Kinect V2-Based Gait Analysis for Children with Cerebral Palsy: Validity and Reliability of Spatial Margin of Stability and Spatiotemporal Variables
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Data Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavão, S.L.; dos Santos, A.N.; Woollacott, M.H.; Rocha, N.A.C.F. Assessment of postural control in children with cerebral palsy: A review. Res. Dev. Disabil. 2013, 34, 1367–1375. [Google Scholar] [CrossRef][Green Version]
- Donker, S.F.; Ledebt, A.; Roerdink, M.; Savelsbergh, G.J.P.; Beek, P.J. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Exp. Brain Res. 2008, 184, 363–370. [Google Scholar] [CrossRef]
- Rose, J.; Wolff, D.R.; Jones, V.K.; Bloch, D.A.; Oehlert, J.W.; Gamble, J.G. Postural balance in children with cerebral palsy. Dev. Med. Child Neurol. 2002, 44, 58–63. [Google Scholar] [CrossRef]
- Alemdaroğlu, E.; Özbudak, S.D.; Mandiroğlu, S.; Biçer, S.A.; Özgirgin, N.; Uçan, H. Predictive Factors for Inpatient Falls among Children with Cerebral Palsy. J. Pediatric Nurs. 2017, 32, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.R.; Patterson, A.A. Gait pathology subtypes are not associated with self-reported fall frequency in children with cerebral palsy. Gait Posture 2018, 63, 189–194. [Google Scholar] [CrossRef]
- Craig, F.; Castelnuovo, R.; Pacifico, R.; Leo, R.; Trabacca, A. Falls in Hospitalized Children With Neurodevelopmental Conditions: A Cross-sectional, Correlational Study. Rehabil. Nurs. J. 2018, 43, 335–342. [Google Scholar] [CrossRef]
- Morgan, P.; McDonald, R.; McGinley, J. Perceived Cause, Environmental Factors, and Consequences of Falls in Adults with Cerebral Palsy: A Preliminary Mixed Methods Study. Rehabil. Res. Pract. 2015, 2015, 196395. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-F.; Wang, T.-M.; Lo, W.-C.; Lu, T.-W.; Hong, S.-W.; Huang, C.-H.; Shieh, J.-Y.; Huang, S.-C. Balance control during level walking in children with spastic diplegic cerebral palsy. Biomed. Eng. Appl. Basis Commun. 2011, 23, 509–517. [Google Scholar] [CrossRef]
- Tracy, J.B.; Petersen, D.A.; Pigman, J.; Conner, B.C.; Wright, H.G.; Modlesky, C.M.; Miller, F.; Johnson, C.L.; Crenshaw, J.R. Dynamic stability during walking in children with and without cerebral palsy. Gait Posture 2019, 72, 182–187. [Google Scholar] [CrossRef]
- Kurz, M.J.; Arpin, D.J.; Corr, B. Differences in the dynamic gait stability of children with cerebral palsy and typically developing children. Gait Posture 2012, 36, 600–604. [Google Scholar] [CrossRef]
- Iosa, M.; Marro, T.; Paolucci, S.; Morelli, D. Stability and harmony of gait in children with cerebral palsy. Res. Dev. Disabil. 2012, 33, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Noreau, L.; Bernard, P.-M. Association between characteristics of locomotion and accomplishment of life habits in children with cerebral palsy. Phys. Ther. 1998, 78, 458–469. [Google Scholar] [CrossRef]
- Sorsdahl, A.B.; Moe-Nilssen, R.; Strand, L.I. Test–retest reliability of spatial and temporal gait parameters in children with cerebral palsy as measured by an electronic walkway. Gait Posture 2008, 27, 43–50. [Google Scholar] [CrossRef]
- Booth, A.T.; Buizer, A.I.; Meyns, P.; Oude Lansink, I.L.; Steenbrink, F.; van der Krogt, M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nandy, A.; Kesar, T.M. Gait deficits and dynamic stability in children and adolescents with cerebral palsy: A systematic review and meta-analysis. Clin. Biomech. 2020, 71, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, S.; Dolatabadi, E.; Ng, K.-D.; Mansfield, A.; Flint, A.; Taati, B.; Iaboni, A. Vision-based assessment of gait features associated with falls in people with dementia. J. Gerontol. Ser. A 2020, 75, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.D.; Mehdizadeh, S.; Iaboni, A.; Mansfield, A.; Flint, A.; Taati, B. Measuring Gait Variables Using Computer Vision to Assess Mobility and Fall Risk in Older Adults With Dementia. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Abd El-Kafy, E.M.; El-Basatiny, H.M.Y.M. Effect of postural balance training on gait parameters in children with cerebral palsy. Am. J. Phys. Med. Rehabil. 2014, 93, 938–947. [Google Scholar] [CrossRef]
- Elnahhas, A.M.; Elshennawy, S.; Aly, M.G. Effects of backward gait training on balance, gross motor function, and gait in children with cerebral palsy: A systematic review. Clin. Rehabil. 2019, 33, 3–12. [Google Scholar] [CrossRef]
- Moreau, N.G.; Bodkin, A.W.; Bjornson, K.; Hobbs, A.; Soileau, M.; Lahasky, K. Effectiveness of rehabilitation interventions to improve gait speed in children with cerebral palsy: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 1938–1954. [Google Scholar] [CrossRef]
- Hof, A.; Gazendam, M.; Sinke, W. The condition for dynamic stability. J. Biomech. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness Control of Balance in Quiet Standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Young, P.M.M.; Wilken, J.M.; Dingwell, J.B. Dynamic margins of stability during human walking in destabilizing environments. J. Biomech. 2012, 45, 1053–1059. [Google Scholar] [CrossRef]
- Hak, L.; Houdijk, H.; Van Der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; Van Dieën, J.H. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin. Biomech. 2013, 28, 1041–1048. [Google Scholar] [CrossRef]
- Martelli, D.; Luo, L.; Kang, J.; Kang, U.J.; Fahn, S.; Agrawal, S.K. Adaptation of stability during perturbed walking in Parkinson’s disease. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- van Vugt, Y.; Stinear, J.; Davies, T.C.; Zhang, Y. Postural stability during gait for adults with hereditary spastic paraparesis. J. Biomech. 2019, 88, 12–17. [Google Scholar] [CrossRef]
- Delabastita, T.; Desloovere, K.; Meyns, P. Restricted arm swing affects gait stability and increased walking speed alters trunk movements in children with cerebral palsy. Front. Hum. Neurosci. 2016, 10, 354. [Google Scholar] [CrossRef]
- Peebles, A.T.; Reinholdt, A.; Bruetsch, A.P.; Lynch, S.G.; Huisinga, J.M. Dynamic margin of stability during gait is altered in persons with multiple sclerosis. J. Biomech. 2016, 49, 3949–3955. [Google Scholar] [CrossRef]
- Ejupi, A.; Gschwind, Y.J.; Valenzuela, T.; Lord, S.R.; Delbaere, K. A kinect and inertial sensor-based system for the self-assessment of fall risk: A home-based study in older people. Hum.–Comput. Interact. 2016, 31, 261–293. [Google Scholar] [CrossRef]
- Zhong, R.; Rau, P.-L.P. Are cost-effective technologies feasible to measure gait in older adults? A systematic review of evidence-based literature. Arch. Gerontol. Geriatr. 2020, 87, 103970. [Google Scholar] [CrossRef]
- Bower, K.; Thilarajah, S.; Pua, Y.-H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. Neuroeng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Yogev Seligmann, G. Validity of the kinect for gait assessment: A focused review. Sensors 2016, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Mentiplay, B.F.; Hough, E.; Pua, Y.H. Three-dimensional cameras and skeleton pose tracking for physical function assessment: A review of uses, validity, current developments and Kinect alternatives. Gait Posture 2019, 68, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.K. 5—Validity, in Rehabilitation Outcome Measures; Stokes, E.K., Ed.; Churchill Livingstone: Edinburgh, UK, 2011; pp. 35–46. [Google Scholar]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Pua, Y.-H.; McGaw, R.; Heywood, S.; Clark, R.A. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J. Biomech. 2015, 48, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.; Llorens, R.; Colomer, C.; Alcañiz, M. Reliability and comparison of Kinect-based methods for estimating spatiotemporal gait parameters of healthy and post-stroke individuals. J. Biomech. 2018, 72, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Barreira, C.C.; Forner-Cordero, A.; Grangeiro, P.M.; Moura, R.T. Kinect v2 based system for gait assessment of children with cerebral palsy in rehabilitation settings. J. Med. Eng. 2020, 44, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Sutherland, D.H. The evolution of clinical gait analysis: Part II Kinematics. Gait Posture 2002, 16, 159–179. [Google Scholar] [CrossRef]
- Mackey, A.H.; Walt, S.E.; Lobb, G.A.; Stott, N.S. Reliability of upper and lower limb three-dimensional kinematics in children with hemiplegia. Gait Posture 2005, 22, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; McGorry, R.W.; Chou, L.-S.; Lin, J.-H.; Chang, C.-C. Accuracy of the Microsoft Kinect™ for measuring gait parameters during treadmill walking. Gait Posture 2015, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J., Jr.; Richards, J.; Higginson, J. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Bruening, D.A.; Ridge, S.T. Automated event detection algorithms in pathological gait. Gait Posture 2014, 39, 472–477. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Oh, J.; Kuenze, C.; Signorile, J. Improved kinect-based spatiotemporal and kinematic treadmill gait assessment. Gait Posture 2017, 51, 77–83. [Google Scholar] [CrossRef]
- Summa, S.; Tartarisco, G.; Favetta, M.; Buzachis, A.; Romano, A.; Bernava, G.; Sancesario, A.; Vasco, G.; Pioggia, G.; Petrarca, M. Validation of low-cost system for gait assessment in children with ataxia. Comput. Methods Programs Biomed. 2020, 196, 105705. [Google Scholar] [CrossRef]
- Summa, S.; Tartarisco, G.; Favetta, M.; Buzachis, A.; Romano, A.; Bernava, G.; Vasco, G.; Pioggia, G.; Petrarca, M.; Castelli, E. Spatio-temporal parameters of ataxia gait dataset obtained with the Kinect. Data in Brief 2020, 32, 106307. [Google Scholar] [CrossRef]
- Whittle, M.W. Three-dimensional motion of the center of gravity of the body during walking. Hum. Mov. Sci. 1997, 16, 347–355. [Google Scholar] [CrossRef]
- Gibson, T.; Jeffery, R.; Bakheit, A. Comparison of three definitions of the mid-stance and mid-swing events of the gait cycle in children. Disabil. Rehabil. 2006, 28, 625–628. [Google Scholar] [CrossRef]
- Fleiss, J.L. Design and Analysis of Clinical Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 73. [Google Scholar]
- Denegar, C.R.; Ball, D.W. Assessing reliability and precision of measurement: an introduction to intraclass correlation and standard error of measurement. J. Sport Rehabil. 1993, 2, 35–42. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. Strength. Cond. J. 2005, 19, 231–240. [Google Scholar]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Van Bloemendaal, M.; Beelen, A.; Kleissen, R.F.; Geurts, A.C.; Nollet, F.; Bus, S.A. Concurrent validity and reliability of a low-cost gait analysis system for assessment of spatiotemporal gait parameters. J. Rehabil. Med. 2019, 51, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, E.; Taati, B.; Mihailidis, A. Concurrent validity of the Microsoft Kinect for Windows v2 for measuring spatiotemporal gait parameters. Med. Eng. Phys. 2016, 38, 952–958. [Google Scholar] [CrossRef]
- Clark, R.A.; Vernon, S.; Mentiplay, B.F.; Miller, K.J.; McGinley, J.L.; Pua, Y.H.; Paterson, K.; Bower, K.J. Instrumenting gait assessment using the Kinect in people living with stroke: reliability and association with balance tests. J. Neuroeng. Rehabil. 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Otte, K.; Kayser, B.; Mansow-Model, S.; Verrel, J.; Paul, F.; Brandt, A.U.; Schmitz-Hübsch, T. Accuracy and reliability of the kinect version 2 for clinical measurement of motor function. PLoS ONE 2016, 11, e0166532. [Google Scholar] [CrossRef]
- Grobelny, A.; Behrens, J.R.; Mertens, S.; Otte, K.; Mansow-Model, S.; Kruger, T.; Gusho, E.; Bellmann-Strobl, J.; Paul, F.; Brandt, A.U.; et al. Maximum walking speed in multiple sclerosis assessed with visual perceptive computing. PLoS ONE 2017, 12, e0189281. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.; Colomer, C.; Alcañiz, M.; Llorens, R. Gait analysis with the Kinect v2: normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J. Neuroeng. Rehabil. 2019, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.; Sattler, I.; Otte, K.; Röhling, H.; Mansow-Model, S.; Müller-Werdan, U. Using new camera-based technologies for gait analysis in older adults in comparison to the established GAITRite system. Sensors 2020, 20, 125. [Google Scholar] [CrossRef]
- Wren, T.A.; Rethlefsen, S.; Kay, R.M. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. J. Pediatr. Orthop. 2005, 25, 79–83. [Google Scholar] [PubMed]
- Auvinet, E.; Multon, F.; Manning, V.; Meunier, J.; Cobb, J. Validity and sensitivity of the longitudinal asymmetry index to detect gait asymmetry using Microsoft Kinect data. Gait Posture 2017, 51, 162–168. [Google Scholar] [CrossRef]
- Auvinet, E.; Multon, F.; Aubin, C.-E.; Meunier, J.; Raison, M. Detection of gait cycles in treadmill walking using a Kinect. Gait Posture 2015, 41, 722–725. [Google Scholar] [CrossRef]
- Motiian, S.; Pergami, P.; Guffey, K.; Mancinelli, C.A.; Doretto, G. Automated extraction and validation of children’s gait parameters with the Kinect. Biomed. Eng. Online 2015, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kuenze, C.; Jacopetti, M.; Signorile, J.F.; Eltoukhy, M. Validity of the microsoft kinect™ in assessing spatiotemporal and lower extremity kinematics during stair ascent and descent in healthy young individuals. Med. Eng. Phys. 2018, 60, 70–76. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.; van Dijsseldonk, R.; Keijsers, N.; Groen, B. Test-retest reliability of stability outcome measures during treadmill walking in patients with balance problems and healthy controls. Gait Posture 2020, 76, 92–97. [Google Scholar] [CrossRef]
- Redekop, S.; Andrysek, J.; Wright, V. Single-session reliability of discrete gait parameters in ambulatory children with cerebral palsy based on GMFCS level. Gait Posture 2008, 28, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Virtual reality enhances gait in cerebral palsy: A training dose-response meta-analysis. Front. Neurol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Dumas, R.; Cheze, L.; Verriest, J.-P. Adjustments to McConville et al. and Young et al. body segment inertial parameters. J. Biomech. 2007, 40, 543–553. [Google Scholar] [CrossRef]
- Devetak, G.F.; Bohrer, R.C.D.; Rodacki, A.L.F.; Manffra, E.F. Center of mass in analysis of dynamic stability during gait following stroke: A systematic review. Gait Posture 2019, 72, 154–166. [Google Scholar] [CrossRef]
- González, A.; Hayashibe, M.; Bonnet, V.; Fraisse, P. Whole body center of mass estimation with portable sensors: Using the statically equivalent serial chain and a kinect. Sensors 2014, 14, 16955–16971. [Google Scholar] [CrossRef]
- Skals, S.; Rasmussen, K.P.; Bendtsen, K.M.; Yang, J.; Andersen, M.S. A musculoskeletal model driven by dual Microsoft Kinect Sensor data. Multibody Syst. Dyn. 2017, 41, 297–316. [Google Scholar] [CrossRef]
- Jansi, R.; Amutha, R. Detection of fall for the elderly in an indoor environment using a tri-axial accelerometer and Kinect depth data. Multidimens. Syst. Signal Process. 2020, 31, 1207–1225. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Salgado, D.P.; Catháin, C.Ó.; O’Connor, N.; Murray, N. Human gait assessment using a 3D marker-less multimodal motion capture system. Multimed. Tools Appl. 2020, 79, 2629–2651. [Google Scholar] [CrossRef]
- Albert, J.A.; Owolabi, V.; Gebel, A.; Brahms, C.M.; Granacher, U.; Arnrich, B. Evaluation of the Pose Tracking Performance of the Azure Kinect and Kinect v2 for Gait Analysis in Comparison with a Gold Standard: A Pilot Study. Sensors 2020, 20, 5104. [Google Scholar] [CrossRef] [PubMed]
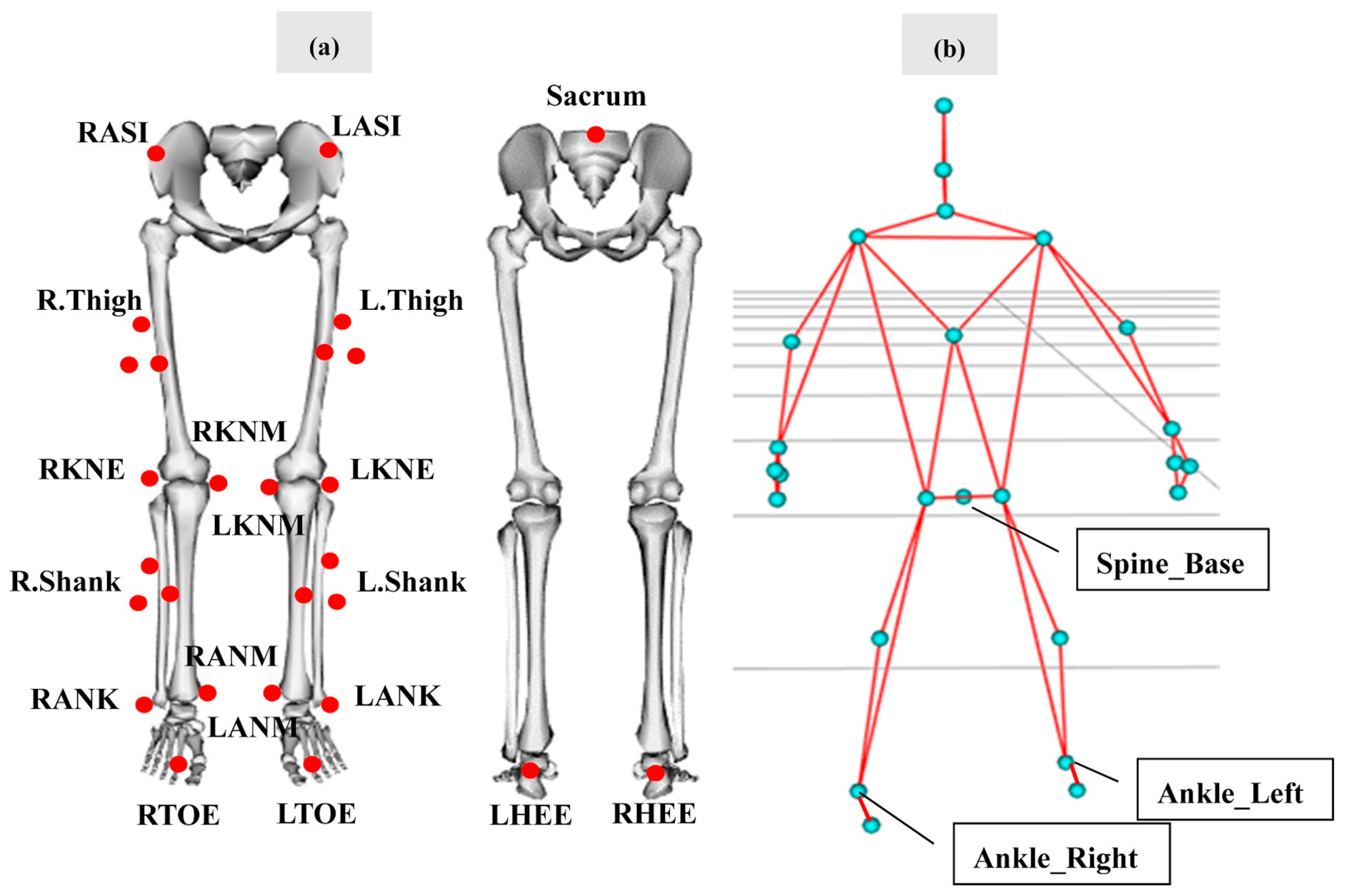
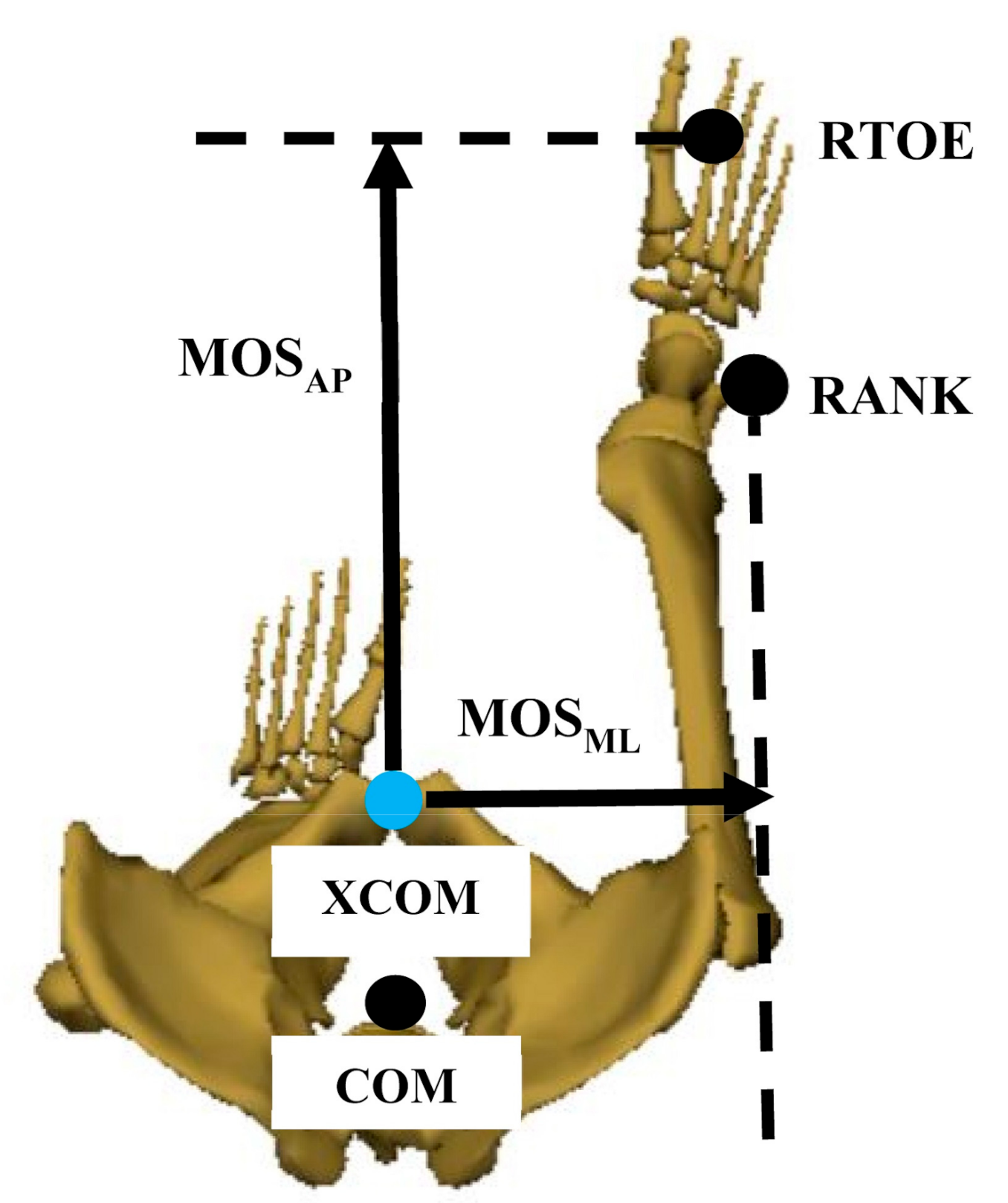
| Motion Analysis | Kinect V2 | |
|---|---|---|
| Step Length (m) | Distance between the heel markers at the left and right foot strike | Distance between the ankles at the left and right foot strike |
| Stride Length (m) | Distance between RHEEs at the two consecutive right foot strike | Distance between the “ankle right” markers at the two consecutive right foot strike |
| Step Width (m) | Orthogonal distance from the LHEE to the vector formed by RHEEs in two consecutive foot strike | Orthogonal distance from the left ankles to the vector formed by the right ankles in two consecutive foot strike |
| Gait Speed (m/s) | Mean resultant velocity of the COM during the gait cycle | Mean resultant velocity of the “spine base” marker during the gait cycle |
| Step Time (s) | The time between the left and right foot strike | As per the Motion Analysis |
| Stride Time (s) | The time between two consecutive right foot strike | As per the Motion Analysis |
| Motion Analysis | ||
|---|---|---|
| Step Length (m) | (1) | |
| Stride Length (m) | (2) | |
| Step Width (m) | (3) | |
| Gait Speed (m/s) | (4) | |
| Step Time (s) | (5) | |
| Stride Time (s) | (6) |
| Motion Analysis | Kinect | ICC2,k | SEM | Relative Errors (%) | |
|---|---|---|---|---|---|
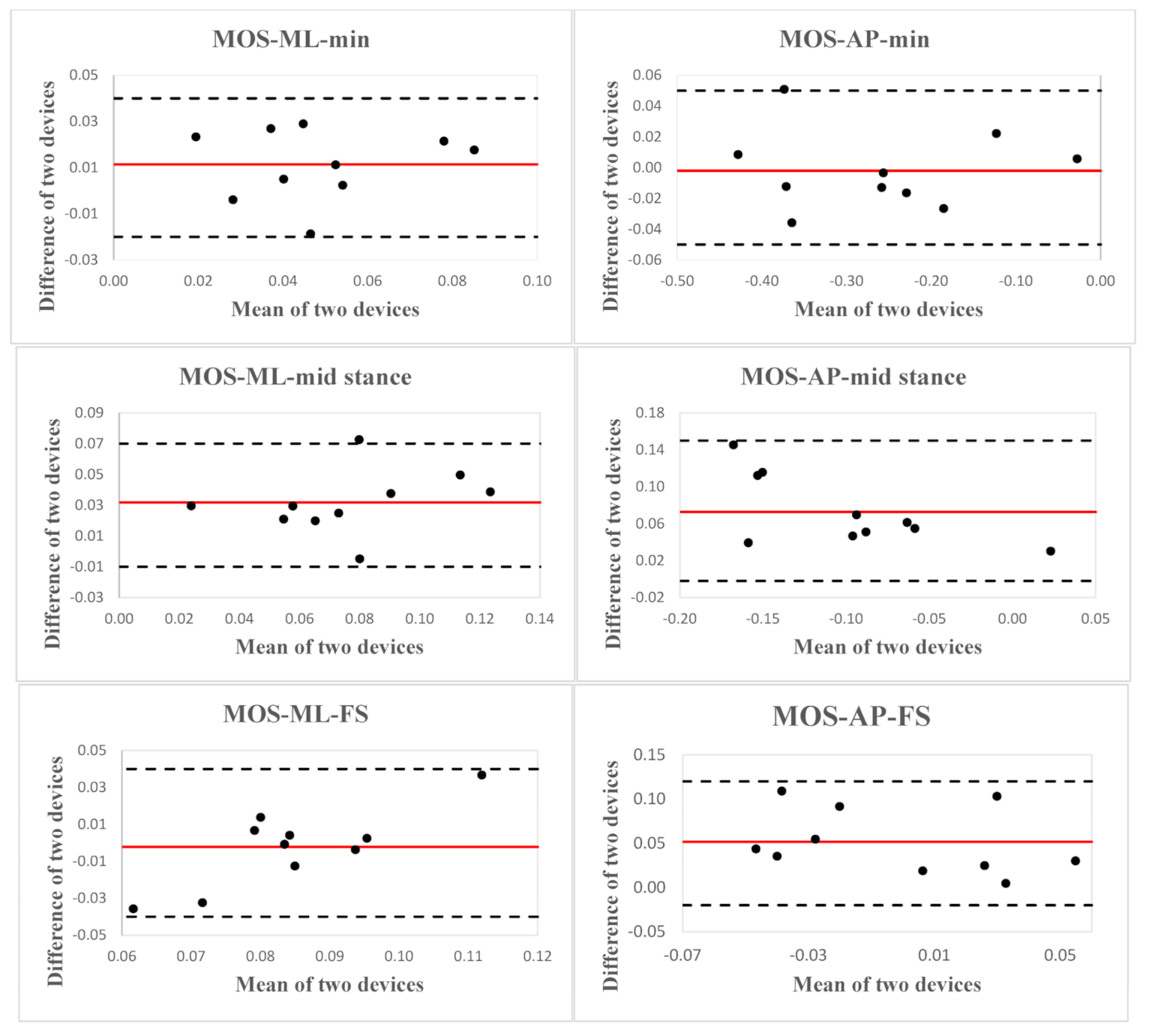
| MOS-ML-min (m) | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.81 (0.20, 0.95) | 0.004 | 32.04 ± 23.06 |
| MOS-ML-mid stance (m) | 0.09 ± 0.03 | 0.06 ± 0.03 | 0.68 (−0.25, 0.93) | 0.01 | 37.06 ± 19.61 |
| MOS-ML-FS (m) | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.42 (−1.78, 0.86) | 0.004 | 22.06 ± 27.15 |
| MOS-AP-min (m) | −0.26 ± 0.13 | −0.26 ± 0.13 | 0.99 (0.96, 1.00) | 0.03 | 9.83 ± 7.51 |
| MOS-AP-mid stance (m) | −0.06 ± 0.05 | −0.14 ± 0.07 | 0.66 (−0.21, 0.92) | 0.06 | 112.82 ± 50.42 |
| MOS-AP-FS (m) | 0.02 ± 0.03 | −0.03 ± 0.05 | 0.51 (−0.29, 0.86) | 0.03 | 186.40 ± 163.49 |
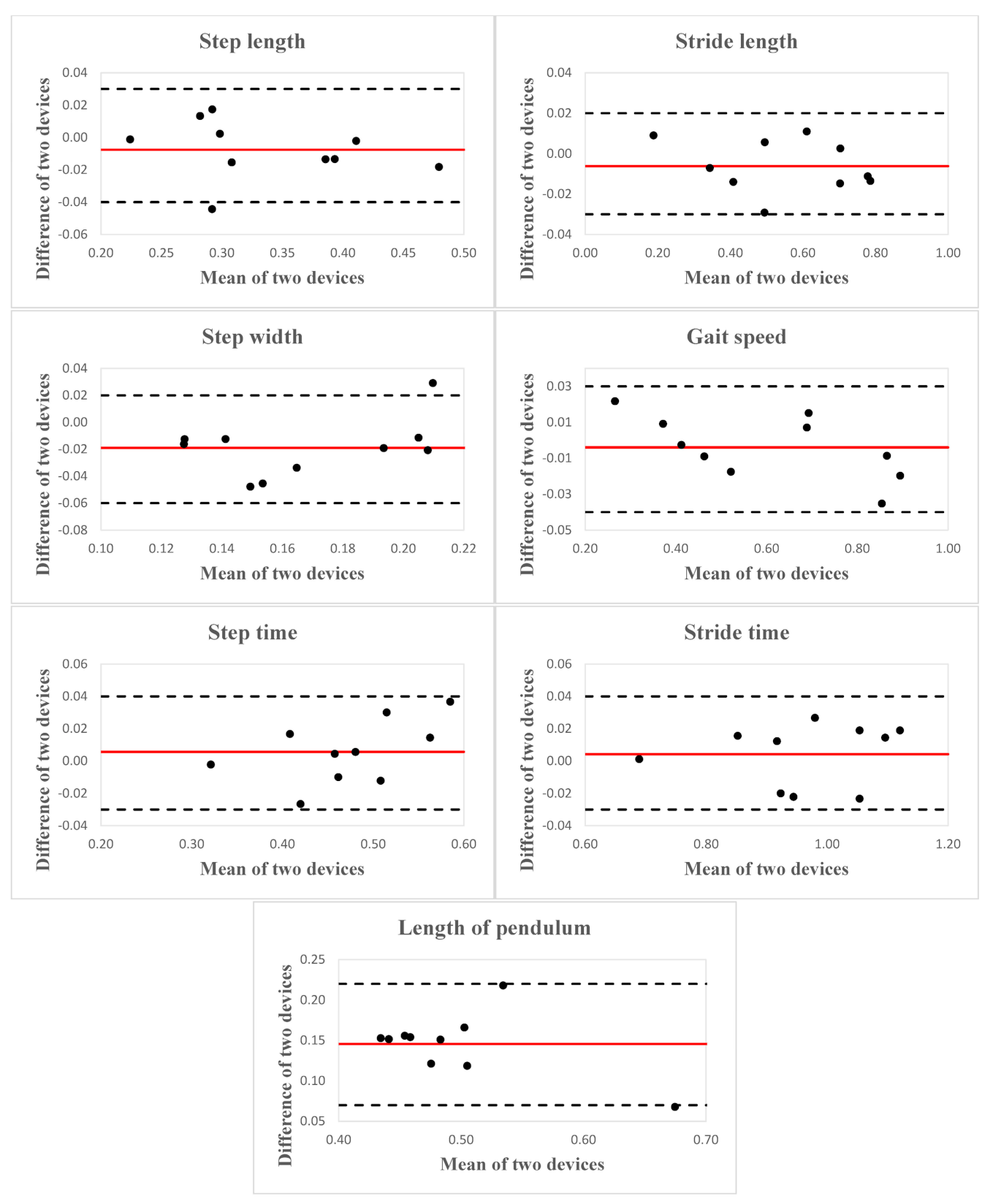
| Step Length (m) | 0.33 ± 0.08 | 0.34 ± 0.08 | 0.99 (0.94, 0.99) | 0.01 | 4.47 ± 4.62 |
| Stride Length (m) | 0.55 ± 0.20 | 0.55 ± 0.20 | 0.99 (0.99, 0.99) | 0.02 | 2.49 ± 1.75 |
| Step Width (m) | 0.16 ± 0.04 | 0.18 ± 0.03 | 0.83 (0.17, 0.96) | 0.01 | 16.81 ± 11.24 |
| Gait Speed (m/s) | 0.60 ± 0.22 | 0.60 ± 0.23 | 0.99 (0.99, 0.99) | 0.03 | 2.69 ± 2.12 |
| Step Time (s) | 0.47 ± 0.08 | 0.47 ± 0.07 | 0.98 (0.94, 0.99) | 0.01 | 3.23 ± 2.21 |
| Stride Time (s) | 0.97 ± 0.13 | 0.96 ± 0.13 | 0.99 (0.98, 0.99) | 0.02 | 1.75 ± 0.72 |
| L (m) | 0.57 ± 0.06 | 0.42 ± 0.08 | 0.45 (−0.07, 0.85) | 0.14 | 25.97 ± 6.84 |
| Parameter | Mean Difference | LoA | Lower LoA | Upper LoA |
|---|---|---|---|---|
| MOS-ML-min (m) | 0.01 | 0.03 | −0.02 | 0.04 |
| MOS-ML-mid stance (m) | 0.03 | 0.04 | −0.01 | 0.07 |
| MOS-ML-FS (m) | −0.002 | 0.04 | −0.04 | 0.04 |
| MOS-AP-min (m) | −0.002 | 0.05 | −0.05 | 0.05 |
| MOS-AP-mid stance (m) | 0.07 | 0.08 | −0.002 | 0.15 |
| MOS-AP-FS (m) | 0.05 | 0.07 | −0.02 | 0.12 |
| Step Length (m) | −0.01 | 0.03 | −0.04 | 0.03 |
| Stride Length (m) | −0.01 | 0.03 | −0.03 | 0.02 |
| Step Width (m) | −0.02 | 0.04 | −0.06 | 0.02 |
| Gait Speed (m/s) | −0.004 | 0.03 | −0.04 | 0.03 |
| Step Time (s) | 0.01 | 0.04 | −0.03 | 0.04 |
| Stride Time (s) | 0.004 | 0.04 | −0.03 | 0.04 |
| L (m) | 0.15 | 0.08 | 0.07 | 0.22 |
| Day 1 | Day 2 | ICC2,k | SEM | |
|---|---|---|---|---|
| MOS-ML-min (m) | 0.03 ± 0.03 | 0.02 ± 0.03 | 0.63 (−0.22, 0.90) | 0.01 |
| MOS-ML-mid stance (m) | 0.05 ± 0.04 | 0.03 ± 0.02 | 0.28 (−1.47, 0.81) | 0.01 |
| MOS-ML-FS (m) | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.56 (−1.01, 0.89) | 0.01 |
| MOS-AP-min (m) | −0.22 ± 0.11 | −0.31 ± 0.10 | 0.64 (−0.24, 0.91) | 0.15 |
| MOS-AP-mid stance (m) | −0.17 ± 0.06 | −0.14 ± 0.07 | 0.69 (−0.06, 0.92) | 0.05 |
| MOS-AP-FS (m) | −0.03 ± 0.07 | 0.01 ± 0.05 | 0.52 (−0.54, 0.87) | 0.05 |
| Step Length (m) | 0.40 ± 0.08 | 0.40 ± 0.10 | 0.82 (0.23, 0.96) | 0.06 |
| Stride Length (m) | 0.71 ± 0.19 | 0.70 ± 0.17 | 0.83 (0.27, 0.96) | 0.24 |
| Step Width (m) | 0.17 ± 0.04 | 0.16 ± 0.04 | 0.88 (0.56, 0.97) | 0.01 |
| Gait Speed (m/s) | 0.76 ± 0.23 | 0.76 ± 0.25 | 0.78 (0.06, 0.95) | 0.49 |
| Step Time (s) | 0.49 ± 0.09 | 0.48 ± 0.06 | 0.85 (0.41, 0.96) | 0.04 |
| Stride Time (s) | 1.00 ± 0.15 | 0.96 ± 0.13 | 0.82 (0.35, 0.96) | 0.15 |
| L (m) | 0.44 ± 0.05 | 0.43 ± 0.04 | 0.92 (0.69, 0.98) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Mithraratne, K.; Wilson, N.; Zhang, Y.; Wang, X. Kinect V2-Based Gait Analysis for Children with Cerebral Palsy: Validity and Reliability of Spatial Margin of Stability and Spatiotemporal Variables. Sensors 2021, 21, 2104. https://doi.org/10.3390/s21062104
Ma Y, Mithraratne K, Wilson N, Zhang Y, Wang X. Kinect V2-Based Gait Analysis for Children with Cerebral Palsy: Validity and Reliability of Spatial Margin of Stability and Spatiotemporal Variables. Sensors. 2021; 21(6):2104. https://doi.org/10.3390/s21062104
Chicago/Turabian StyleMa, Yunru, Kumar Mithraratne, Nichola Wilson, Yanxin Zhang, and Xiangbin Wang. 2021. "Kinect V2-Based Gait Analysis for Children with Cerebral Palsy: Validity and Reliability of Spatial Margin of Stability and Spatiotemporal Variables" Sensors 21, no. 6: 2104. https://doi.org/10.3390/s21062104
APA StyleMa, Y., Mithraratne, K., Wilson, N., Zhang, Y., & Wang, X. (2021). Kinect V2-Based Gait Analysis for Children with Cerebral Palsy: Validity and Reliability of Spatial Margin of Stability and Spatiotemporal Variables. Sensors, 21(6), 2104. https://doi.org/10.3390/s21062104






