Stain Style Transfer for Histological Images Using S3CGAN
Abstract
:1. Introduction
2. Related Work
2.1. Conventional Color Normalization
2.2. Color Normalization Methods Based on GANs
3. Proposed Method
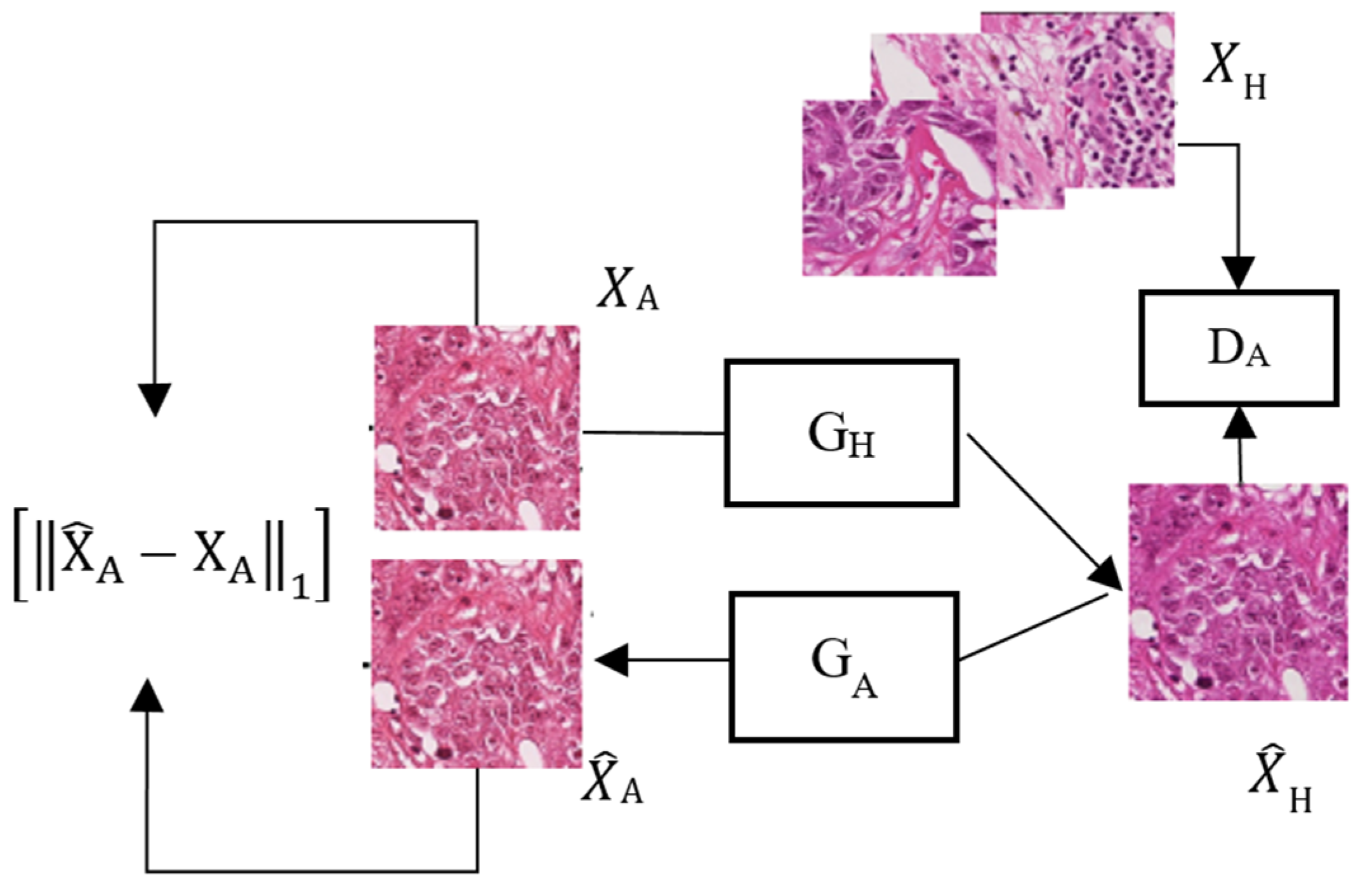
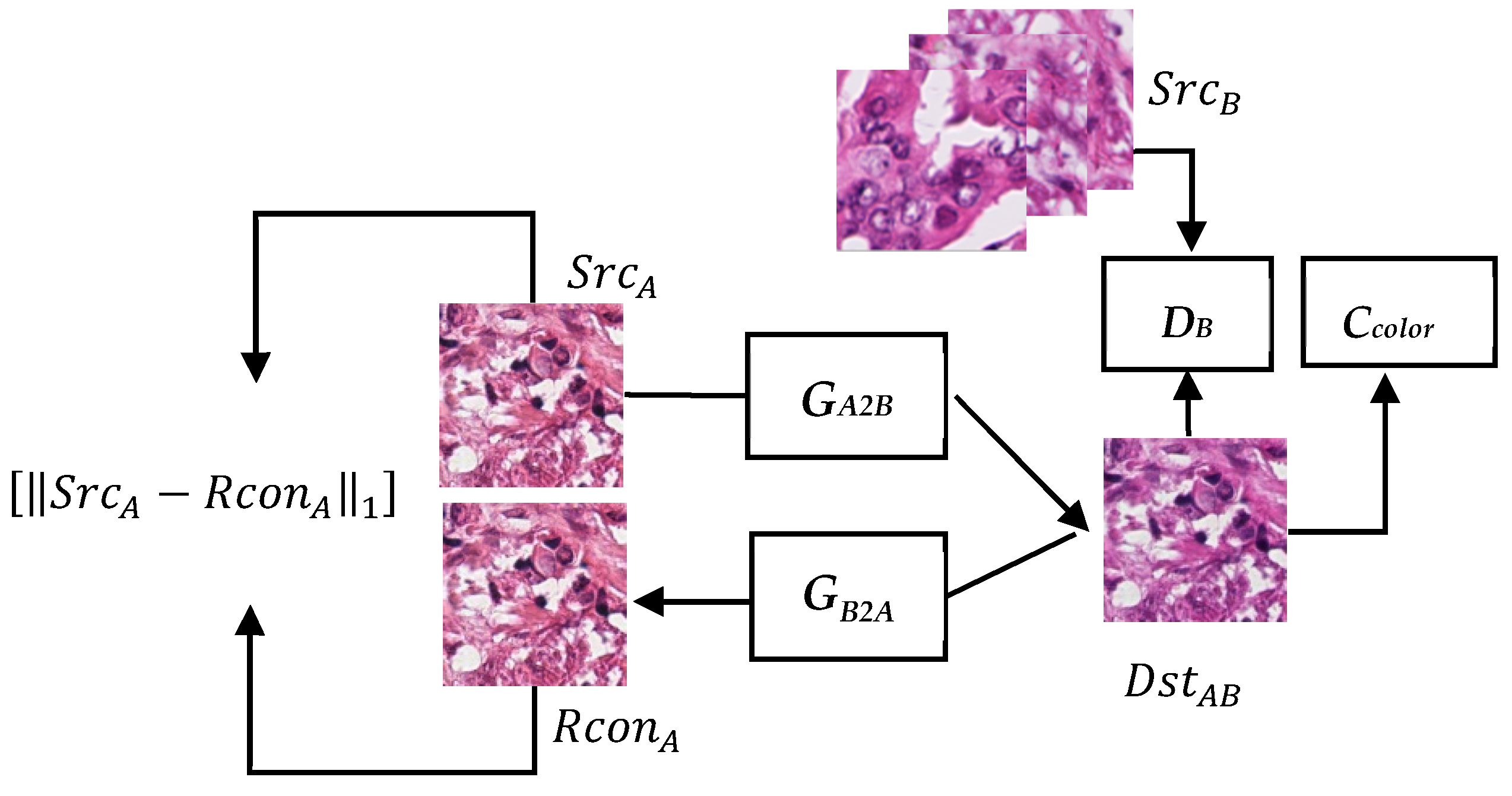
3.1. System Architecture
3.2. Generator
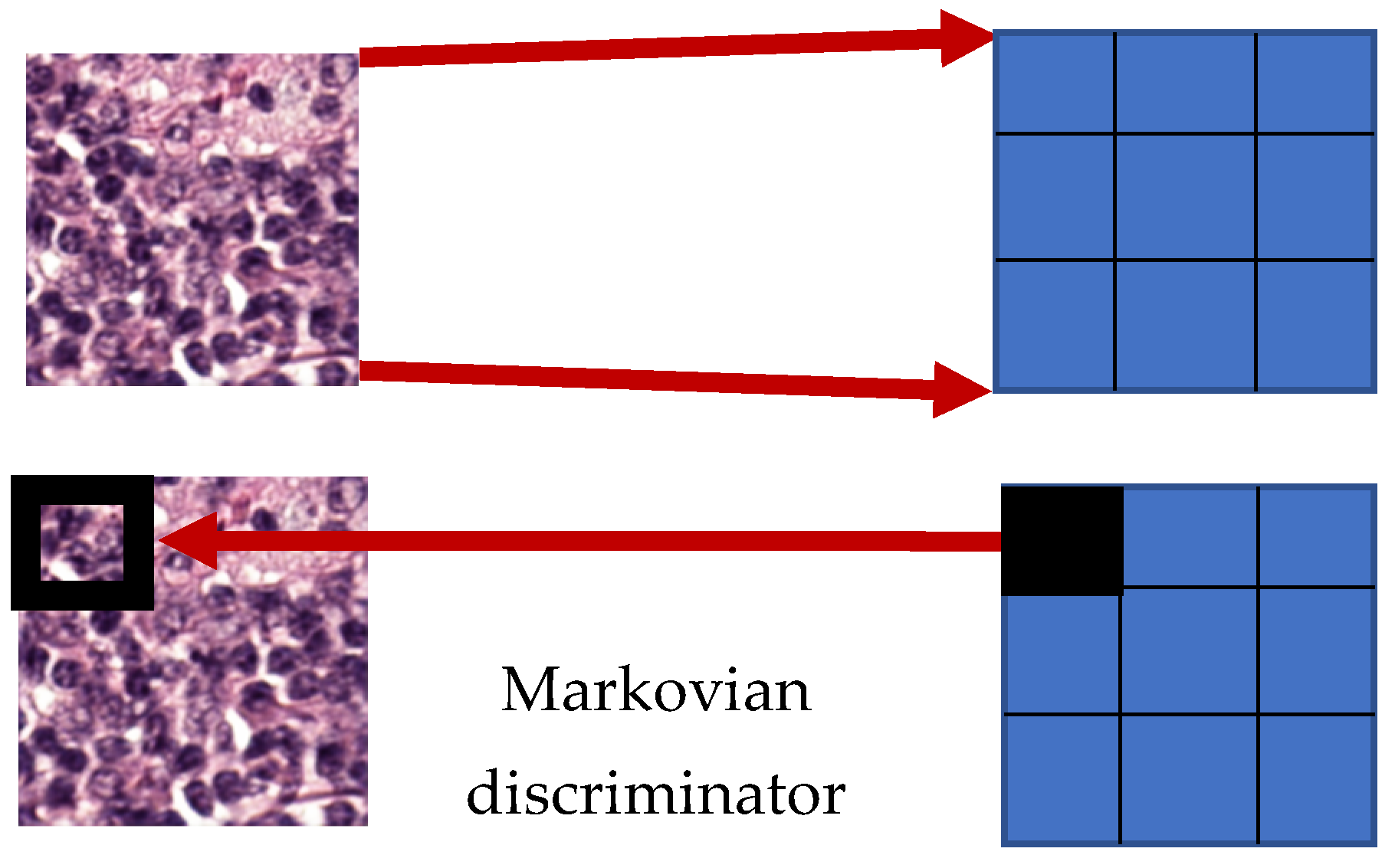
3.3. Discriminator
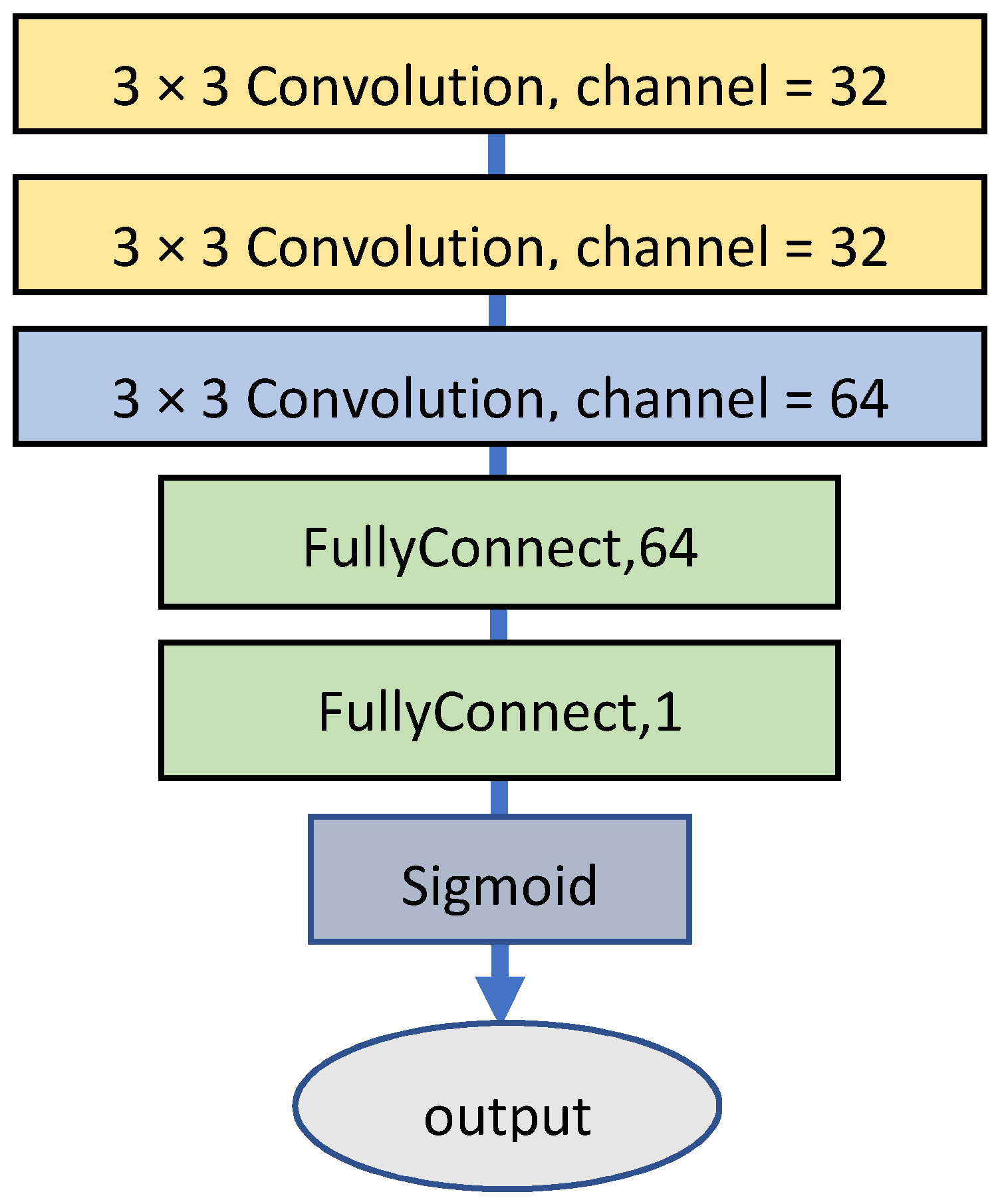
3.4. Specialized Color Classifier
3.5. Training Process
4. Experiments
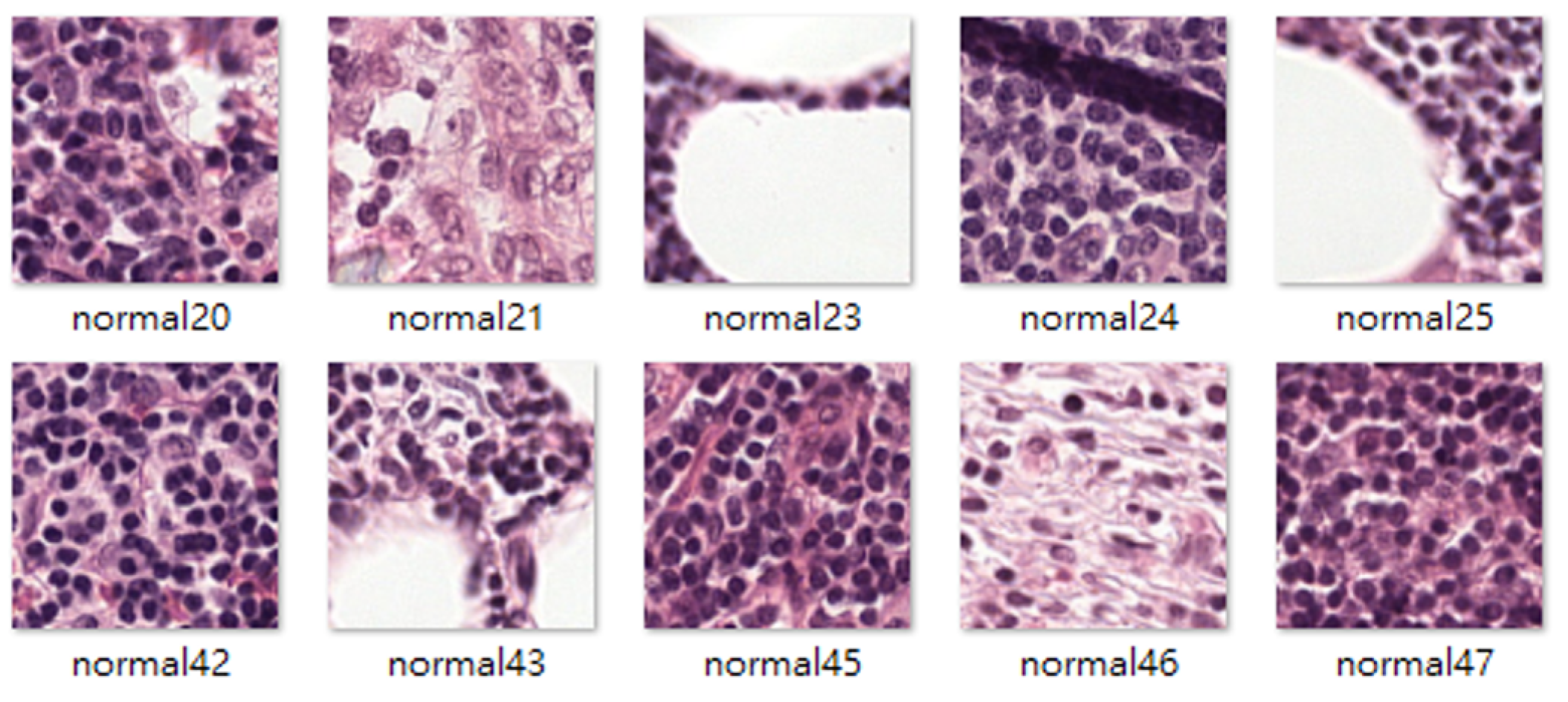
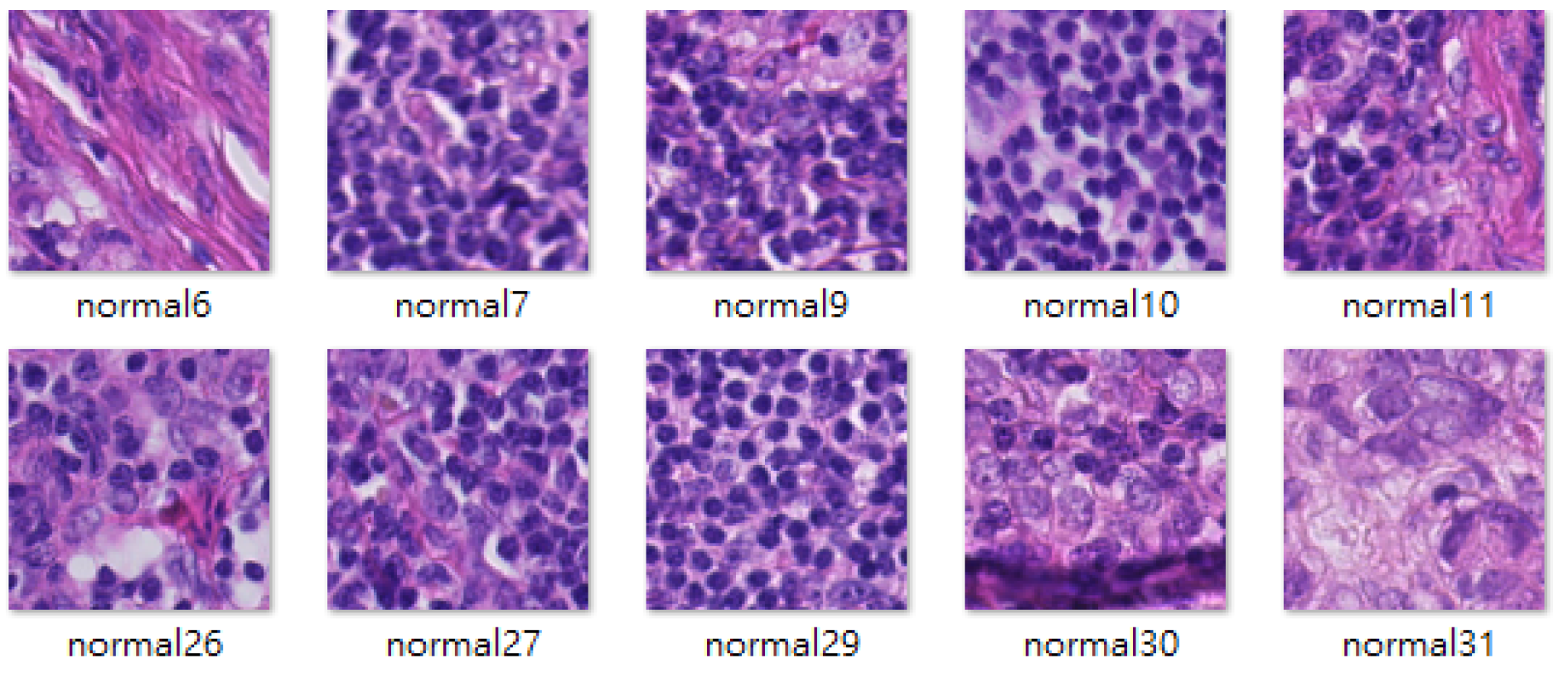
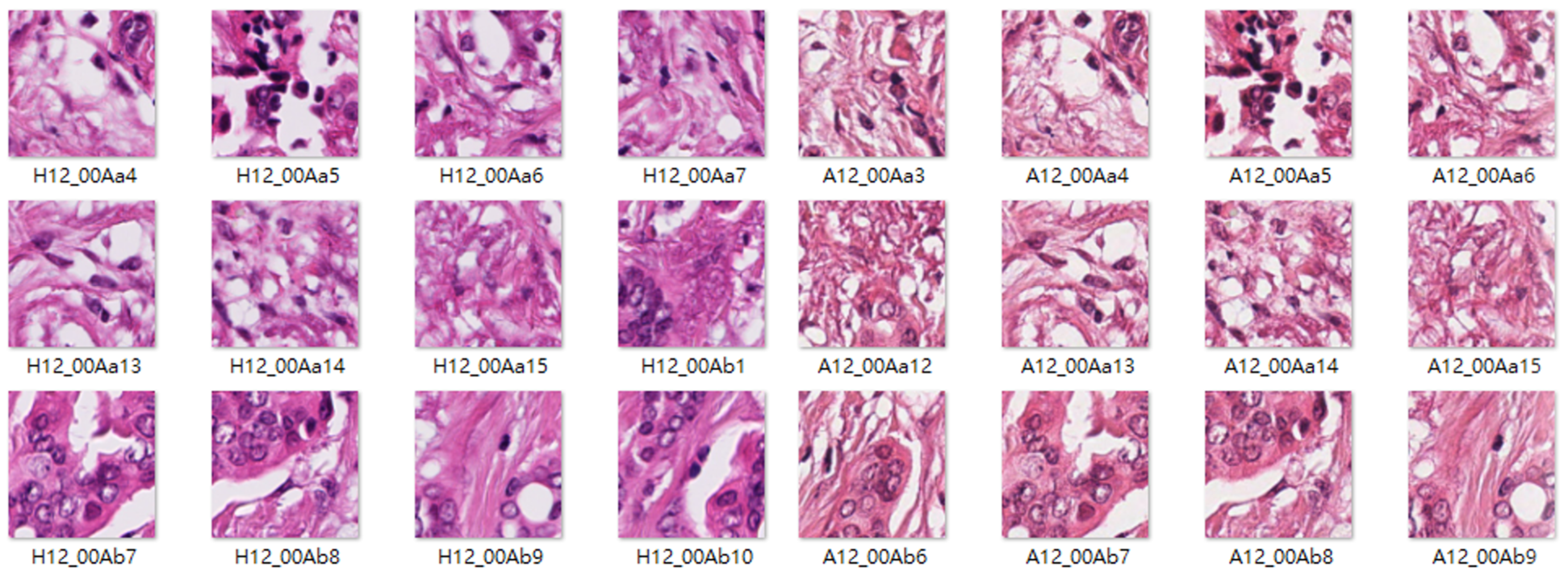
4.1. Datasets
4.2. Evaluating the Tumor Classification Performance
4.3. Mitos-Atypia-14 Experiment
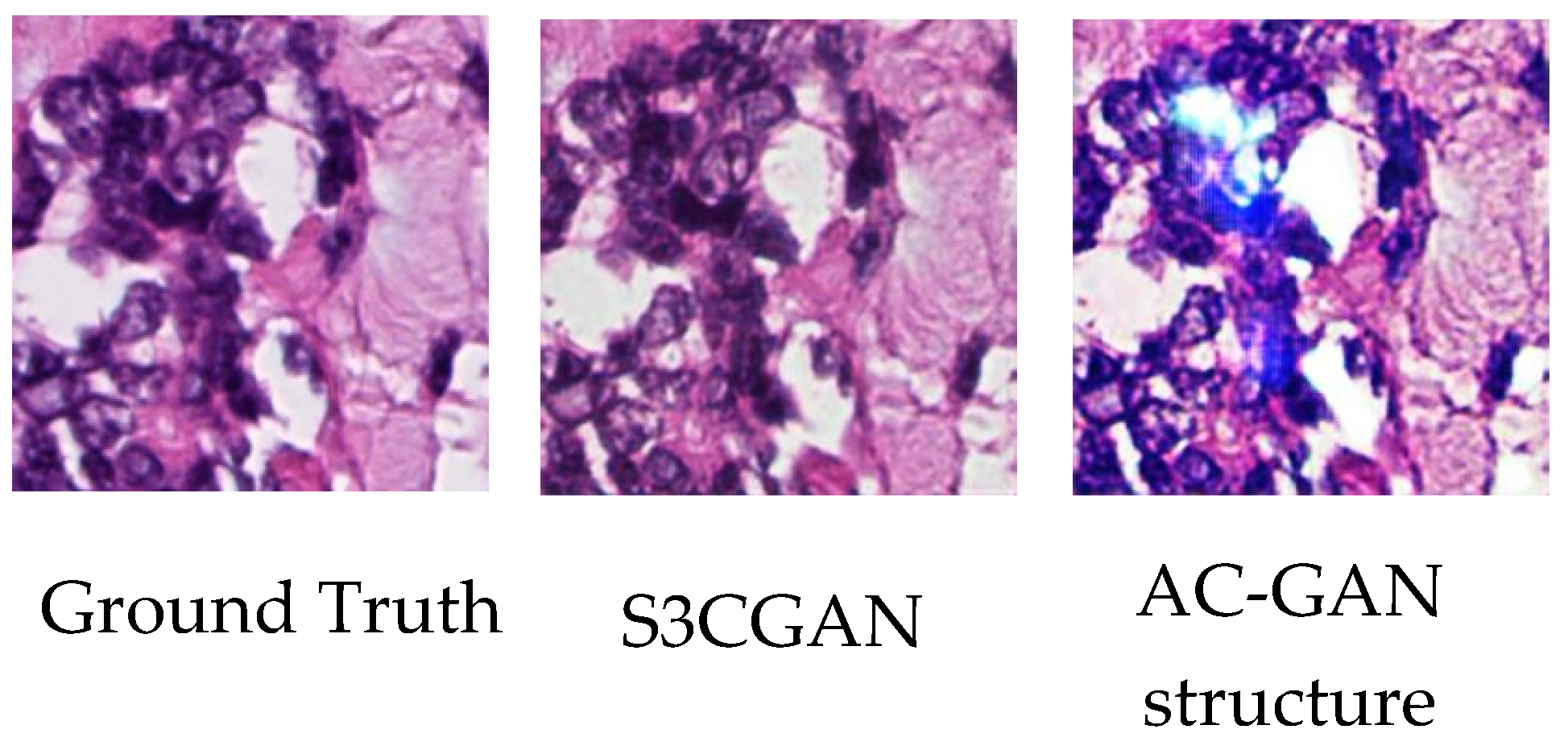
4.4. Specialized Color Classifier vs. Embedded Classifier
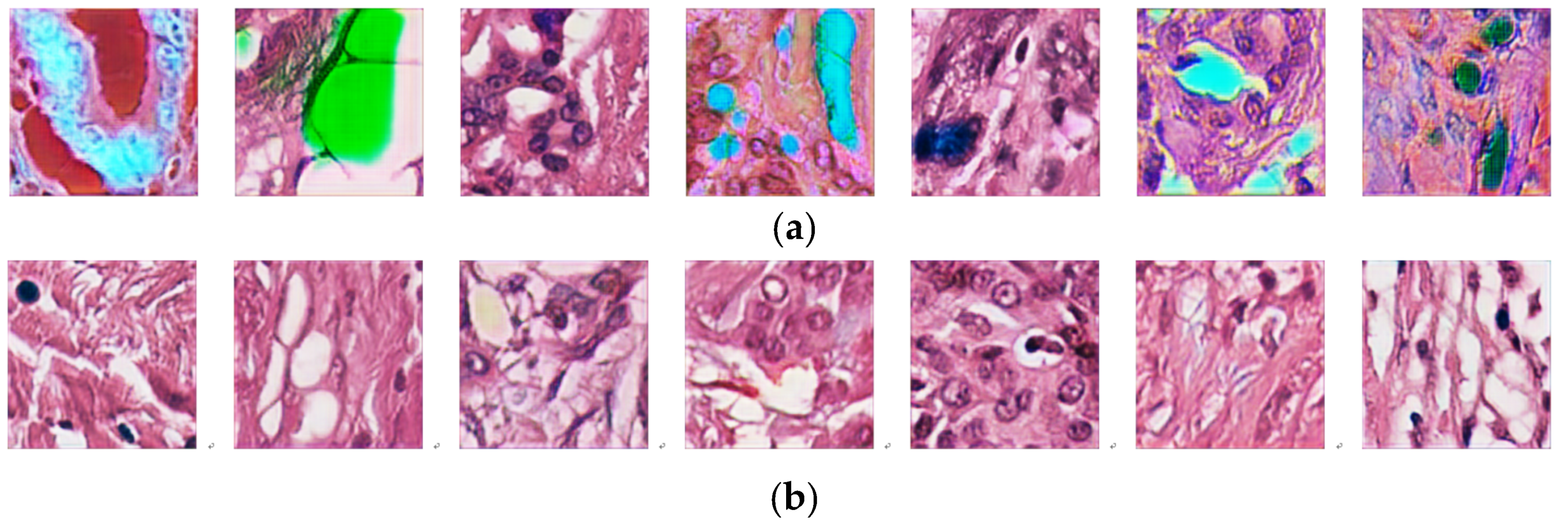
4.5. Training Stability and the Hyperparameters of the Specialized Color Classifier
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinhard, E.; Adhikhmin, M.; Gooch, B.; Shirley, P. Color transfer between images. IEEE Comput. Graph. Appl. 2001, 21, 34–41. [Google Scholar] [CrossRef]
- Macenko, M.; Niethammer, M.; Marron, J.S.; Borland, D.; Woosley, J.T.; Guan, X.; Schmitt, C.; Thomas, N.E. A method for normalizing histology slides for quantitative analysis. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, ISBI’09, Boston, MA, USA, 28 June–1 July 2009; pp. 1107–1110. [Google Scholar]
- Vahadane, A.; Peng, T.; Sethi, A.; Albarqouni, S.; Wang, L.; Baust, M.; Navab, N.; Steiger, K.; Schlitter, A.M.; Esposito, I. Structure-preserving color normalization and sparse stain separation for histological images. IEEE Trans. Med. Imag. 2016, 35, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Rajpoot, N.; Treanor, D.; Magee, D. A nonlinear mapping approach to stain normalization in digital histopathology images using image-specific color deconvolution. IEEE Trans. Biomed. Eng. 2014, 61, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial networks. In Proceedings of the Advances in Neural Information Processing Systems 27 (NIPS 2014), Montreal, QC, Canada, 8–13 December 2014; pp. 2672–2680. [Google Scholar]
- Mirza, M.; Osindero, S. Conditional Generative Adversarial Nets. arXiv 2014, arXiv:1411.1784. [Google Scholar]
- Cho, H.; Lim, S.; Choi, G.; Min, H. Neural Stain-Style Transfer Learning Using Gan for Histopathological Images. arXiv 2017, arXiv:1710.08543. [Google Scholar]
- Zhu, J.; Park, T.; Isola, P.; Efros, A.A. Unpaired Image-to-Image Translation Using Cycle-Consistent Adversarial Networks. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 2242–2251. [Google Scholar] [CrossRef] [Green Version]
- Shaban, M.T.; Baur, C.; Navab, N.; Albarqouni, S. Staingan: Stain Style Transfer for Digital Histological Images. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 953–956. [Google Scholar] [CrossRef] [Green Version]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015. [Google Scholar]
- Isola, P.; Zhu, J.-Y.; Zhou, T.; Efros, A.A. Image-to-Image Translation with Conditional Adversarial Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Gulrajani, I.; Ahmed, F.; Arjovsky, M.; Dumoulin, V.; Courville, A.C. Improved training of wasserstein gans. arXiv 2017, arXiv:1704.00028. [Google Scholar]
- Odena, A.; Olah, C.; Shlens, J. Conditional image synthesis with auxiliary classifier gans. arXiv 2016, arXiv:1610.09585. [Google Scholar]
- Camelyon16. Available online: https://camelyon16.grand-challenge.org/ (accessed on 8 June 2020).
- Mitos-Atypia-14. Available online: https://mitos-atypia-14.grand-challenge.org/ (accessed on 8 June 2020).
- Jiang, Y.; Chen, L.; Zhang, H.; Xiao, X. Breast cancer histopathological image classification using convolutional neural networks with small SE-ResNet module. PLoS ONE 2019, 14, e0214587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]


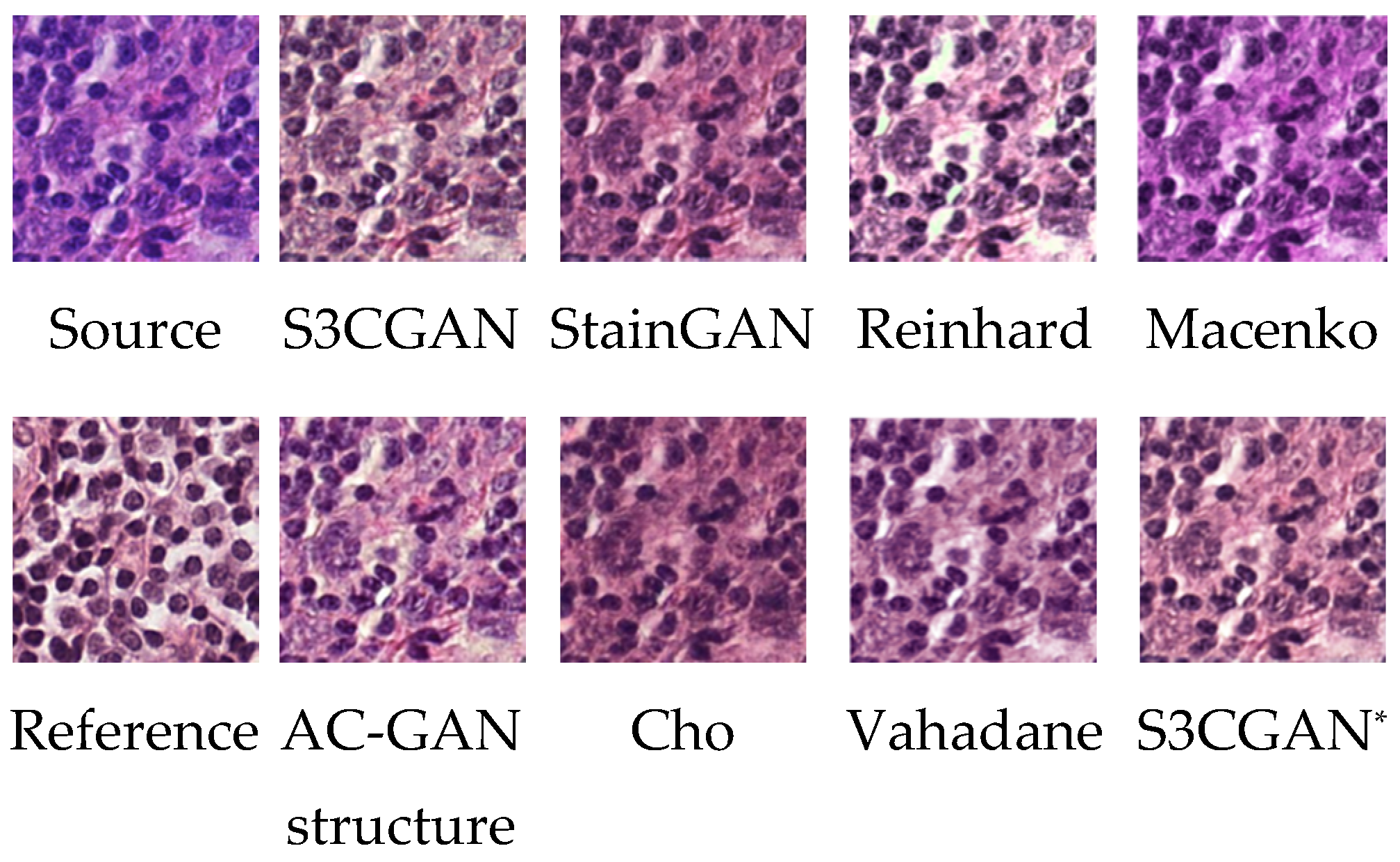
| Methods | AUC (Simple Classifier) | AUC (Complicated Classifier) |
|---|---|---|
| Reinhard [1] | 0.58 | 0.83 |
| Macenko [2] | 0.65 | 0.85 |
| Vahadane [3] | 0.77 | 0.87 |
| Cho [7] | 0.73 | 0.90 |
| StainGAN [9] | 0.79 | 0.91 |
| AC-GAN structure | 0.81 | 0.91 |
| S3CGAN* | 0.81 | 0.91 |
| S3CGAN | 0.83 | 0.92 |
| SSIM/p-Value | PSNR/p-Value | |
|---|---|---|
| Reinhard [1] | 0.58/0.00 | 13.4/0.00 |
| Macenko [2] | 0.67/0.00 | 14.0/0.00 |
| Vahadane [3] | 0.65/0.00 | 14.2/0.00 |
| Cho [7] | 0.68/0.00 | 20.4/0.00 |
| StainGAN [9] | 0.73/0.00 | 23.0/0.00 |
| AC-GAN structure | 0.69/0.00 | 21.1/0.00 |
| S3CGAN* | 0.75 | 24.7 |
| S3CGAN | 0.76 | 24.9 |
| Source | β = 0.1 | β = 0.3 | β = 0.4 | β = 1 | |
|---|---|---|---|---|---|
| Stain A to B |  |  |  | 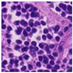 | 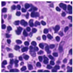 |
| Stain A to B | 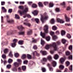 |  | 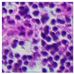 | 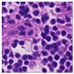 |  |
| Stain B to A | 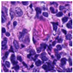 |  | 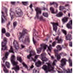 |  | 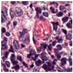 |
| Stain B to A | 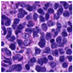 | 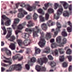 | 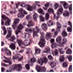 | 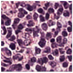 | 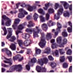 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Ma, Y.-X. Stain Style Transfer for Histological Images Using S3CGAN. Sensors 2022, 22, 1044. https://doi.org/10.3390/s22031044
Lee J-S, Ma Y-X. Stain Style Transfer for Histological Images Using S3CGAN. Sensors. 2022; 22(3):1044. https://doi.org/10.3390/s22031044
Chicago/Turabian StyleLee, Jiann-Shu, and Yao-Xian Ma. 2022. "Stain Style Transfer for Histological Images Using S3CGAN" Sensors 22, no. 3: 1044. https://doi.org/10.3390/s22031044
APA StyleLee, J.-S., & Ma, Y.-X. (2022). Stain Style Transfer for Histological Images Using S3CGAN. Sensors, 22(3), 1044. https://doi.org/10.3390/s22031044





