Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder
Abstract
:1. Introduction
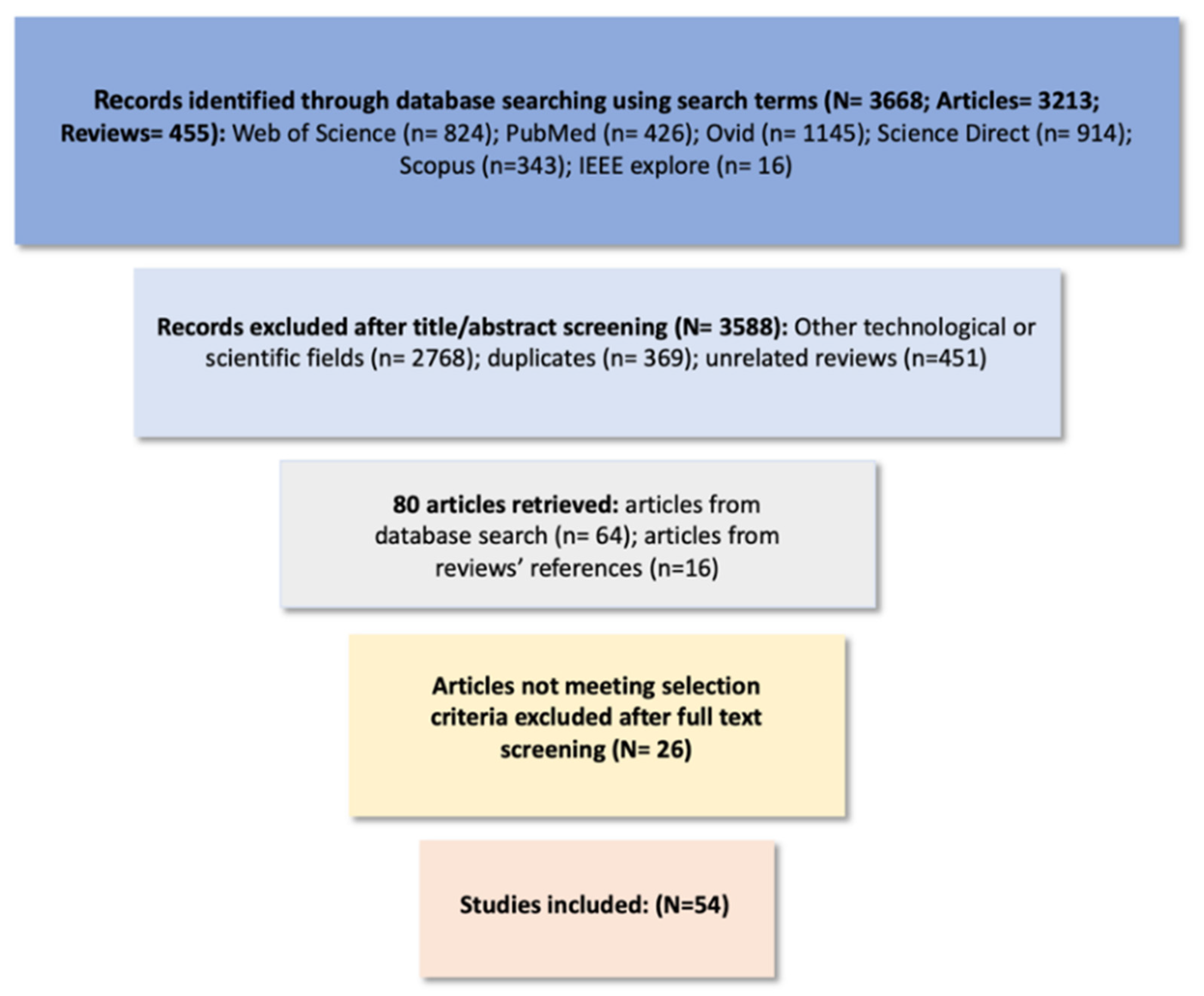
2. Methods
3. Therapeutic Monitoring of Lithium Levels in Different Biological Fluids
4. Conventional Lithium-Monitoring Techniques
5. Advances in Therapeutic Monitoring of Lithium Levels
5.1. Optical Methods
5.1.1. Spectrophotometry
5.1.2. Fluorometry
5.1.3. In Vivo Optical Methods
| Reference | Type | Sensing Platform | Testing Matrix | Lithium Ligand/Detection Method |
|---|---|---|---|---|
| Cash et al. [67] | Optical | lithium-sensitive (optical) nanosensors | In vivo monitoring | Photoacoustic imaging |
| Di et al. [19] | Optical | Erythrocyte-camouflaged florescence-based microsensor | In vivo monitoring | Diffuse in vivo flow cytometry |
| Smith et al. [18] | Optical | 3 T clinical scanner | In vivo monitoring, human brain | Spectroscopic imaging |
| Albero et al. [60] | Spectrophotometric | Flow-through spectrophotometric bulk optode | Saliva and pharmaceuticals | Ionophore-based poly(vinyl) chloride membrane |
| Rumbelow et al. [54] | Spectrophotometric | Hitachi 917 analyzer | Serum | Substituted porphyrin compound |
| Tabata et al. [53] | Spectrophotometric | Shimadzu UV-2100 and Jasco Ubest spectrophotometers | Serum | Porphyrin (octabromoporphyrin) |
| Trautman et al. [51] | Spectrophotometric | Varian Superscan 3 ultraviolet-visible double-beam spectrophotometer | Blood | Thoron [l-(o-arseno- phenylazo)-2-naphthol-3,&disulphonic acid, sodium salt] |
| Zhai et al. [59] | Spectrophotometric | UV-visible absorption spectrometer | Serum | Titrimetric detection based on complexation of the lithium with Li titration reagent in dichloromethane (CH2Cl2) |
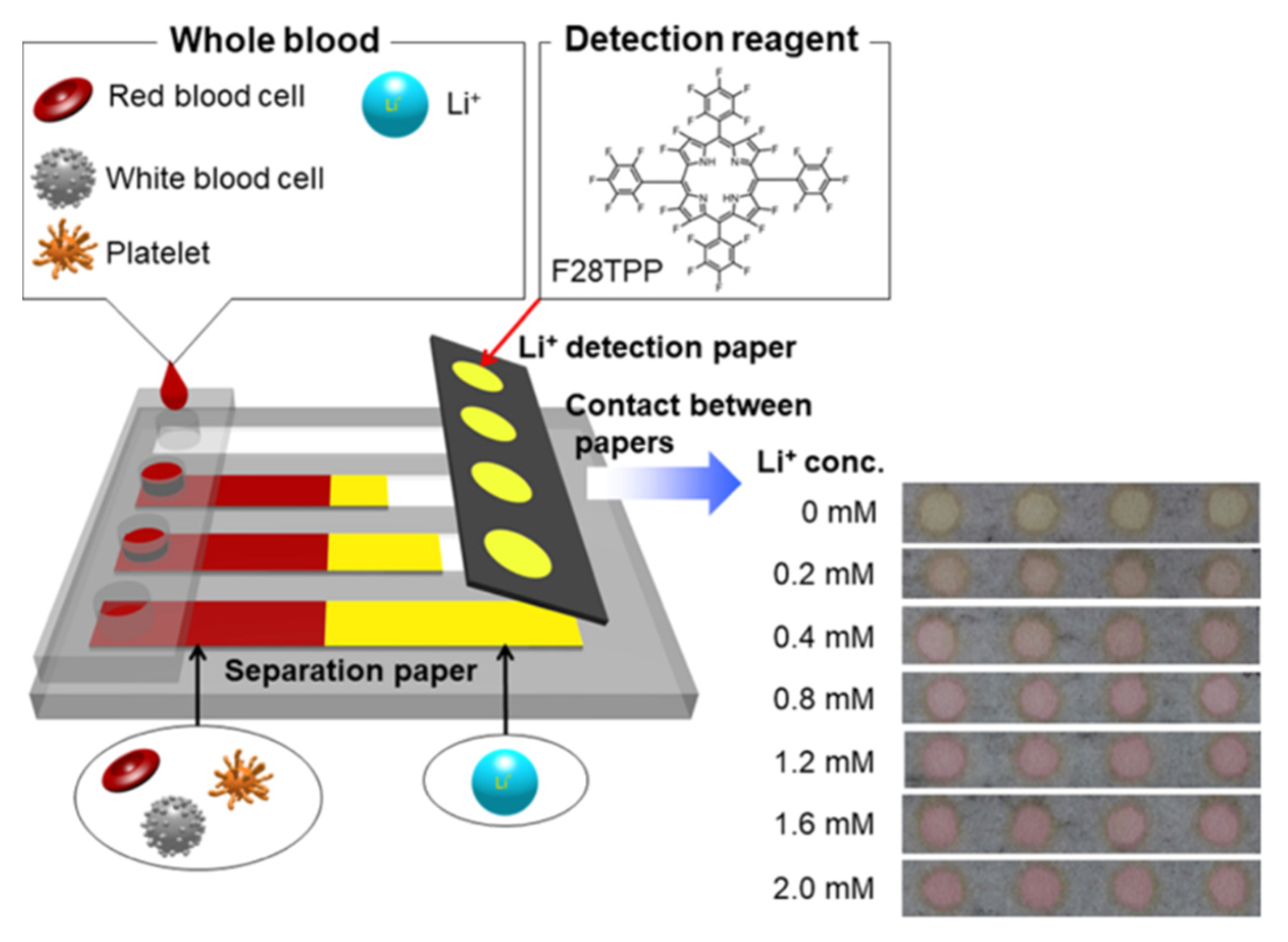
| Komatsu et al. [46] | Optical, colorimetric | Colorimetric paper-based device consisting of a blood cell separation unit and a colorimetric detection unit | Whole blood | F28 tetraphenylporphyrin (F28TPP) was used as the detection reagent |
| Gorham et al. [62] | Optical, colorimetric | Dry slide-based serum lithium assay | Blood | Absorbance change based on binding of Li+ to a crown-ether azo dye |
| Iwai et al. [35] | Optical, colorimetric | Lithium assay kit LS coupled with microplate reader | Whole blood and urine | Colorimetric response based on binding of Li+ and polyfluoroporphyrin as chromogen |
| Gruson et al. [64] | Optical, colorimetric | Dimension Xpand analyzer | Blood | Absorbance change based on the formation of a noncovalent binary complex between Li and 7- nitro-2,12-dicarboxyl-16, 17dihydro-5H, 15H-dibenzo wb,ix (1), 11, (4, 5, 7, 8) dioxatetraaza-cyclotetra-decine in an alkaline mixture |
| Qassem et al. [52,55,56] | Optical, colorimetric | Optical and electrical impedance spectroscopy | Blood | Combination of optical and electrical impedance spectroscopy, optical detection based on the reaction between Li and quinizarin |
| Zhang et al. [63] | Optical, colorimetric | Spectra detected on UV-Vis spectrophotometer | Methanol and water solution | Absorbance change and a colorimetric response based on macrocyclic Sm(III) complex serving as a colorimetric ligand for Li+ |
| Obare et al. [43,44] | Optical, colorimetric | Gold nanoparticles | Tested in aqueous solution | Absorbance change and colorimetric response based on binding of Li+ with 1, 10-phenanthroline ligand |
| Gunnlaugsson et al. [45] | Optical, fluorometric | Fluorescent PET Li+ chemosensor | Tested in queous solution | Diaza-9-crown-3 as the Li+ receptor |
| Kim et al. [25] | Optical, fluorometric | UV-Vis spectrophotometer | Saliva | 1,4-dihydroxyanthraquinone (quinizarin) |
5.2. Electrochemical Methods
5.2.1. Ion-Selective Electrodes (ISEs)
Potentiometric
Voltammetric
5.2.2. Capillary Electrophoresis
| Reference | Type | Sensing Platform | Testing Matrix | Surface Modification/Lithium Ligand |
|---|---|---|---|---|
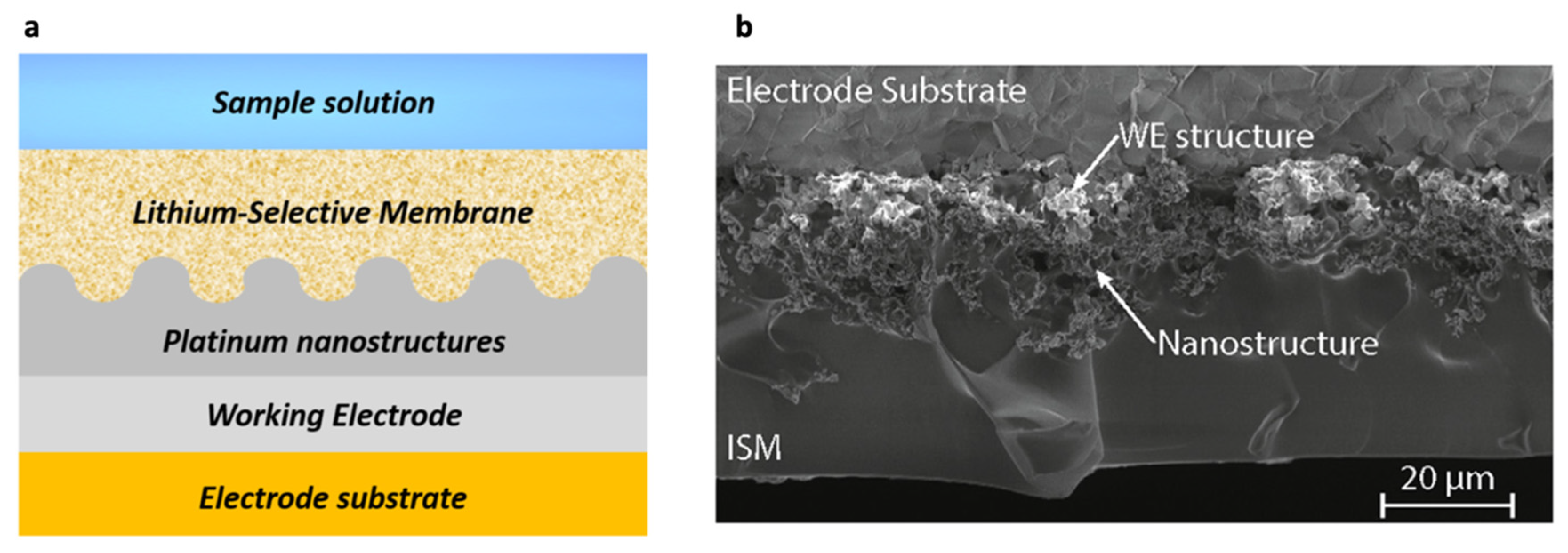
| Criscuolo et al. [14,29,30,31] | ISE, potentiometric | Metal nanostructures (SC-ISEs) | Sweat | ISM containing poly(vinyl chloride) and Li ionophore VI (6,6- Dibenzyl-1,4,8-11-tetraoxacyclotetradecane) |
| Sweilam et al. [17,75] | ISE, potentiometric | Cotton-fiber-based lithium sensor | ISF | Lithium sensor fabricated by dipping a cotton thread in SWCNT ink and lithium membrane solution |
| Lindino et al. [70] | ISE, potentiometric | Gold electrode | Serum | Conducting polymer [poly(o-methoxyaniline)] |
| Hanitra et al. [73,74] | ISE, potentiometric | Multi-channel electrochemical sensing | Water | ISM containing poly(vinyl chloride) and Li ionophore VI (6,6-Dibenzyl-1,4, 8-11-tetraoxacyclotetradecane) |
| Singh et al. [76] | ISE, voltammetric | Screen-printed sensor strips | Serum | 14-crown-4 ether (6,6′-dibenzyl- 14-crown-4 ether)-based ionophore |
| Coldur et al. [49,71,72] | ISE, potentiometric | Potentiometric flow injection system | Serum | Solvent polyvinyl chloride (PVC) membrane |
| Suherman et al. [24] | ISE, voltammetric | (LiMn2O4)-modified glassy carbon electrodes (LMO-GCEs) and screen-printed electrodes (LMO-SPEs) | Saliva | Electrochemical sensing of lithium based on the galvanostatic delithiation of LMO followed by linear stripping voltammetry (LSV) |
| Metzger et al. [40] | ISE, potentiometric | Ag/AgCl electrodes | Serum | PVC membrane containing N,N-dlcyciohexyi-N’,N’-diiso- butyCclscyclohexane-l,2dicarboxamlde (ETH 1810) |
| Bertholf et al. [41] | ISE | ISEs coupled with Du Pont Na/K/Li analyzer | Serum | PVC membrane |
| Novell et al. [15] | ISE, potentiometric | Paper-based potentiometric cell | Blood | Polymeric membrane |
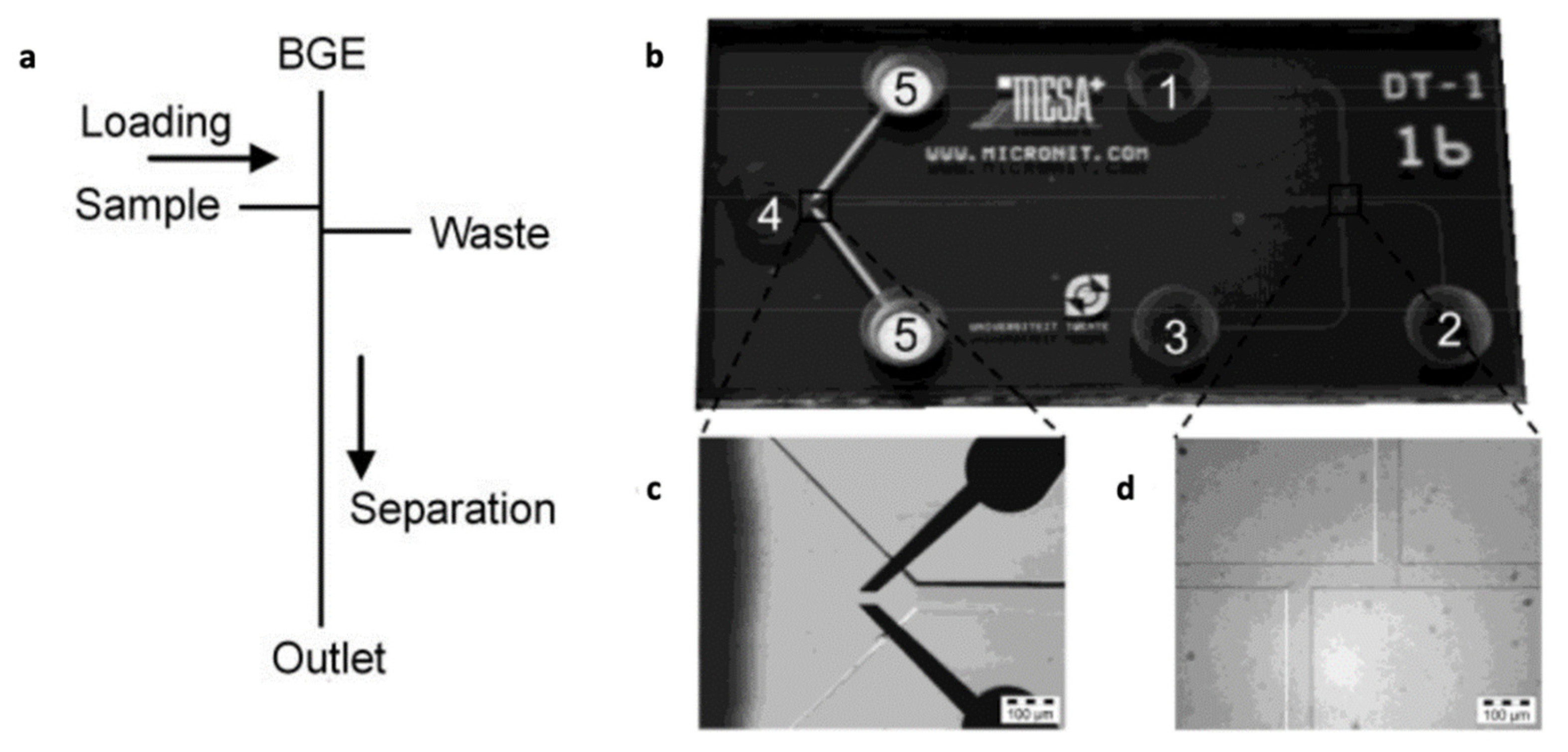
| Floris et al. [79] | Microchip capillary electrophoresis | Conductivity detection | Blood | N/A |
| Jamal et al. [36] | Capillary zone electrophoresis | Indirect UV detection | Serum and urine | N/A |
| Vrouwe et al. [42,77,78] | Microchip capillary electrophoresis | Conductivity detection | Blood | N/A |
| Kuban et al. [37] | Microchip capillary electrophoresis | Conductivity detection | Serum and urine | N/A |
6. Discussion
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shorter, E. The history of lithium therapy. Bipolar Disord. 2009, 11, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craddock, N.; Sklar, P. Genetics of bipolar disorder. Lancet 2013, 381, 1654–1662. [Google Scholar] [CrossRef]
- Harrison, P.J.; Geddes, J.R.; Tunbridge, E. The Emerging Neurobiology of Bipolar Disorder. Trends Neurosci. 2018, 41, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef]
- de Mendiola, X.P.; Hidalgo-Mazzei, D.; Vieta, E.; González-Pinto, A. Overview of lithium’s use: A nationwide survey. Int. J. Bipolar Disord. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Matsuura, H.; Kimoto, S.; Harada, I.; Naemura, S.; Yamamuro, K.; Kishimoto, T. Lithium carbonate as a treatment for paliperidone extended-release-induced leukopenia and neutropenia in a patient with schizoaffective disorder; a case report. BMC Psychiatry 2016, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Murru, A.; for the International Group for The Study of Lithium Treated Patients (IGSLi); Manchia, M.; Hajek, T.; Nielsen, R.E.; Rybakowski, J.K.; Sani, G.; Schulze, T.G.; Tondo, L.; Bauer, M. Lithium’s antiviral effects: A potential drug for CoViD-19 disease? Int. J. Bipolar Disord. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Birch, N.J. Inorganic Pharmacology of Lithium. Chem. Rev. 1999, 99, 2659–2682. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Camus, M.; Baron, G.; Peytavin, G.; Massias, L.; Farinotti, R. Comparison of lithium concentrations in red blood cells and plasma in samples collected for TDM, acute toxicity, or acute-on-chronic toxicity. Eur. J. Clin. Pharmacol. 2003, 59, 583–587. [Google Scholar] [CrossRef]
- El Balkhi, S.; Megarbane, B.; Poupon, J.; Baud, F.J.; Galliot-Guilley, M. Lithium poisoning: Is determination of the red blood cell lithium concentration useful? Clin. Toxicol. 2009, 47, 8–13. [Google Scholar] [CrossRef]
- Couffignal, C.; Chevillard, L.; El Balkhi, S.; Cisternino, S.; Declèves, X. The Pharmacokinetics of Lithium. In The Science and Practice of Lithium Therapy; Malhi, G.S., Masson, M., Bellivier, F., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–53. [Google Scholar] [CrossRef]
- Öhlund, L.; Ott, M.; Oja, S.; Bergqvist, M.; Lundqvist, R.; Sandlund, M.; Renberg, E.S.; Werneke, U. Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 2018, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Criscuolo, F.; Cantu, F.; Taurino, I.; Carrara, S.; De Micheli, G. Flexible sweat sensors for non-invasive optimization of lithium dose in psychiatric disorders. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27-30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Novell, M.; Guinovart, T.; Blondeau, P.; Rius, F.X.; Andrade, F.J. A paper-based potentiometric cell for decentralized monitoring of Li levels in whole blood. Lab Chip 2013, 14, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Eltayib, E.; Brady, A.J.; Caffarel-Salvador, E.; Gonzalez-Vazquez, P.; Alkilani, A.Z.; McCarthy, H.O.; McElnay, J.C.; Donnelly, R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016, 102, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sweilam, M.N.; Cordery, S.F.; Totti, S.; Velliou, E.G.; Campagnolo, P.; Varcoe, J.R.; Delgado-Charro, M.B.; Crean, C. Textile-based non-invasive lithium drug monitoring: A proof-of-concept study for wearable sensing. Biosens. Bioelectron. 2020, 150, 111897. [Google Scholar] [CrossRef]
- Smith, F.E.; Cousins, D.A.; Thelwall, P.E.; Ferrier, I.N.; Blamire, A.M. Quantitative lithium magnetic resonance spectroscopy in the normal human brain on a 3 T clinical scanner: Lithium Brain MRS in Healthy Controls at 3 T′. Magn. Reson. Med. 2011, 66, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Tan, X.; Calderon, I.A.C.; Reilly, A.E.N.; Niedre, M.; Clark, H.A. Real-time particle-by-particle detection of erythrocyte-camouflaged microsensor with extended circulation time in the bloodstream. Proc. Natl. Acad. Sci. USA 2020, 117, 3509–3517. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lokhov, P.G. Evaluation of Dried Blood Spot Sampling for Clinical Metabolomics: Effects of Different Papers and Sample Storage Stability. Metabolites 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfro, I.D.; Tegner, M.; Krutzmann, M.E.; Artmann, A.D.C.; Brandeburski, M.R.; Peteffi, G.P.; Linden, R.; Antunes, M.V. Determination of lithium in dried blood spots and dried plasma spots by graphite furnace atomic absorption spectrometry: Method development, validation and clinical application. Talanta 2020, 216, 120907. [Google Scholar] [CrossRef]
- Zakaria, R.; Allen, K.; Koplin, J.; Roche, P.; Greaves, R.F. Advantages and Challenges of Dried Blood Spot Analysis by Mass Spectrometry Across the Total Testing Process. EJIFCC 2016, 27, 288–317. [Google Scholar]
- Serdarević, N.; Kozjek, F.; Malešič, I. Saliva and Serum Lithium Monitoring in Hospitalized Patients and Possibility to Replace Serum to Saliva. Bosn. J. Basic Med. Sci. 2008, 6, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Suherman, A.L.; Rasche, B.; Godlewska, B.R.; Nicholas, P.; Herlihy, S.; Caiger, N.; Cowen, P.J.; Compton, R.G. Electrochemical Detection and Quantification of Lithium Ions in Authentic Human Saliva Using LiMn2O4-Modified Electrodes. ACS Sens. 2019, 4, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Diamond, D.; Lau, K.T. Development of non-invasive biochemical device for monitoring the lithium level from saliva for bipolar disorder patients. In Proceedings of the 2011 IEEE SENSORS, Limerick, Ireland, 28–31 October 2011; pp. 1744–1747. [Google Scholar] [CrossRef] [Green Version]
- Ben-Aryeh, H.; Naon, H.; Szargel, R.; Gutman, D.; Hefetz, A. Salivary lithium concentration—A tool for monitoring psychiatric patients. Oral Surg. Oral Med. Oral Pathol. 1980, 50, 127–129. [Google Scholar] [CrossRef]
- Murru, A.; Torra, M.; Callari, A.; Pacchiarotti, I.; Romero, S.; de la Presa, B.G.; Varo, C.; Goikolea, J.; Pérez-Sola, V.; Vieta, E.; et al. A study on the bioequivalence of lithium and valproate salivary and blood levels in the treatment of bipolar disorder. Eur. Neuropsychopharmacol. 2017, 27, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Kiang, T.K.; Ranamukhaarachchi, S.A.; Ensom, M.H. Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology. Pharmaceutics 2017, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Criscuolo, F.; Taurino, I.; Carrara, S.; De Micheli, G. A novel electrochemical sensor for non-invasive monitoring of lithium levels in mood disorders. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3825–3828. [Google Scholar] [CrossRef] [Green Version]
- Criscuolo, F.; Taurino, I.; Stradolini, F.; Carrara, S.; De Micheli, G. Highly-stable Li+ ion-selective electrodes based on noble metal nanostructured layers as solid-contacts. Anal. Chim. Acta 2018, 1027, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, F.; Ny Hanitra, I.; Aiassa, S.; Taurino, I.; Oliva, N.; Carrara, S.; De Micheli, G. Wearable multifunctional sweat-sensing system for efficient healthcare monitoring. Sens. Actuators B Chem. 2020, 328, 129017. [Google Scholar] [CrossRef]
- Kiang, T.K.; Schmitt, V.; Ensom, M.H.; Chua, B.; Häfeli, U.O. Therapeutic Drug Monitoring in Interstitial Fluid: A Feasibility Study Using a Comprehensive Panel of Drugs. J. Pharm. Sci. 2012, 101, 4642–4652. [Google Scholar] [CrossRef] [PubMed]
- Leboulanger, B.; Aubry, J.-M.; Bondolfi, G.; Guy, R.; Delgado-Charro, M.B. Lithium Monitoring by Reverse Iontophoresis in Vivo. Clin. Chem. 2004, 50, 2091–2100. [Google Scholar] [CrossRef]
- Leboulanger, B.; Fathi, M.; Guy, R.H.; Delgado-Charro, M.B. Reverse iontophoresis as a noninvasive tool for lithium monitoring and pharmacokinetic profiling. Pharm. Res. 2004, 21, 1214–1222. [Google Scholar] [CrossRef]
- Iwai, M.; Kondo, F.; Suzuki, T.; Ogawa, T.; Seno, H. Application of Lithium Assay kit LS for quantification of lithium in whole blood and urine. Leg. Med. 2020, 49, 101834. [Google Scholar] [CrossRef]
- Jamal, T.; Hennequin, C.; Gahoual, R.; Leyris, A.; Beaudeux, J.-L.; Baud, F.J.; Houzé, P. Is Capillary Electrophoresis a New Tool to Monitor Acute Lithium Poisoning in Human? J. Anal. Toxicol. 2019, 43, 571–578. [Google Scholar] [CrossRef]
- Kubáň, P.; Hauser, P.C. Evaluation of microchip capillary electrophoresis with external contactless conductivity detection for the determination of major inorganic ions and lithium in serum and urine samples. Lab Chip 2008, 8, 1829–1836. [Google Scholar] [CrossRef]
- Bowman, J. The application of resonance monochromators to the determination of lithium in blood serum by atomic absorption spectro photometry. Anal. Chim. Acta 1967, 37, 465–471. [Google Scholar] [CrossRef]
- Doku, G.N.; Gadzekpo, V.P. Simultaneous determination of lithium, sodium and potassium in blood serum by flame photometric flow-injection analysis. Talanta 1996, 43, 735–739. [Google Scholar] [CrossRef]
- Metzger, E.; Ammann, D.; Asper, R.; Simon, W. Ion selective liquid membrane electrode for the assay of lithium in blood serum. Anal. Chem. 1986, 58, 132–135. [Google Scholar] [CrossRef]
- Bertholf, R.L.; Savory, M.G.; Winborne, K.H.; Hundley, J.C.; Plummer, G.M.; Savory, J. Lithium determined in serum with an ion-selective electrode. Clin. Chem. 1988, 34, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Vrouwe, E.X.; Luttge, R.; Berg, A.V.D. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis 2004, 25, 1660–1667. [Google Scholar] [CrossRef]
- Obare, S.O.; Hollowell, A.R.E.; Murphy, C.J. Sensing Strategy for Lithium Ion Based on Gold Nanoparticles. Langmuir 2002, 18, 10407–10410. [Google Scholar] [CrossRef]
- Obare, S.O.; Murphy, C.J. A Two-Color Fluorescent Lithium Ion Sensor. Inorg. Chem. 2001, 40, 6080–6082. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Bichell, B.; Nolan, C. A novel fluorescent photoinduced electron transfer (PET) sensor for lithium. Tetrahedron Lett. 2002, 43, 4989–4992. [Google Scholar] [CrossRef]
- Komatsu, T.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Paper-Based Device for the Facile Colorimetric Determination of Lithium Ions in Human Whole Blood. ACS Sens. 2020, 5, 1287–1294. [Google Scholar] [CrossRef]
- Dimeski, G.; Badrick, T.; John, A.S. Ion Selective Electrodes (ISEs) and interferences—A review. Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance Characterization and Modeling of Electrodes for Biomedical Applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef]
- Coldur, F.; Andac, M. A Flow-Injection Potentiometric System for Selective and Sensitive Determination of Serum Lithium Level. Electroanalysis 2013, 25, 732–740. [Google Scholar] [CrossRef]
- Villemin, E.; Raccurt, O. Optical lithium sensors. Coord. Chem. Rev. 2021, 435, 213801. [Google Scholar] [CrossRef]
- Trautman, J.K.; Gadzekpo, V.Y.P.; Christian, G.D. Spectrophotometric determination of lithium in blood serum with thoron. Talanta 1983, 30, 587–591. [Google Scholar] [CrossRef]
- Qassem, M.; Hickey, M.; Kyriacou, P.A. Colorimetric determinations of lithium levels in drop-volumes of human plasma for monitoring patients with bipolar mood disorder. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5160–5163. [Google Scholar] [CrossRef]
- Tabata, M.; Nishimoto, J.; Kusano, T. Spectrophotometric determination of lithium ion using a water-soluble octabromoporphyrin in aqueous solution. Talanta 1998, 46, 703–709. [Google Scholar] [CrossRef]
- Rumbelow, B.; Peake, M. Performance of a novel spectrophotometric lithium assay on a routine biochemistry analyser. Ann. Clin. Biochem. Int. J. Lab. Med. 2001, 38, 684–686. [Google Scholar] [CrossRef]
- Constantinou, L.; Triantis, I.; Hickey, M.; Kyriacou, P.A. On the merits of tetrapolar impedance spectroscopy for monitoring lithium concentration variations in human blood plasma. IEEE Trans. Biomed. Eng. 2016, 64, 601–609. [Google Scholar] [CrossRef]
- Qassem, M.; Constantinou, L.; Triantis, I.F.; Hickey, M.; Palazidou, E.; Kyriacou, P.A. A Method for Rapid, Reliable, and Low-Volume Measurement of Lithium in Blood for Use in Bipolar Disorder Treatment Management. IEEE Trans. Biomed. Eng. 2018, 66, 130–137. [Google Scholar] [CrossRef]
- Qassem, M.; Triantis, I.; Hickey, M.; Palazidou, E.; Kyriacou, P. Methodology for rapid assessment of blood lithium levels in ultramicro volumes of blood plasma for applications in personal monitoring of patients with bipolar mood disorder. J. Biomed. Opt. 2018, 23, 107004. [Google Scholar] [CrossRef]
- May, J.; Hickey, M.; Triantis, I.; Palazidou, E.; Kyriacou, P. Spectrophotometric analysis of lithium carbonate used for bipolar disorder. Biomed. Opt. Express 2015, 6, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Xie, X.; Cherubini, T.; Bakker, E. Ionophore-Based Titrimetric Detection of Alkali Metal Ions in Serum. ACS Sens. 2017, 2, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Albero, M.; Ortuño, J.; Garcia, M.; Cuartero, M.; Alcaraz, M. Novel flow-through bulk optode for spectrophotometric determination of lithium in pharmaceuticals and saliva. Sens. Actuators B Chem. 2010, 145, 133–138. [Google Scholar] [CrossRef]
- Monogarova, O.V.; Oskolok, K.V.; Apyari, V.V. Colorimetry in Chemical Analysis. J. Anal. Chem. 2018, 73, 1076–1084. [Google Scholar] [CrossRef]
- Gorham, J.D.; Walton, K.G.; McClellan, A.C.; Scott, M.G. Evaluation of a New Colorimetric Assay for Serum Lithium. Ther. Drug Monit. 1994, 16, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, T.-T.; Zhang, L.-F.; Ma, S.; Shen, Y.-J.; Feng, C.-C.; Nie, P.-P.; Yang, Z.-R.; Zhu, C. The selective colorimetric probe based on a macrocyclic Sm(III) complex for detecting lithium ion and its performance in the psychiatric drug. Dye. Pigment. 2019, 174, 108027. [Google Scholar] [CrossRef]
- Gruson, D.; Lallali, A.; Furlan, V.; Taburet, A.M.; Legrand, A.; Conti, M. Evaluation of a new lithium colorimetric assay performed on the Dade Behring Dimension® X-pand™ system. Clin. Chem. Lab. Med. 2004, 42, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, K.; Nomoto, M.; Sugihara, H.; Okada, T. Behaviour of 2,9-disubstituted 1, 10-phenanthroline derivatives as specific lithium ion fluorophores. Analyst 1992, 117, 1491–1495. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cash, K.J.; Li, C.; Xia, J.; Wang, L.V.; Clark, H.A. Optical Drug Monitoring: Photoacoustic Imaging of Nanosensors to Monitor Therapeutic Lithium in Vivo. ACS Nano 2015, 9, 1692–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliasgharpour, M.; Hagani, H. Evaluation of lithium determination in three analyzers: Flame emission, flame atomic absorption spectroscopy and ion selective electrode. N. Am. J. Med. Sci. 2009, 1, 4. [Google Scholar]
- Coldur, F.; Andac, M.; Isildak, I.; Saka, T. A micro-sized PVC membrane Li+-selective electrode without internal filling solution and its medical applications. J. Electroanal. Chem. 2009, 626, 30–35. [Google Scholar] [CrossRef]
- Lindino, C.A.; Casagrande, M.; Peiter, A.; Ribeiro, C. Poly(o-methoxyaniline) modified electrode for detection of lithium ions. Quím. Nova 2012, 35, 449–453. [Google Scholar] [CrossRef]
- Christian, G.D. Reagents for Lithium Electrodes and Sensors for Blood Serum Analysis. Sensors 2002, 2, 432–435. [Google Scholar] [CrossRef]
- Coldur, F.; Andaç, M. All-Solid-State Polyvinyl Chloride Membrane Lithium-Selective Electrode with Improved Selectivity and Its Application in Serum Lithium Assay. Sens. Lett. 2011, 9, 1738–1744. [Google Scholar] [CrossRef]
- Ny Hanitra, I.; Lobello, L.; Stradolini, F.; Tuoheti, A.; Criscuolo, F.; Kilic, T.; Demarchi, D.; Carrara, S.; De Micheli, G. A Flexible Front-End for Wearable Electrochemical Sensing. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Ny Hanitra, I.; Criscuolo, F.; Pankratova, N.; Carrara, S.; De Micheli, G. Multichannel Front-End for Electrochemical Sensing of Metabolites, Drugs, and Electrolytes. IEEE Sens. J. 2019, 20, 3636–3645. [Google Scholar] [CrossRef]
- Sweilam, M.N.; Varcoe, J.R.; Crean, C. Fabrication and Optimization of Fiber-Based Lithium Sensor: A Step toward Wearable Sensors for Lithium Drug Monitoring in Interstitial Fluid. ACS Sens. 2018, 3, 1802–1810. [Google Scholar] [CrossRef]
- Singh, U.; Kumbhat, S. Ready to Use Electrochemical Sensor Strip for Point-of-Care Monitoring of Serum Lithium. Electroanalysis 2020, 33, 393–399. [Google Scholar] [CrossRef]
- Vrouwe, E.X.; Luttge, R.; Olthuis, W.; Berg, A.V.D. Microchip analysis of lithium in blood using moving boundary electrophoresis and zone electrophoresis. Electrophoresis 2005, 26, 3032–3042. [Google Scholar] [CrossRef]
- Vrouwe, E.X.; Luttge, R.; Vermes, I.; Berg, A.V.D. Microchip Capillary Electrophoresis for Point-of-Care Analysis of Lithium. Clin. Chem. 2007, 53, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Staal, S.; Lenk, S.; Staijen, E.; Kohlheyer, D.; Eijkel, J.; Berg, A.V.D. A prefilled, ready-to-use electrophoresis based lab-on-a-chip device for monitoring lithium in blood. Lab Chip 2010, 10, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Torii, H.; Osakai, T.; Kimoto, T. Pulse Amperometric Detection of Lithium in Artificial Serum Using a Flow Injection System with a Liquid/Liquid-Type Ion-Selective Electrode. Anal. Chem. 1998, 70, 4286–4290. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2015, 76, 102–114. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsányi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2008, 13, 51–68. [Google Scholar] [CrossRef]
- Ates, H.C.; Roberts, J.A.; Lipman, J.; Cass, A.E.; Urban, G.A.; Dincer, C. On-Site Therapeutic Drug Monitoring. Trends Biotechnol. 2020, 38, 1262–1277. [Google Scholar] [CrossRef] [PubMed]





| Biological Fluid | Advantages | Limitations |
|---|---|---|
| Blood | Provides accurate measurements | Relative invasiveness, cost, and impracticalities |
| Sweat | Non-invasiveness | Sample collection requires stimulating sweat glands, presence of potential contaminants |
| Saliva | Accessibility, non-invasiveness | Drug instability, presence of potential contaminants, and lack of phase II metabolites |
| Interstitial fluid (ISF) | Good correlation with venous blood, suitable for continuous monitoring, good reproducibility, minimally invasive | Low volume, sample evaporation |
| Dried blood/plasma spots | Small collection volume, minimal discomfort, and easy sample collection | Accuracy of measurement and reproducibility |
| Urine | High concentrations of many drugs and metabolites in urine, non-invasiveness | Unsatisfactory accuracy, the need for pretreatment, and wide variations between patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, M.; Qassem, M.; Triantis, I.F.; Kyriacou, P.A. Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors 2022, 22, 736. https://doi.org/10.3390/s22030736
Sheikh M, Qassem M, Triantis IF, Kyriacou PA. Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors. 2022; 22(3):736. https://doi.org/10.3390/s22030736
Chicago/Turabian StyleSheikh, Mahsa, Meha Qassem, Iasonas F. Triantis, and Panicos A. Kyriacou. 2022. "Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder" Sensors 22, no. 3: 736. https://doi.org/10.3390/s22030736
APA StyleSheikh, M., Qassem, M., Triantis, I. F., & Kyriacou, P. A. (2022). Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors, 22(3), 736. https://doi.org/10.3390/s22030736








