An Inkjet Printed Flexible Electrocorticography (ECoG) Microelectrode Array on a Thin Parylene-C Film
Abstract
:1. Introduction
2. Materials and Methods
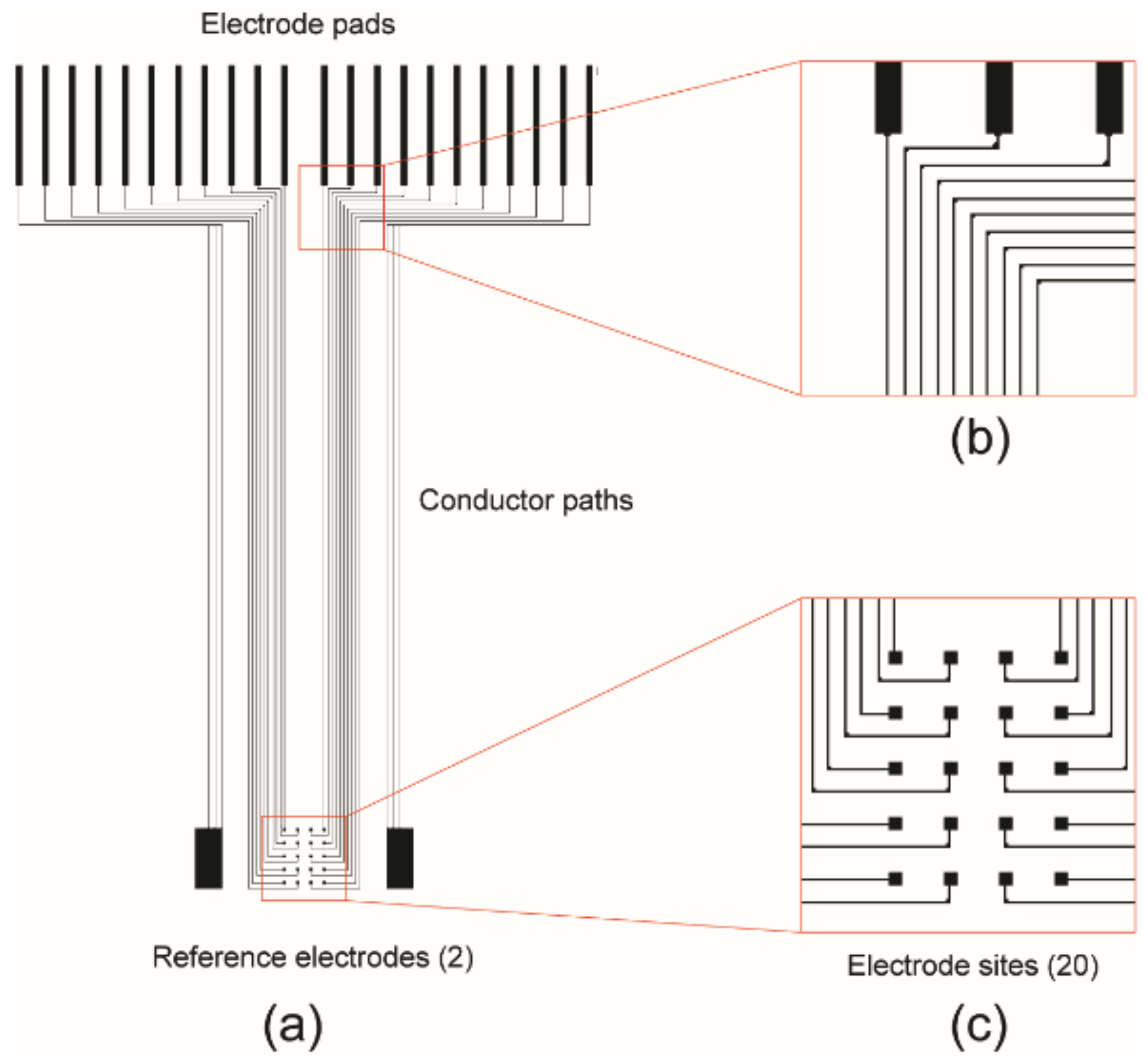
2.1. ECoG Electrode Design and Inkjet Printing
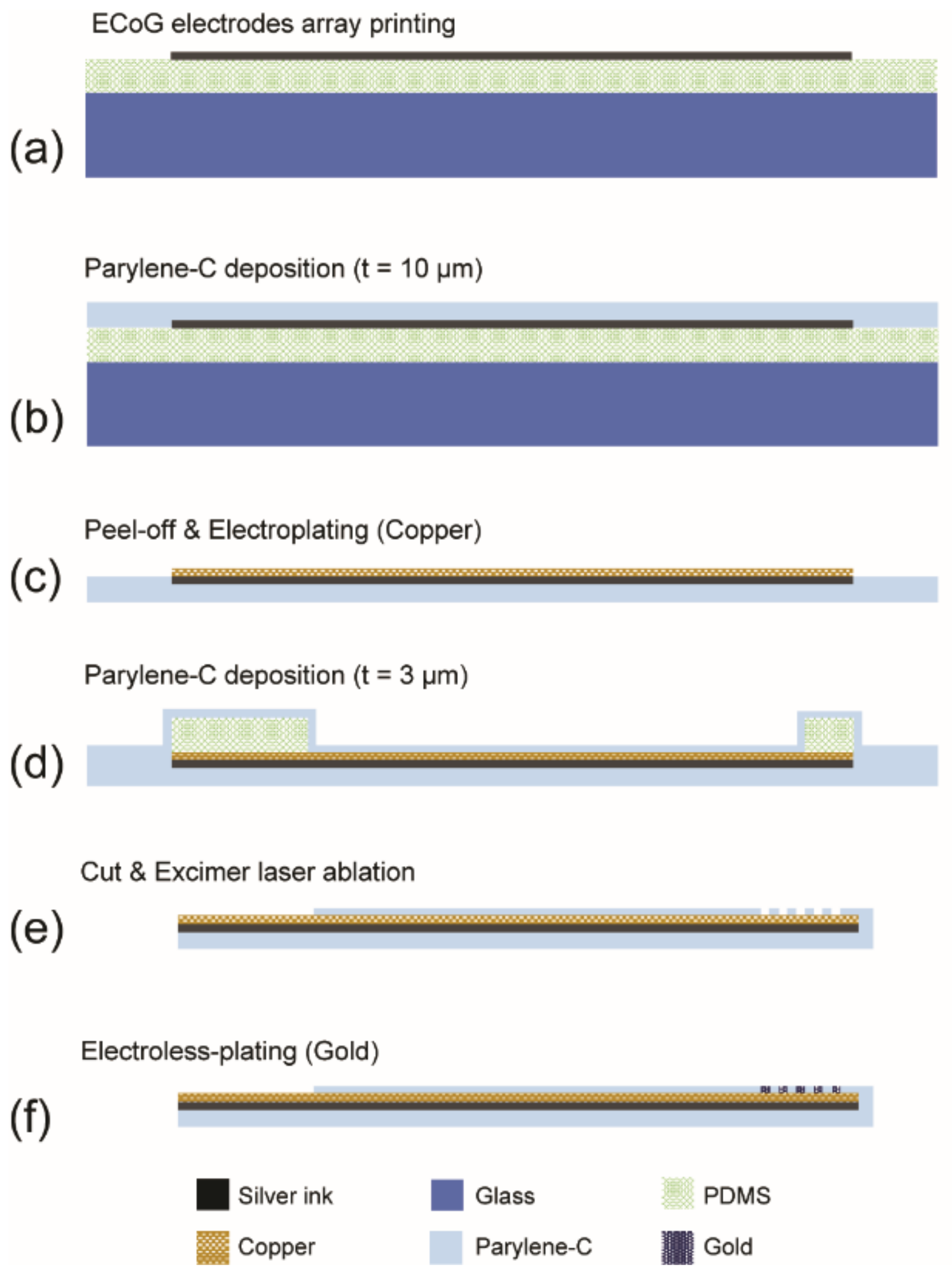
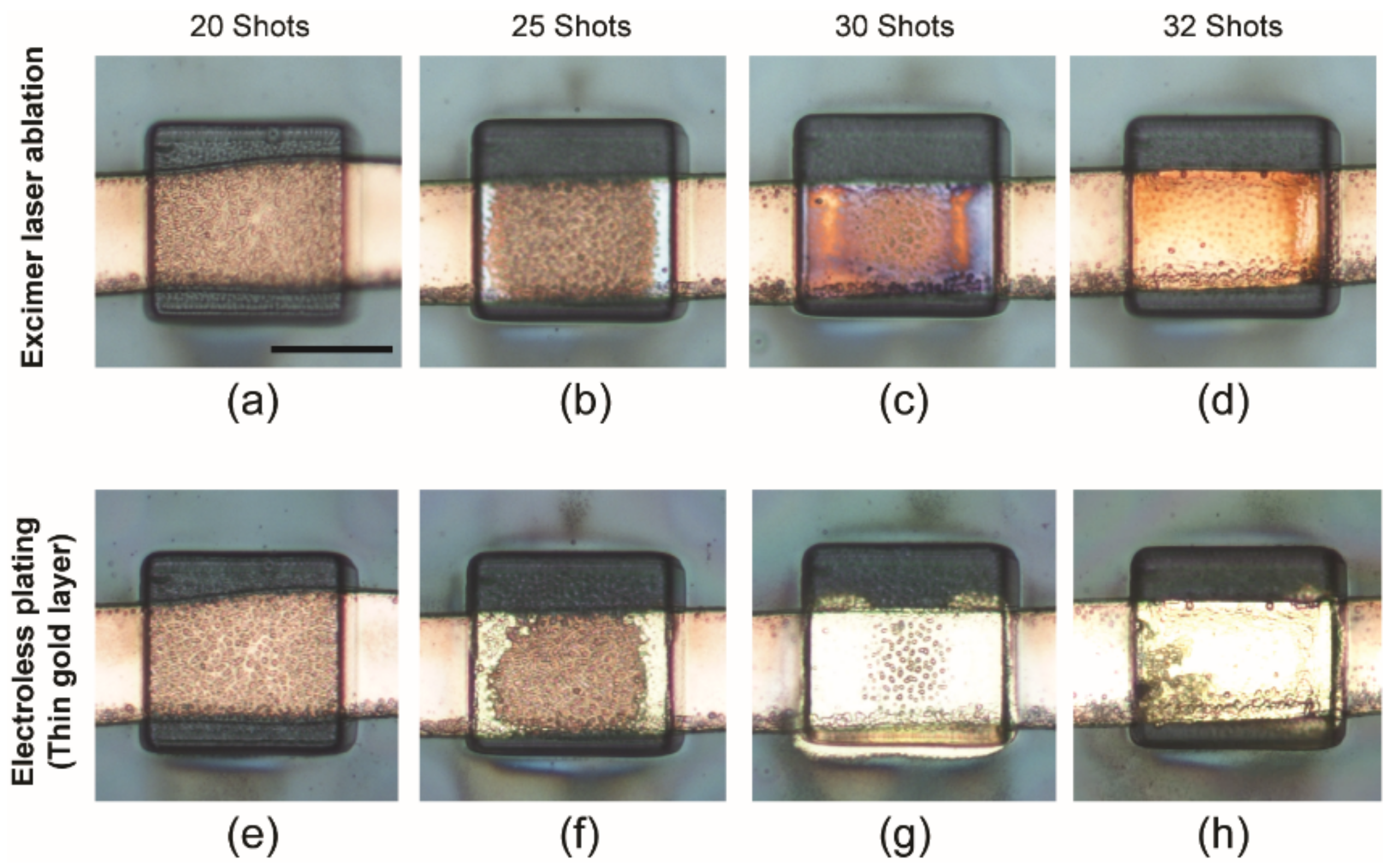
2.2. Fabrication of Thin Flexible Parylene ECoG Electrode Array
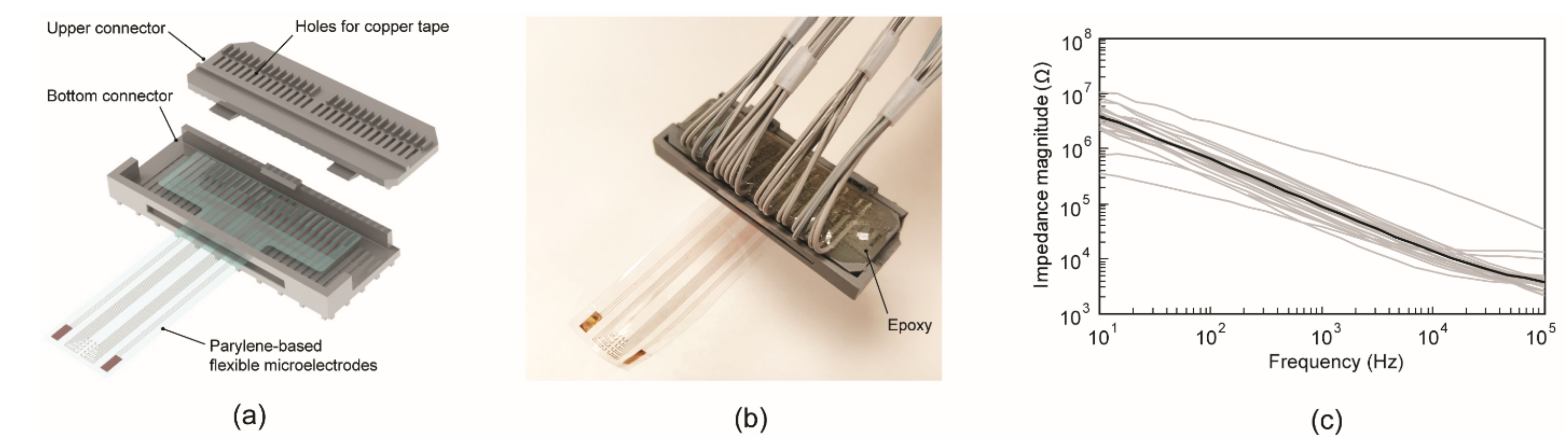
2.3. Electrical Connector and In Vitro Characterization of ECoG Electrode Array
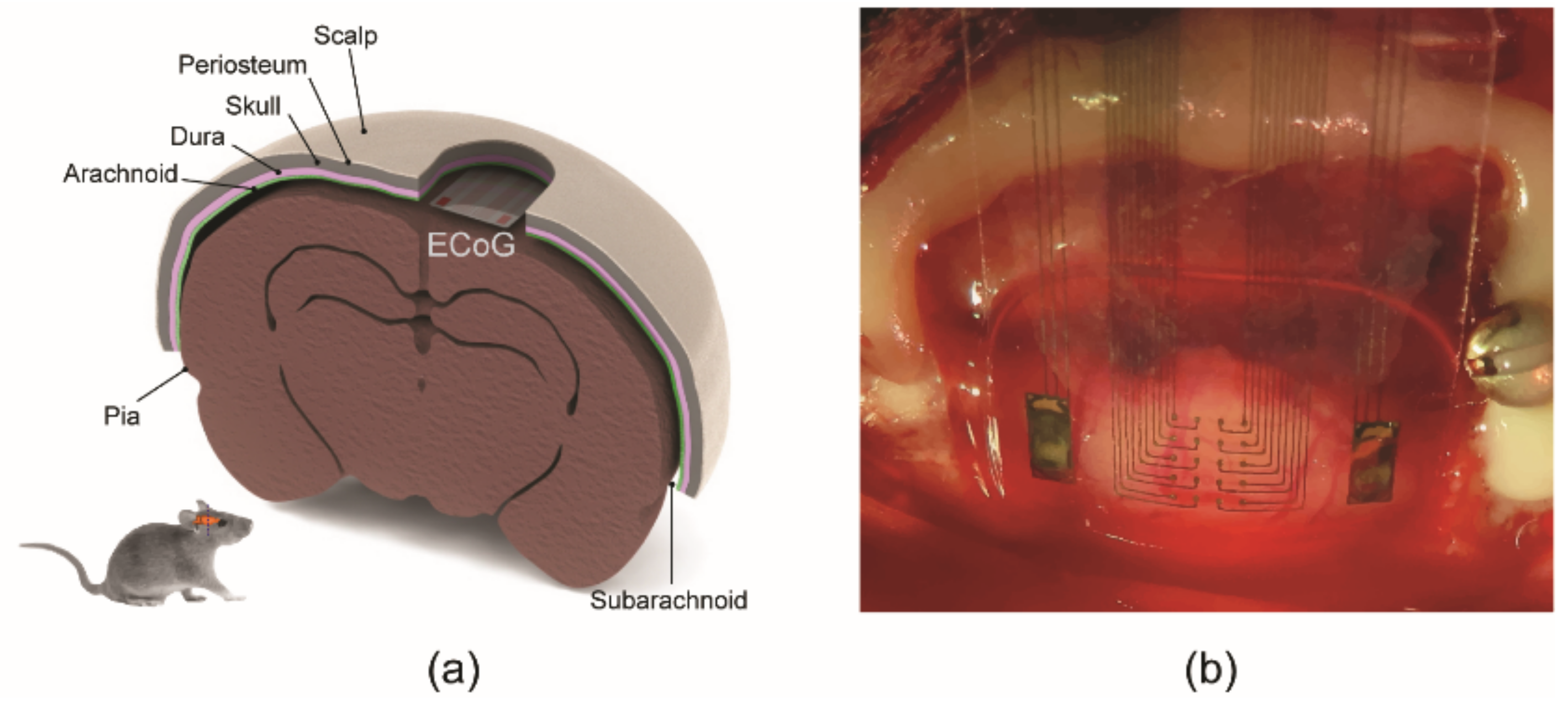
2.4. In Vivo Testing of ECoG Electrode Array in a Rat
3. Results and Discussion
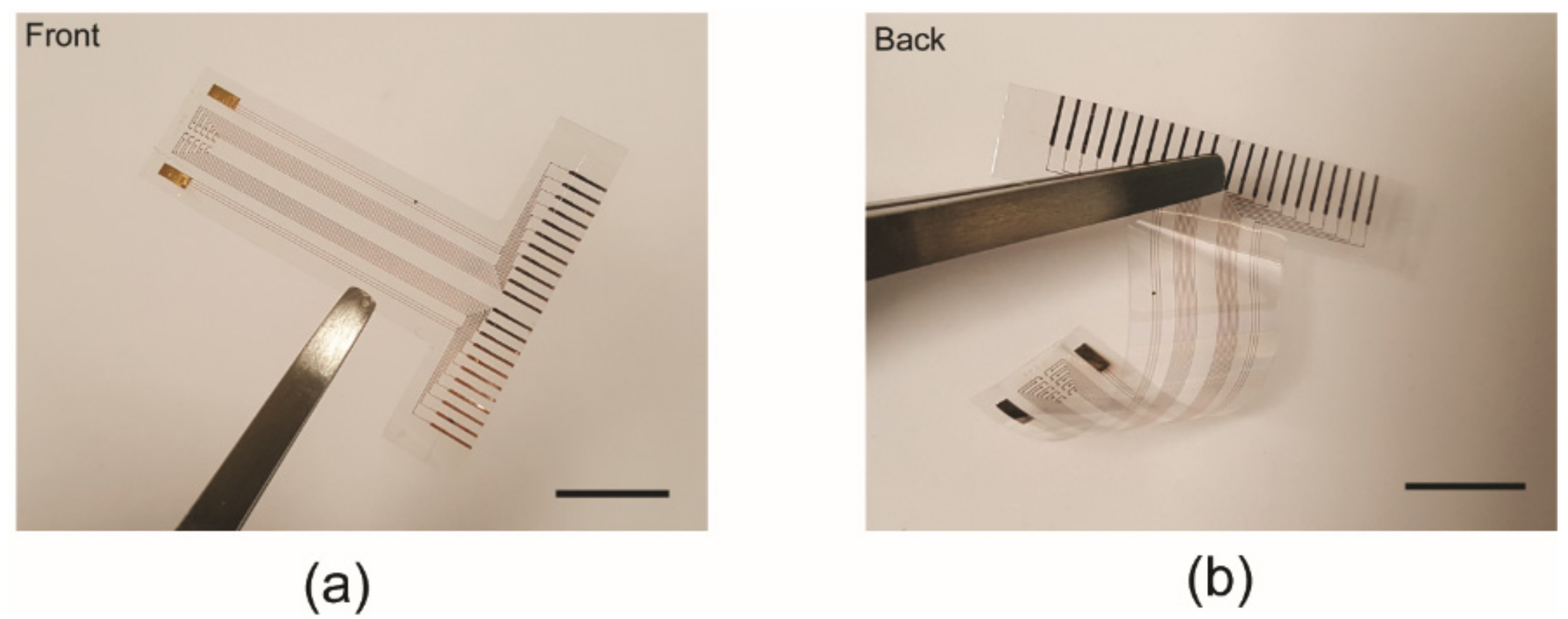
3.1. Fabricated Device (Thin Flexible Parylene ECoG Electrode Array)
3.2. Connector Assembly and Impedance Measurement In Vitro
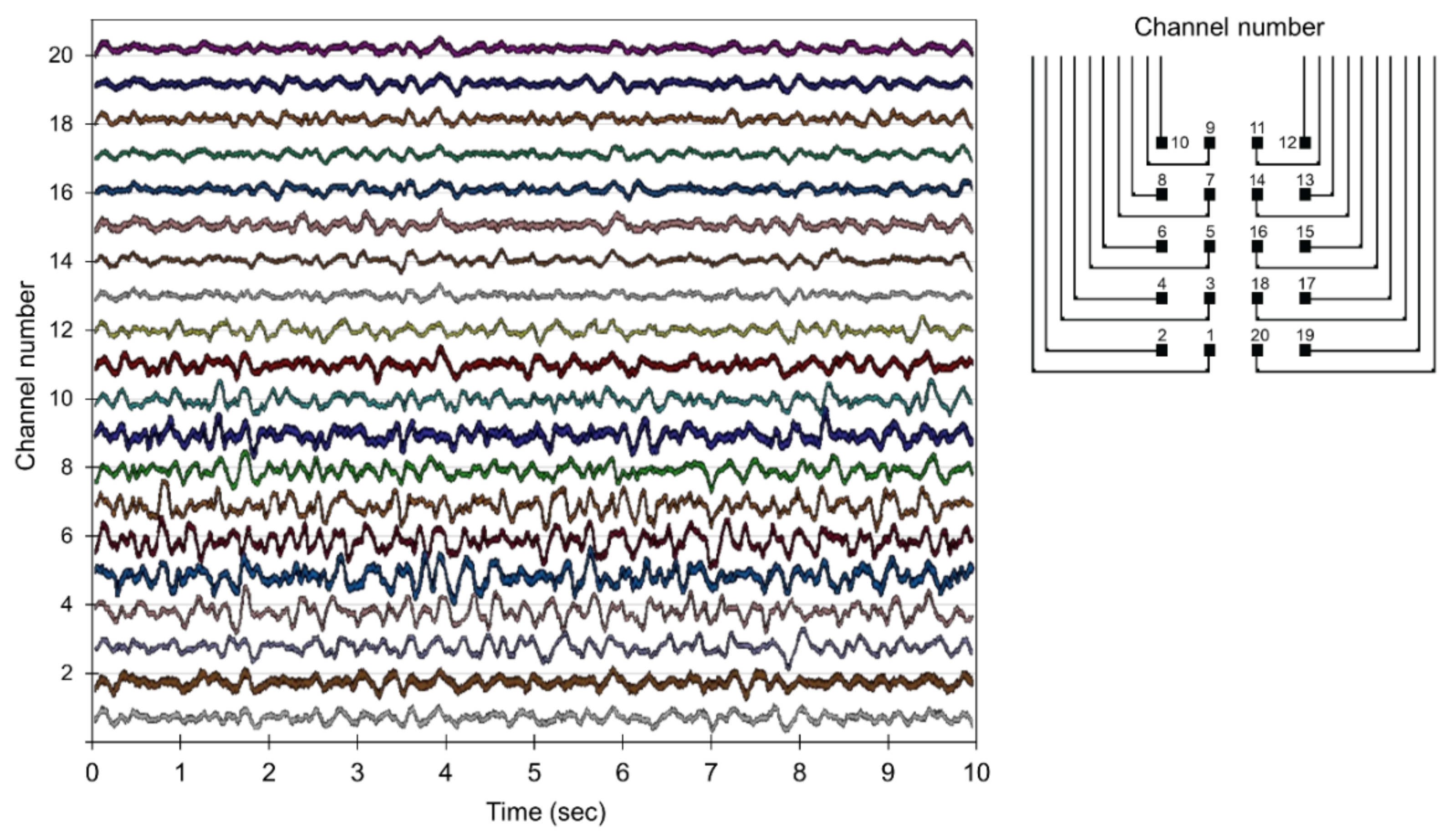
3.3. Local Field Potentials Recording by the Implanted Parylene ECoG Electrode Array
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z.; Pongrácz, A. Multifunctional soft implants to monitor and control neural activity in the central and peripheral nervous system: A review. Sens. Actuators B Chem. 2017, 243, 1214–1223. [Google Scholar] [CrossRef]
- Yang, W.; Gong, Y.; Yao, C.-Y.; Shrestha, M.; Jia, Y.; Qiu, Z.; Fan, Q.H.; Weber, A.; Li, W. A fully transparent, flexible PEDOT:PSS–ITO–Ag–ITO based microelectrode array for ECoG recording. Lab. Chip. 2021, 21, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Jeong, U.-J.; Lee, J.; Chou, N.; Kim, K.; Shin, H.; Chae, U.; Yu, H.-Y.; Cho, I.-J. A minimally invasive flexible electrode array for simultaneous recording of ECoG signals from multiple brain regions. Lab. Chip. 2021, 21, 2383–2397. [Google Scholar] [CrossRef] [PubMed]
- Schander, A.; Strokov, S.; Stemmann, H.; Tebmann, T.; Kreiter, A.K.; Lang, W. A Flexible 202-Channel Epidural ECoG Array with PEDOT: PSS Coated Electrodes for Chronic Recording of the Visual Cortex. IEEE Sens. J. 2019, 19, 820–825. [Google Scholar] [CrossRef]
- Kaiju, T.; Inoue, M.; Hirata, M.; Suzuki, T. High-density mapping of primate digit representations with a 1152-channel µeCoG array. J. Neural Eng. 2021, 18, 036025. [Google Scholar] [CrossRef] [PubMed]
- Slutzky, M.W.; Jordan, L.R.; Krieg, T.; Chen, M.; Mogul, D.J.; Miller, L.E. Optimal spacing of surface electrode arrays for brain-machine interface applications. J. Neural Eng. 2010, 7, 026004. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Marzullo, T.C.; Hooi, F.; Langhals, N.B.; Majewska, A.K.; Brown, E.B.; Kipke, D.R. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J. Neural Eng. 2010, 7, 046011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, M.B.; Pearce, S.M.; Ledbetter, N.M.; Warren, D.J.; Clark, G.A.; Tresco, P.A. The foreign body response to the Utah Slant Electrode Array in the cat sciatic nerve. Acta Biomater. 2014, 10, 4650–4660. [Google Scholar] [CrossRef]
- Muthuswamy, J.; Anand, S.; Sridharan, A. Adaptive movable neural interfaces for monitoring single neurons in the brain. Front. Neurosci. 2011, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Kimtan, T.; Thupmongkol, J.; Williams, J.C.; Thongpang, S. Printable and transparent micro-electrocorticography (μECoG) for optogenetic applications. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 482–485. [Google Scholar] [CrossRef]
- Chou, L.C.; Tsai, S.W.; Chang, W.L.; Chiou, J.C.; Chiu, T.W. A parylene-C based 16 channels flexible bio-electrode for ECoG recording. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 877–880. [Google Scholar] [CrossRef]
- Yamakawa, T.; Inoue, T.; Niwayama, M.; Oka, F.; Imoto, H.; Nomura, S.; Suzuki, M. Implantable Multi-Modality Probe for Subdural Simultaneous Measurement of Electrophysiology, Hemodynamics, and Temperature Distribution. IEEE Trans. Biomed. Eng. 2019, 66, 3204–3211. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.; Garcia-Cortadella, R.; Calia, A.B.; Mavredakis, N.; Illa, X.; Masvidal-Codina, E.; de la Cruz, J.; del Corro, E.; Rodríguez, L.; Prats-Alfonso, E.; et al. Improved metal-graphene contacts for low-noise, high-density microtransistor arrays for neural sensing. Carbon N. Y. 2020, 161, 647–655. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Hartings, J.; Ghosh, S.; Narayan, R.; Ahn, C. Polysilicon-based flexible temperature sensor for brain monitoring with high spatial resolution. J. Micromech. Microeng. 2017, 27, 6497–6500. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.C.; Renaud, P.; Tanila, H.; Djupsund, K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens. Bioelectron. 2007, 22, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, A.; Nguyen, J.K.; Capadona, J.R.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12, 036002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Wang, M.; Ge, C.; Xie, Z.; Guo, Z.; Hong, W.; Gu, X.; Wang, L.; Yi, Z.; Jiang, C.; et al. Flexible bioelectrodes with enhanced wrinkle microstructures for reliable electrochemical modification and neuromodulation in vivo. Biosens. Bioelectron. 2019, 135, 181–191. [Google Scholar] [CrossRef]
- Karlsson, J.M.; Haraldsson, T.; Carlborg, C.F.; Hansson, J.; Russom, A.; Van Der Wijngaart, W. Fabrication and transfer of fragile 3D PDMS microstructures. J. Micromech. Microeng. 2012, 22, 085009. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, G.T.; Ha, J.S. Simple patterning via adhesion between a buffered-oxide etchant-treated PDMS stamp and a SiO2 substrate. Adv. Funct. Mater. 2007, 17, 2125–2132. [Google Scholar] [CrossRef]
- Kim, Y.; Ren, X.; Kim, J.W.W.; Noh, H. Direct inkjet printing of micro-scale silver electrodes on polydimethylsiloxane (PDMS) microchip. J. Micromech. Microeng. 2014, 24, 115010. [Google Scholar] [CrossRef]
- Moon, J.-H.; Baek, D.H.; Choi, Y.Y.; Lee, K.H.; Kim, H.C.; Lee, S.-H. Wearable polyimide–PDMS electrodes for intrabody communication. J. Micromech. Microeng. 2010, 20, 025032. [Google Scholar] [CrossRef]
- Takeuchi, S.; Suzuki, T.; Mabuchi, K.; Fujita, H. 3D flexible multichannel neural probe array. J. Micromech. Microeng. 2004, 14, 104–107. [Google Scholar] [CrossRef]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef]
- Ochoa, M.; Wei, P.; Wolley, A.J.; Otto, K.J.; Ziaie, B. A hybrid PDMS-Parylene subdural multi-electrode array. Biomed. Microdevices 2013, 15, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Huang, X.; Liu, G.; Wang, W.; Dong, F.; Li, Z. Fabrication and characterization of a parylene-based three-dimensional microelectrode array for use in retinal prosthesis. J. Microelectromech. Syst. 2010, 19, 367–374. [Google Scholar] [CrossRef]
- Castagnola, V.; Descamps, E.; Lecestre, A.; Dahan, L.; Remaud, J.; Nowak, L.G.; Bergaud, C. Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens. Bioelectron. 2015, 67, 450–457. [Google Scholar] [CrossRef]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E.; et al. Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Rui, Y.; Liu, J.; Wang, Y.; Yang, C. Parylene-based implantable Pt-black coated flexible 3-D hemispherical microelectrode arrays for improved neural interfaces. Microsyst. Technol. 2011, 17, 437–442. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, R.; Chen, T.; Zhang, K.; Gui, J. 3D bioprinting modified autologous matrix-induced chondrogenesis(AMIC) technique for repair of cartilage defects. Mater. Des. 2021, 203, 109621. [Google Scholar] [CrossRef]
- Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Priedl, J.; Kasper, C.; Egger, D. 3D printing of cell culture devices: Assessment and prevention of the cytotoxicity of photopolymers for stereolithography. Materials 2020, 13, 3011. [Google Scholar] [CrossRef]
- Chen, L.; Deng, C.; Li, J.; Yao, Q.; Chang, J.; Wang, L.; Wu, C. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials 2019, 196, 138–150. [Google Scholar] [CrossRef]
- Wu, J.; Pang, H.; Ding, L.; Wang, Y.; He, X.; Shu, Q.; Xuan, S.; Gong, X. A lightweight, ultrathin aramid-based flexible sensor using a combined inkjet printing and buckling strategy. Chem. Eng. J. 2021, 421, 129830. [Google Scholar] [CrossRef]
- Hussin, H.; Soin, N.; Wan Muhamad Hatta, S.F.; Md Rezali, F.A.; Abdul Wahab, Y. Review—Recent Progress in the Diversity of Inkjet-Printed Flexible Sensor Structures in Biomedical Engineering Applications. J. Electrochem. Soc. 2021, 168, 077508. [Google Scholar] [CrossRef]
- Goel, P.; Singh, S.; Kaur, H.; Mishra, S.; Deep, A. Low-cost inkjet printing of metal–organic frameworks patterns on different substrates and their applications in ammonia sensing. Sens. Actuators B Chem. 2021, 329, 129157. [Google Scholar] [CrossRef]
- Lin, L.K.; Tsai, J.T.; DÃ-Az-Amaya, S.; Oduncu, M.R.; Zhang, Y.; Huang, P.Y.; Ostos, C.; Schmelzel, J.P.; Mohammadrahimi, R.; Xu, P.; et al. Antidelaminating, Thermally Stable, and Cost-Effective Flexible Kapton Platforms for Nitrate Sensors, Mercury Aptasensors, Protein Sensors, and p-Type Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2021, 13, 11369–11384. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.W.; Kim, J.; Noh, M. A novel fabrication method of Parylene-based microelectrodes utilizing inkjet printing. Sens. Actuators B Chem. 2017, 238, 862–870. [Google Scholar] [CrossRef]
- Liu, K.; NiCkolov, Z.; Oh, J.; “Moses” Noh, H. KrF excimer laser micromachining of MEMS materials: Characterization and applications. J. Micromech. Microeng. 2011, 22, 015012. [Google Scholar] [CrossRef]
- Liu, K.; Kim, Y.; Noh, H. (Moses) ArF Excimer Laser Micromachining of MEMS Materials: Characterization and Applications. J. Micro. Nano-Manuf. 2014, 2, 021006. [Google Scholar] [CrossRef]
- Rubehn, B.; Bosman, C.; Oostenveld, R.; Fries, P.; Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 2009, 6, 036003. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Alimperti, S.; Choi, P.; Noh, M. An Inkjet Printed Flexible Electrocorticography (ECoG) Microelectrode Array on a Thin Parylene-C Film. Sensors 2022, 22, 1277. https://doi.org/10.3390/s22031277
Kim Y, Alimperti S, Choi P, Noh M. An Inkjet Printed Flexible Electrocorticography (ECoG) Microelectrode Array on a Thin Parylene-C Film. Sensors. 2022; 22(3):1277. https://doi.org/10.3390/s22031277
Chicago/Turabian StyleKim, Yoontae, Stella Alimperti, Paul Choi, and Moses Noh. 2022. "An Inkjet Printed Flexible Electrocorticography (ECoG) Microelectrode Array on a Thin Parylene-C Film" Sensors 22, no. 3: 1277. https://doi.org/10.3390/s22031277
APA StyleKim, Y., Alimperti, S., Choi, P., & Noh, M. (2022). An Inkjet Printed Flexible Electrocorticography (ECoG) Microelectrode Array on a Thin Parylene-C Film. Sensors, 22(3), 1277. https://doi.org/10.3390/s22031277





