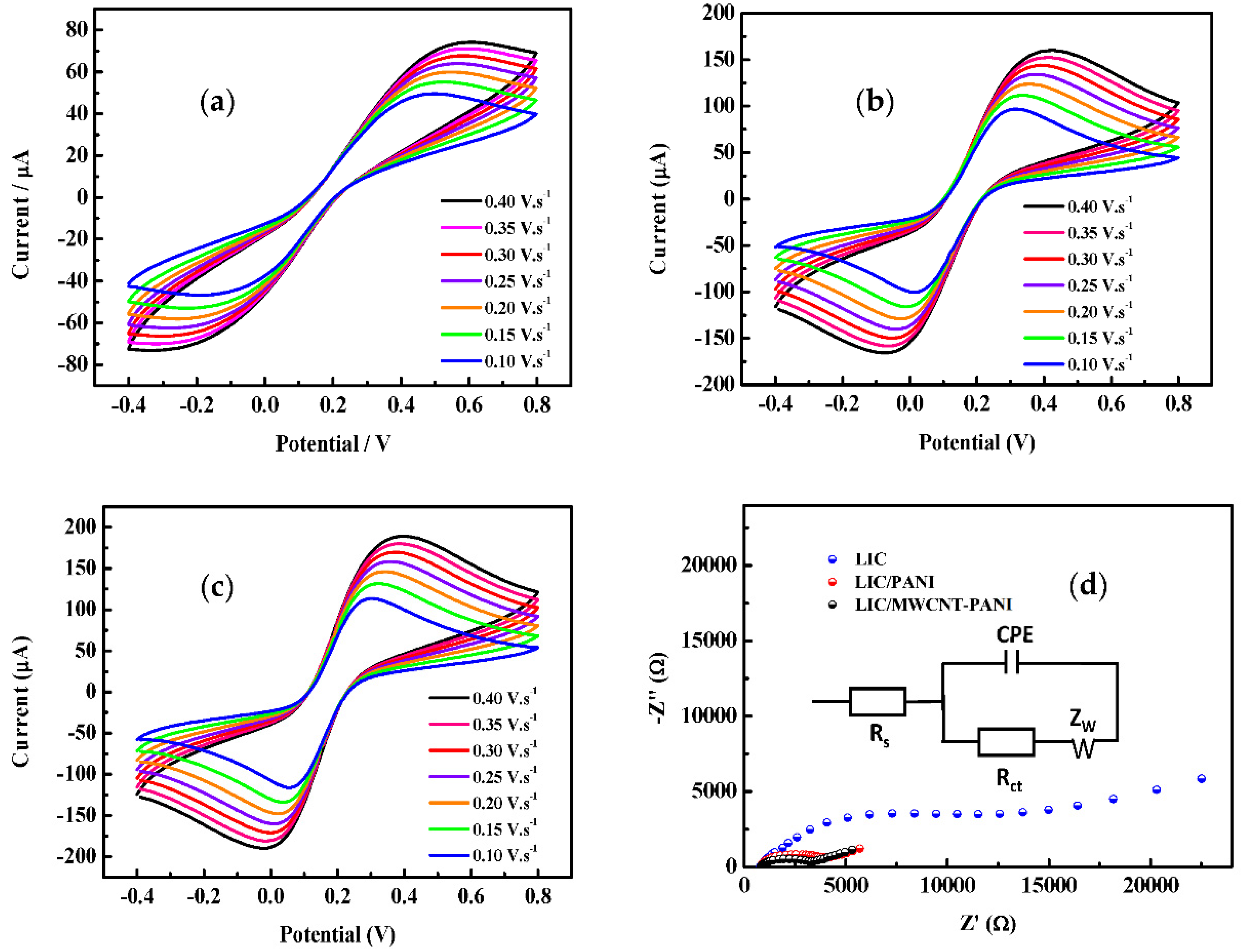
3.3. Surface Area A
3.4. Electrochemical Investigation of 4-AP
- α is the transfer coefficient = 0.5,
- n is the number of electrons transferred,
- F is the Faraday constant, and
- R and T have their usual meanings.
- D diffusion coefficients of the oxidized and reduced form.
- ν scan rate.
- peak-to-peak separation.
3.5. Electrochemical Detection of 4-AP
3.6. Dtermination of 4-AP by SWV
3.6.1. Analytical Curve
| Electrode | LOD (μM) | Range (μM) | References |
|---|---|---|---|
| Au/Pd/rGO/GCE | 0.12 | 1–300 | [6] |
| Cu-Au MWCNT nanocomposite/GCE | 0.105 | 0.5–1.6 | [32] |
| Graphene chitosan/GCE | 0.057 | 0.2–550 | [33] |
| Bis-schiff Base Cobalt Complexes/GCE | 2.08 | 5–30 | [34] |
| AuNPs and a Layered Double Hydroxide Sodium/GCE | 0.1 | 0.5–400 | [63] |
| Graphene-Polyaniline | 15.68 | 50–500 | [64] |
| Fc-PAA-GNPs/GCE | 7.61 | 30–1064 | [65] |
| LIC/MAWCNT-PANI | 0.006 | 0.1–55 | This work |
3.6.2. Reproducibility, Stability and Selectivity
3.6.3. Determination of 4-AP in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.303 (accessed on 5 April 2021).
- US EPA. Water Quality Standards Regulations: New York. Available online: https://www.epa.gov/wqs-tech/water-quality-standards-regulations-new-york (accessed on 5 April 2021).
- Vilian, A.T.E.; Veeramani, V.; Chen, S.-M.; Madhu, R.; Huh, Y.S.; Han, Y.-K. Preparation of a Reduced Graphene Oxide/Poly-l -Glutathione Nanocomposite for Electrochemical Detection of 4-Aminophenol in Orange Juice Samples. Anal. Methods 2015, 7, 5627–5634. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, R.; Liu, C.; Yang, S.; Lu, Z.; Luo, S. Electrochemical Detection of 4-Nitrophenol Based on a Glassy Carbon Electrode Modified with a Reduced Graphene Oxide/Au Nanoparticle Composite. Anal. Methods 2013, 5, 5508. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Electrochemical Sensor Based on Palladium-Reduced Graphene Oxide Modified with Gold Nanoparticles for Simultaneous Determination of Acetaminophen and 4-Aminophenol. Talanta 2018, 178, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, P.; Wu, F.; Zhao, J.; Han, D.; Cui, G. Significant Enhancement in the Electrochemical Determination of 4-Aminophenol from Nanoporous Gold by Decorating with a Pd@CeO2 Composite Film. New J. Chem. 2020, 44, 3087–3096. [Google Scholar] [CrossRef]
- El Harrad, L.; Bourais, I.; Mohammadi, H.; Amine, A. Recent Advances in Electrochemical Biosensors Based on Enzyme Inhibition for Clinical and Pharmaceutical Applications. Sensors 2018, 18, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, K.; Dale, C.; Hedley, J.; Kowal, M.D.; Kaner, R.B.; Keegan, N. Laser-Scribed Graphene Presents an Opportunity to Print a New Generation of Disposable Electrochemical Sensors. Nanoscale 2014, 6, 13613–13622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randviir, E.P.; Brownson, D.A.C.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The Fabrication, Characterisation and Electrochemical Investigation of Screen-Printed Graphene Electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598. [Google Scholar] [CrossRef] [Green Version]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New Directions in Screen Printed Electroanalytical Sensors: An Overview of Recent Developments. Analyst 2011, 136, 1067. [Google Scholar] [CrossRef]
- Honeychurch, K.C. Screen-Printed Electrochemical Biosensors and Sensors for Monitoring Metal Pollutants. Insci. J. 2012, 2, 1–51. [Google Scholar] [CrossRef]
- Li, W.; Tan, C.; Lowe, M.A.; Abruña, H.D.; Ralph, D.C. Electrochemistry of Individual Monolayer Graphene Sheets. ACS Nano 2011, 5, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Medarametla, S.P.; Li, H.; Zhou, C.; Lin, D. 3D Printing of Graphene Aerogels. Small 2016, 12, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, M.; Di Marcello, M.; Perugini, M.; Ponzielli, V.; Sergi, M.; Mascini, M.; Compagnone, D. Electrochemical DNA Biosensor for Polycyclic Aromatic Hydrocarbon Detection. Microchim. Acta 2008, 163, 163–169. [Google Scholar] [CrossRef]
- Lucarelli, F.; Authier, L.; Bagni, G.; Marrazza, G.; Baussant, T.; Aas, E.; Mascini, M. DNA Biosensor Investigations in Fish Bile for Use as a Biomonitoring Tool. Anal. Lett. 2003, 36, 1887–1901. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Chaubey, A. Biosensors for Clinical Diagnostics Industry. Sens. Actuators B Chem. 2003, 91, 117–127. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- de Araujo, W.R.; Frasson, C.M.R.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R.L.C. Single-Step Reagentless Laser Scribing Fabrication of Electrochemical Paper-Based Analytical Devices. Angew. Chem. Int. Ed. 2017, 56, 15113–15117. [Google Scholar] [CrossRef]
- Mendes, L.F.; de Siervo, A.; Reis de Araujo, W.; Longo Cesar Paixão, T.R. Reagentless Fabrication of a Porous Graphene-like Electrochemical Device from Phenolic Paper Using Laser-Scribing. Carbon 2020, 159, 110–118. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, T.; Pak, J. Fabrication and Transfer of Laser Induced Graphene (LIG) Electrode for Flexible Substrate-Based Electrochemical Sensor Applicatins. Trans. Korean Inst. Electr. Eng. 2018, 67, 406–412. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Bakhtiyari, A.N.; Zheng, H. A Comparative Study of Laser-Induced Graphene by CO2 Infrared Laser and 355 Nm Ultraviolet (UV) Laser. Micromachines 2020, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Mamleyev, E.R.; Heissler, S.; Nefedov, A.; Weidler, P.G.; Nordin, N.; Kudryashov, V.V.; Länge, K.; MacKinnon, N.; Sharma, S. Laser-Induced Hierarchical Carbon Patterns on Polyimide Substrates for Flexible Urea Sensors. NPJ Flex. Electron. 2019, 3, 2. [Google Scholar] [CrossRef]
- Getachew, B.A.; Bergsman, D.S.; Grossman, J.C. Laser-Induced Graphene from Polyimide and Polyethersulfone Precursors as a Sensing Electrode in Anodic Stripping Voltammetry. ACS Appl. Mater. Interfaces 2020, 12, 48511–48517. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, J.; Wang, Z.; Song, W.; Cao, G. Recent Advances in Preparation and Application of Laser-Induced Graphene in Energy Storage Devices. Mater. Today Energy 2020, 18, 100569. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical Sensor for Nitrite Detection in Water Samples Using Flexible Laser-Induced Graphene Electrodes Functionalized by CNT Decorated by Au Nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Anurag, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Investigation of Laser Induced Graphene Electrodes Modified by MWNT/AuNPs for Detection of Nitrite. In Proceedings of the 2019 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; pp. 615–620. [Google Scholar]
- Lin, X.; Lu, Z.; Dai, W.; Liu, B.; Zhang, Y.; Li, J.; Ye, J. Laser Engraved Nitrogen-Doped Graphene Sensor for the Simultaneous Determination of Cd(II) and Pb(II). J. Electroanal. Chem. 2018, 828, 41–49. [Google Scholar] [CrossRef]
- Ramasubramanian, P.A.; Thangavel, S.; Nallamuthu, G.; Kirabakaran, K.; Vasudevan, V.; Ravichandran, K.; Venugopal, G. A Novel MoS2 Structures for Electrochemical Detection of 4-Aminophenol. J. Mater. Sci. Mater. Electron. 2018, 29, 5696–5701. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Madhusudana Reddy, T.; Palakollu, V.N.; Karpoormath, R.; Subba Rao, Y.; Venkataprasad, G.; Gopal, T.V.; Gopal, P. Multi Walled Carbon Nanotubes Supported CuO-Au Hybrid Nanocomposite for the Effective Application towards the Electrochemical Determination of Acetaminophen and 4-Aminophenol. Synth. Met. 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Yin, H.; Ma, Q.; Zhou, Y.; Ai, S.; Zhu, L. Electrochemical Behavior and Voltammetric Determination of 4-Aminophenol Based on Graphene–Chitosan Composite Film Modified Glassy Carbon Electrode. Electrochim. Acta 2010, 55, 7102–7108. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Z.; Liang, C.; Han, G.; Zhang, S.; Feng, X. Electrochemical Simultaneous Detection of Paracetamol and 4- Aminophenol Based on Bis-Schiff Base Cobalt Complex. Int. J. Electrochem. Sci. 2019, 14, 7178–7201. [Google Scholar] [CrossRef]
- Dou, N.; Zhang, S.; Qu, J. Simultaneous Detection of Acetaminophen and 4-Aminophenol with an Electrochemical Sensor Based on Silver–Palladium Bimetal Nanoparticles and Reduced Graphene Oxide. RSC Adv. 2019, 9, 31440–31446. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anodic Pretreatment of Glassy Carbon: Impacts on Structural and Electrochemical Characteristics of Niox Nanoparticles—MedCrave Online. Available online: https://medcraveonline.com/IJBSBE/anodic-pretreatment-of-glassy-carbon-impacts-on-structural-and-electrochemical-characteristics-of-niox-nanoparticles.html (accessed on 24 December 2021).
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of Conducting Polymer Composites to Electrochemical Sensors: A Review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting Polymers Prepared by Oxidative Polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sens. 2019, 2019, 4257125. [Google Scholar] [CrossRef] [Green Version]
- Stanford, M.G.; Zhang, C.; Fowlkes, J.D.; Hoffman, A.; Ivanov, I.N.; Rack, P.D.; Tour, J.M. High-Resolution Laser-Induced Graphene. Flexible Electronics beyond the Visible Limit. ACS Appl. Mater. Interfaces 2020, 12, 10902–10907. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussein, M.A.; Alamry, K.A.; Al-Shehry, F.M.; Asiri, A.M. Polyaniline/Graphene/Carbon Nanotubes Nanocomposites for Sensing Environmentally Hazardous 4-Aminophenol. Nano-Struct. Nano-Objects 2018, 15, 63–74. [Google Scholar] [CrossRef]
- Mazzeu, M.A.C.; Faria, L.K.; Cardoso, A.D.M.; Gama, A.M.; Baldan, M.R.; Gonçalves, E.S. Structural and Morphological Characteristics of Polyaniline Synthesized in Pilot Scale. J. Aerosp. Technol. Manag. 2017, 9, 39–47. [Google Scholar] [CrossRef]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly Sensitive, Room Temperature Gas Sensor Based on Polyaniline-Multiwalled Carbon Nanotubes (PANI/MWCNTs) Nanocomposite for Trace-Level Ammonia Detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Rasheed, H.K.; Kareem, A.A. Effect of Multiwalled Carbon Nanotube Reinforcement on the Opto-Electronic Properties of Polyaniline/c-Si Heterojunction. J. Opt. Commun. 2018, 42, 25–29. [Google Scholar] [CrossRef]
- Das, A.K.; Bhowmik, R.; Meikap, A.K. Surface Functionalized Carbon Nanotube with Polyvinylidene Fluoride: Preparation, Characterization, Current-Voltage and Ferroelectric Hysteresis Behaviour of Polymer Nanocomposite Films. AIP Adv. 2017, 7, 045110. [Google Scholar] [CrossRef] [Green Version]
- Sreekala, P.S.; John, H.; Aanandan, C.K. Studies on Anomalous Dispersion Behavior of PANI–CNT Composites for Enhanced Shielding Effectiveness in Various Microwave Bands. Appl. Phys. A 2020, 126, 389. [Google Scholar] [CrossRef]
- Halvaee, M.; Didehban, K.; Goodarzi, V.; Ghaffari, M.; Ehsani, M.; Saeb, M.R. Comparison of Pristine and Polyaniline-Grafted MWCNTs as Conductive Sensor Elements for Phase Change Materials: Thermal Conductivity Trend Analysis. J. Appl. Polym. Sci. 2017, 134, 45389. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C. Measuring Electrochemical Surface Area of Nanomaterials versus the Randles−Ševčík Equation. ChemElectroChem 2020, 7, 3414–3415. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for Measuring the Surface Areas of Metal Oxide Electrocatalysts for Determining Their Intrinsic Electrocatalytic Activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Sharifi-viand, A.; Mahjani, M.G.; Jafarian, M. Determination of Fractal Rough Surface of Polypyrrole Film: AFM and Electrochemical Analysis. Synth. Met. 2014, 191, 104–112. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Khamis, E.; Salman, R.M.; Rahal, H.T.; El Morr, Z. Electrochemical Synthesis and Corrosion Behaviour of Polyaniline on Stainless Steel in Sodium Hydroxide Solutions. Chem. Eng. Commun. 2020, 208, 271–280. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.; Kelly, P.; Brownson, D.; Banks, C. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; Yang, X.; Li, H.; Shi, Q.; Wang, K. Chiral Voltammetric Sensor for Tryptophan Enantiomers by Using a Self-Assembled Multiwalled Carbon Nanotubes/Polyaniline/Sodium Alginate Composite. Chirality 2021, 33, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Klingler, R.J.; Kochi, J.K. Electron-Transfer Kinetics from Cyclic Voltammetry. Quantitative Description of Electrochemical Reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wasfi, A.S. A Comparative Study of Polyaniline/MWCNT with Polyaniline/SWCNT Nanocomposite Films Synthesized by Microwave Plasma Polymerization. Synth. Met. 2019, 250, 49–54. [Google Scholar] [CrossRef]
- Scandurra, G.; Antonella, A.; Ciofi, C.; Saitta, G.; Lanza, M. Electrochemical Detection of P-Aminophenol by Flexible Devices Based on Multi-Wall Carbon Nanotubes Dispersed in Electrochemically Modified Nafion. Sensors 2014, 14, 8926–8939. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Shi, Z.; Liu, Z.; Low, S.S.; Zhu, J.; Zhang, T.; Chen, Z.; Yu, X.; Lu, Y.; Lu, D.; et al. Smartphone-Based Square Wave Voltammetry System with Screen-Printed Graphene Electrodes for Norepinephrine Detection. Smart Mater. Med. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Guziejewski, D. Electrode Mechanisms with Coupled Chemical Reaction—Amplitude Effect in Square-Wave Voltammetry. J. Electroanal. Chem. 2020, 870, 114186. [Google Scholar] [CrossRef]
- Üzer, A.; Sağlam, Ş.; Can, Z.; Erçağ, E.; Apak, R. Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode. Int. J. Mol. Sci. 2016, 17, 1253. [Google Scholar] [CrossRef]
- Yin, H.; Shang, K.; Meng, X.; Ai, S. Voltammetric Sensing of Paracetamol, Dopamine and 4-Aminophenol at a Glassy Carbon Electrode Coated with Gold Nanoparticles and an Organophillic Layered Double Hydroxide. Microchim. Acta 2011, 175, 39–46. [Google Scholar] [CrossRef]
- Rattanarat, P.; Suea-Ngam, A.; Ruecha, N.; Siangproh, W.; Henry, C.S.; Srisa-Art, M.; Chailapakul, O. Graphene-Polyaniline Modified Electrochemical Droplet-Based Microfluidic Sensor for High-Throughput Determination of 4-Aminophenol. Anal. Chim. Acta 2016, 925, 51–60. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Senthilkumar, S. Redox-Active Gold Nanoparticle-Encapsulated Poly(Amidoamine) Dendrimer for Electrochemical Sensing of 4-Aminophenol. J. Mol. Liq. 2021, 325, 115131. [Google Scholar] [CrossRef]


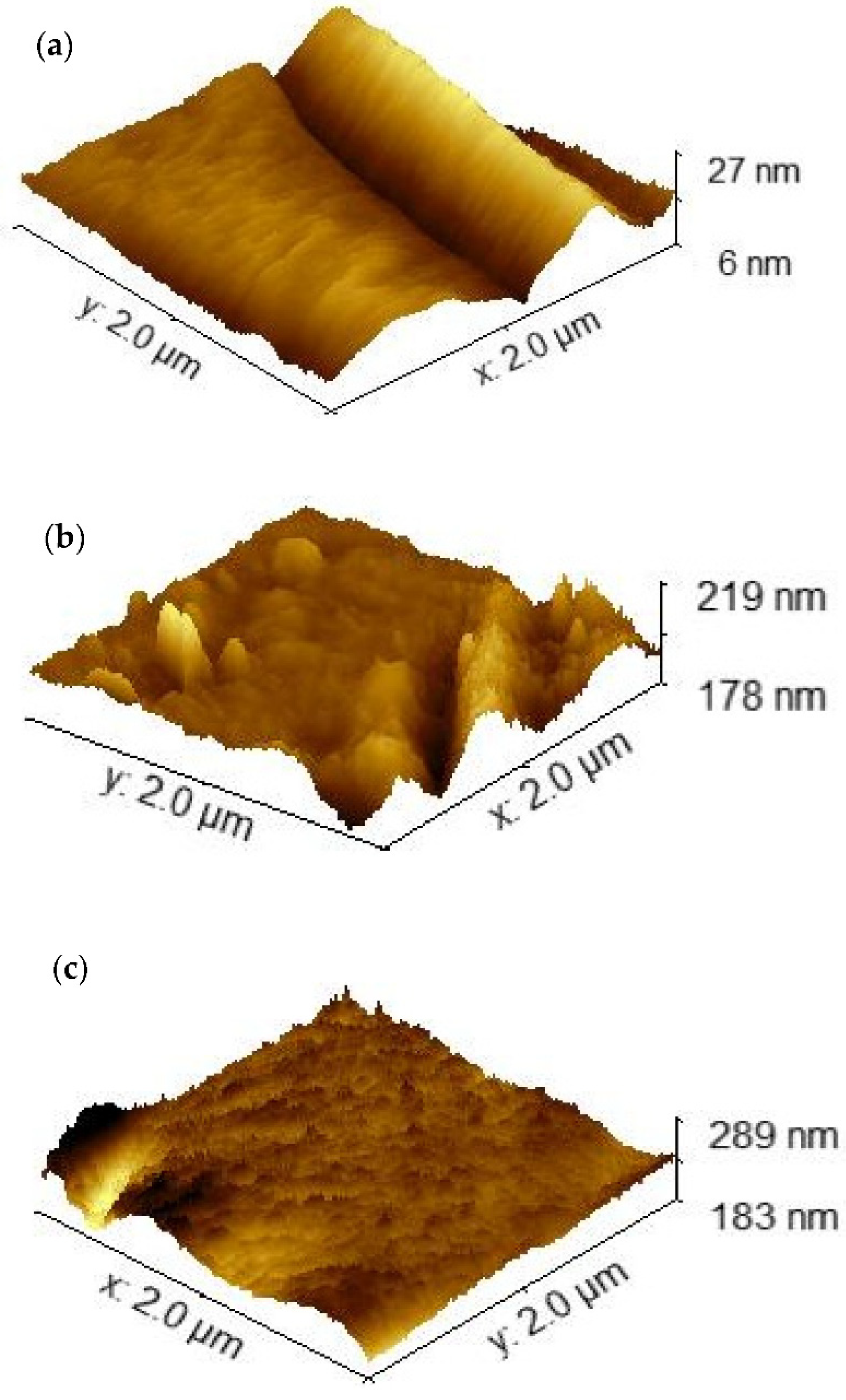



| Electrodes | Surface Area (μm2) | Roughness (nm) |
|---|---|---|
| LIC | 4.08 ± 0.49 | 2.58 ± 0.31 |
| LIC/PANI | 4.16 ± 0.48 | 2.88 ± 0.33 |
| LIC/MWCNT-PANI | 4.82 ± 0.48 | 4.96 ± 0.51 |
| Samples | Added Concentration (μM) | Current (µA) | RSD (%) (n = 3) | Recovery (%) |
|---|---|---|---|---|
| 1 | 5 | 108.22 | 3.03 | 97.63 |
| 2 | 10 | 134.75 | 2.71 | 96.40 |
| 3 | 15 | 188.75 | 2.86 | 98.17 |
| Samples | Added Concentration (μM) | Current (µA) | RSD (%) (n = 3) | Recovery (%) |
|---|---|---|---|---|
| 1 | 5 | 113.06 | 2.81 | 102.00 |
| 2 | 10 | 135.60 | 3.42 | 100.63 |
| 3 | 15 | 198.97 | 3.11 | 105.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasraoui, S.; Ameur, S.; Al-Hamry, A.; Ben Ali, M.; Kanoun, O. Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser Induced Graphene Electrodes Modified with MWCNT-PANI. Sensors 2022, 22, 833. https://doi.org/10.3390/s22030833
Nasraoui S, Ameur S, Al-Hamry A, Ben Ali M, Kanoun O. Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser Induced Graphene Electrodes Modified with MWCNT-PANI. Sensors. 2022; 22(3):833. https://doi.org/10.3390/s22030833
Chicago/Turabian StyleNasraoui, Salem, Sami Ameur, Ammar Al-Hamry, Mounir Ben Ali, and Olfa Kanoun. 2022. "Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser Induced Graphene Electrodes Modified with MWCNT-PANI" Sensors 22, no. 3: 833. https://doi.org/10.3390/s22030833
APA StyleNasraoui, S., Ameur, S., Al-Hamry, A., Ben Ali, M., & Kanoun, O. (2022). Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser Induced Graphene Electrodes Modified with MWCNT-PANI. Sensors, 22(3), 833. https://doi.org/10.3390/s22030833