Use of Robot-Assisted Gait Training in Pediatric Patients with Cerebral Palsy in an Inpatient Setting—A Randomized Controlled Trial
Abstract
1. Introduction
2. Participants and Methods
2.1. Study Design
2.2. Recruitment and Inclusion and Exclusion Criteria
2.3. Randomization and Blinding
2.4. Study Protocol
2.5. Hybrid Assistive Limb
2.6. Therapy Concept (Intervention)
2.7. Outcome Measures and Statistical Analysis
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Outcome
3.3. Secondary Outcome
3.3.1. 10-Meter Walking Test (Max)
3.3.2. 6-Minute Walking Tes
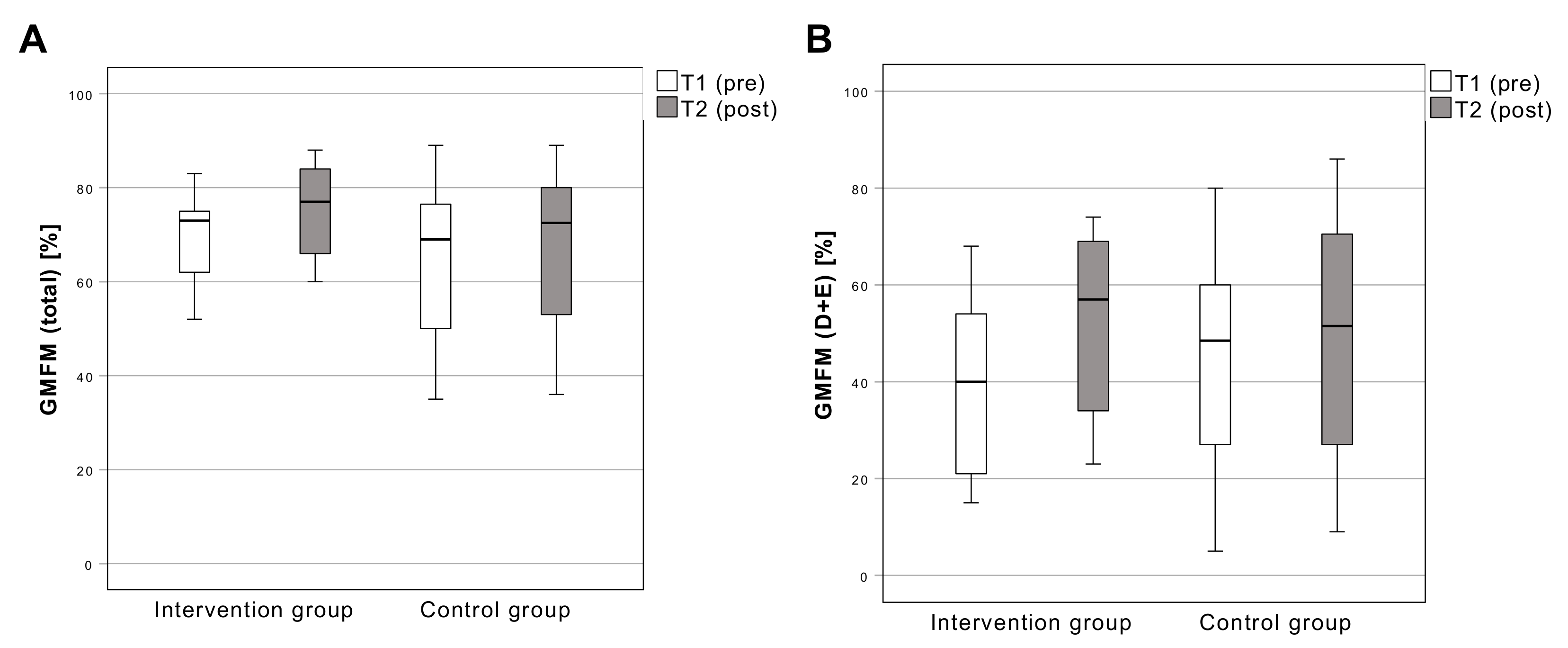
3.3.3. Gross Motor Function Measure
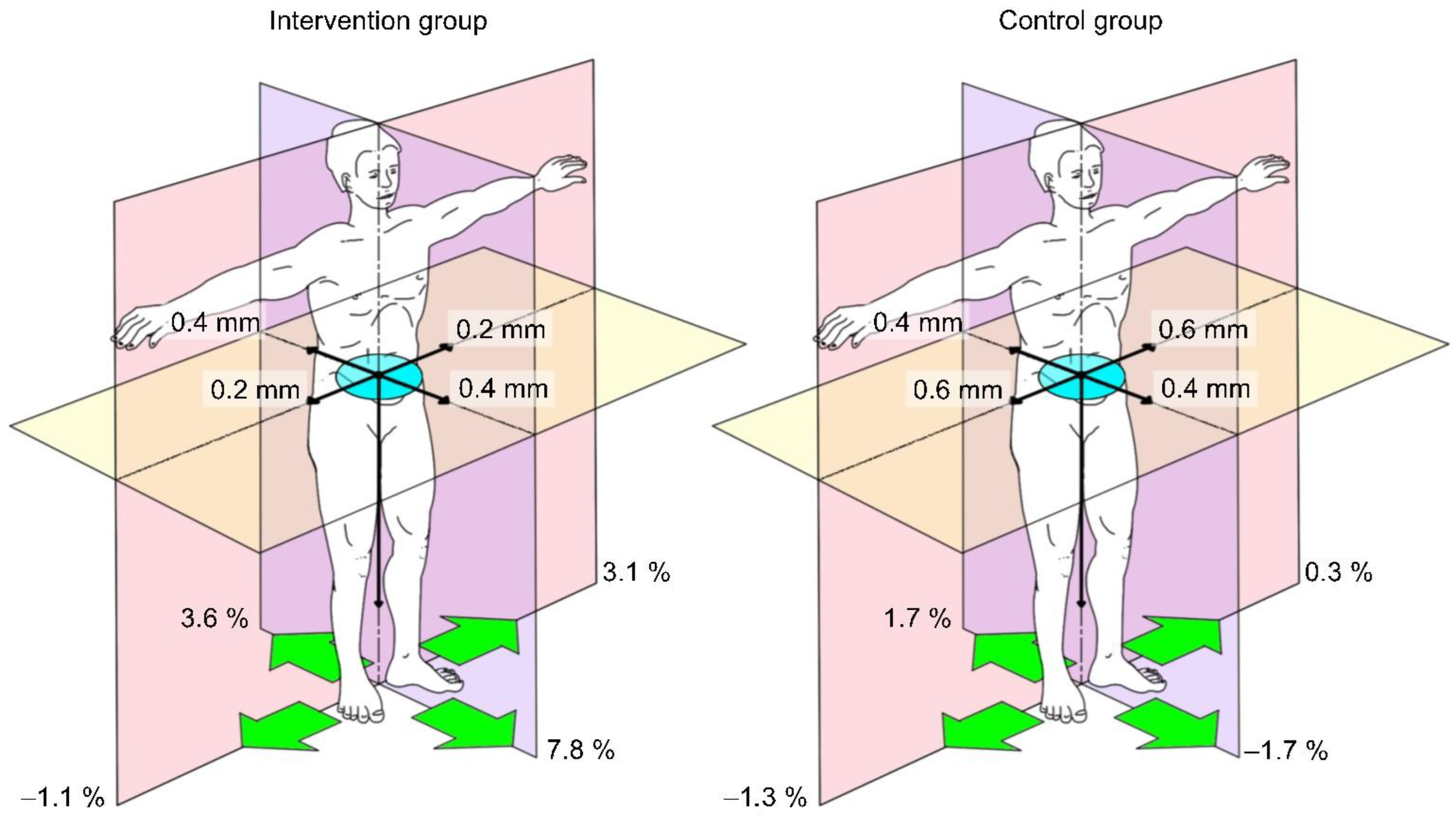
3.3.4. Pedobarography
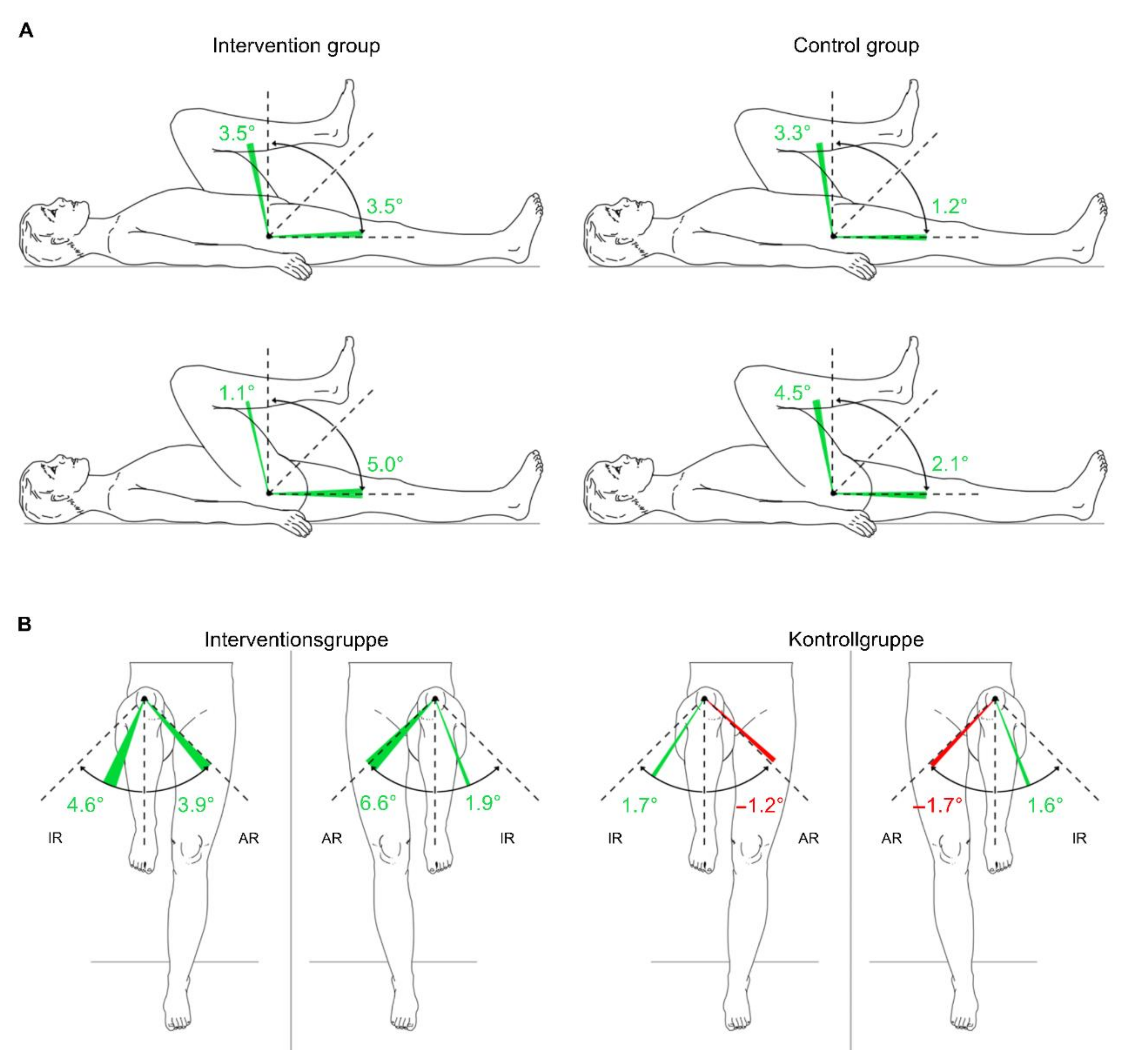
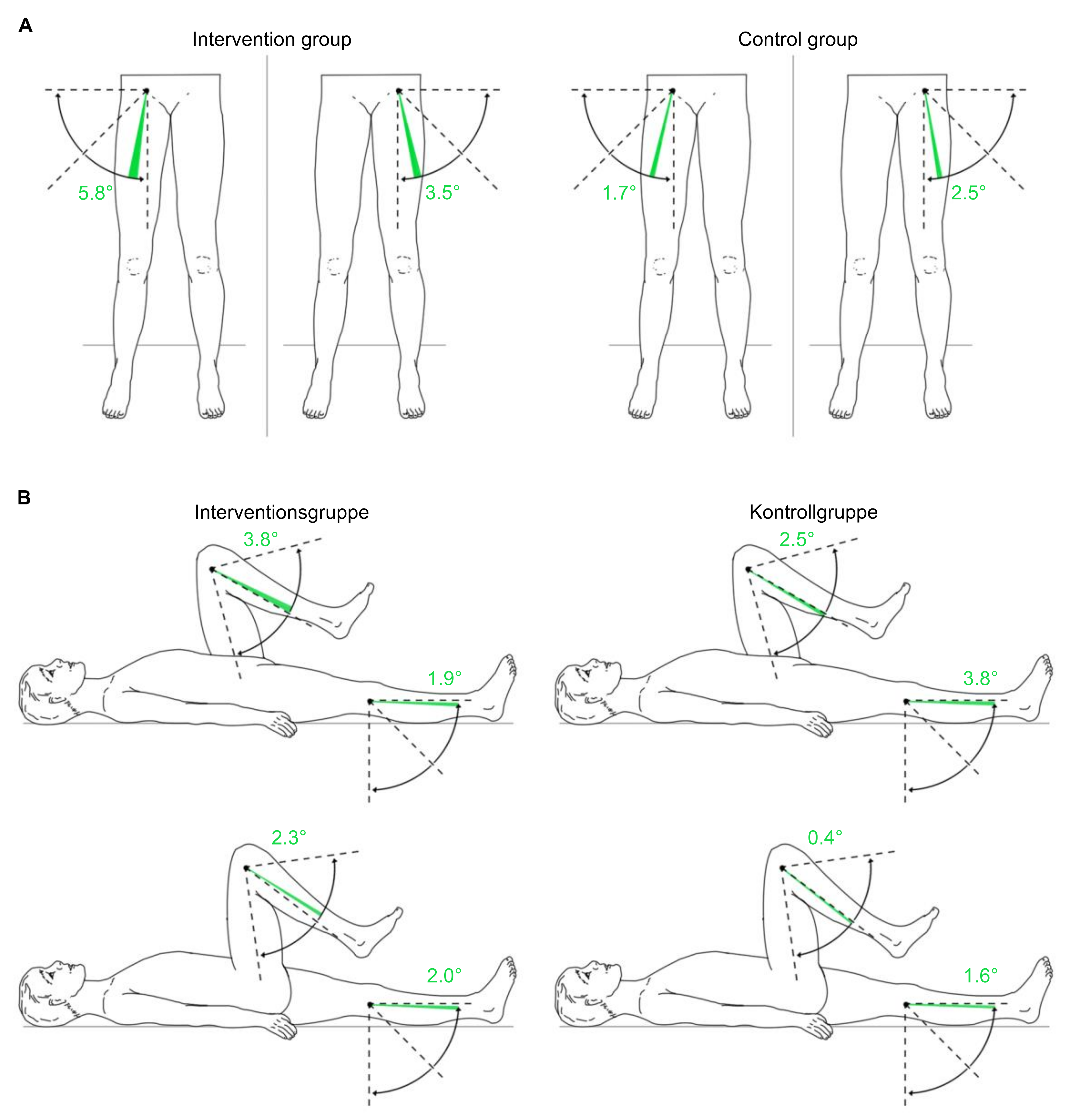
3.3.5. Passive Range of Motion of the Lower Extremities
4. Discussion
4.1. Limitations
4.2. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Intervention | Why? | Who? | How Much? |
|---|---|---|---|
| DocManMed | Treatment of local movement disorders; pain modulation; education of patients and attendants | Doctor with postgraduate training in manualmedicine | 4×/week, 30 min |
| PhysioNeuro | Facilitation of movements and movement transitions; education; improvement of coordination; promotion of independent and directed movements; increase in concentration and reception skills;promotion of participation. | Physiotherapist with postgraduate training in physiotherapy on a neurophysiological basis | 5×/week, 30 min |
| PhysioMT | Treatment of local movement disorders; education; pain modulation | Physiotherapist with postgraduate training in musculoskeletal therapy | |
| MET | Development of functional strength; training control; coordination training; promotion of independent and directed movements;promotion of participation. | Physiotherapist with postgraduate training in medical exercise therapy | 5×/week, 30 min |
| MassTher | Regulation of muscle tone; pain modulation; physical and mental relaxation; local blood circulation stimulation | Massage therapist | 5×/week, 30 min |
| HAL | Facilitation of movement transitions; coordination and harmonization of standing leg to swing leg phase transition; postural alignment; contextual gait training; support and completion of movementto promote proprioception. | Specially trained physiotherapist | 5×/week, 30 min |
References
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An Update on the Prevalence of Cerebral Palsy: A Systematic Review and Meta-Analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral Palsy. Nat. Rev. Dis. Prim. 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.R. Gait Pathology in Individuals with Cerebral Palsy. In The Identification and Treatment of Gait Problems in Cerebral Palsy; Gage, J.R., Schwartz, M.H., Koop, S.E., Eds.; Mac Keith Press: London, UK, 2009; pp. 11–171. ISBN 978-1-898683-65-0. [Google Scholar]
- Rosenbaum, P.L.; Walter, S.D.; Hanna, S.E.; Palisano, R.J.; Russell, D.J.; Raina, P.; Wood, E.; Bartlett, D.J.; Galuppi, B.E. Prognosis for Gross Motor Function in Cerebral Palsy: Creation of Motor Development Curves. JAMA 2002, 288, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Shortland, A. Muscle Deficits in Cerebral Palsy and Early Loss of Mobility: Can We Learn Something from Our Elders? Dev. Med. Child Neurol. 2009, 51 (Suppl. S4), 59–63. [Google Scholar] [CrossRef]
- Garvey, M.A.; Giannetti, M.L.; Alter, K.E.; Lum, P.S. Cerebral Palsy: New Approaches to Therapy. Curr. Neurol. Neurosci. Rep. 2007, 7, 147–155. [Google Scholar] [CrossRef]
- Novak, I.; McIntyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.-A.; Goldsmith, S. A Systematic Review of Interventions for Children with Cerebral Palsy: State of the Evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef]
- Hill, M.; Healy, A.; Chockalingam, N. Effectiveness of Therapeutic Footwear for Children: A Systematic Review. J. Foot Ankle Res. 2020, 13, 23. [Google Scholar] [CrossRef]
- Damiano, D.L. Activity, Activity, Activity: Rethinking Our Physical Therapy Approach to Cerebral Palsy. Phys. Ther. 2006, 86, 1534–1540. [Google Scholar] [CrossRef]
- Barreto, T.M.; Bento, M.N.; Barreto, T.M.; Jagersbacher, J.G.; Jones, N.S.; Lucena, R.; Bandeira, I.D. Prevalence of Depression, Anxiety, and Substance-Related Disorders in Parents of Children with Cerebral Palsy: A Systematic Review. Dev. Med. Child Neurol. 2020, 62, 163–168. [Google Scholar] [CrossRef]
- Pirpiris, M.; Gates, P.E.; McCarthy, J.J.; D’Astous, J.; Tylkowksi, C.; Sanders, J.O.; Dorey, F.J.; Ostendorff, S.; Robles, G.; Caron, C.; et al. Function and Well-Being in Ambulatory Children With Cerebral Palsy. J. Pediatr. Orthop. 2006, 26, 119–124. [Google Scholar] [CrossRef]
- Rosen, J.; Ferguson, P.W. (Eds.) Wearable Robotics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–3. ISBN 978-0-12-814659-0. [Google Scholar]
- Bayón, C.; Martín-Lorenzo, T.; Moral-Saiz, B.; Ramírez, Ó.; Pérez-Somarriba, Á.; Lerma-Lara, S.; Martínez, I.; Rocon, E. A Robot-Based Gait Training Therapy for Pediatric Population with Cerebral Palsy: Goal Setting, Proposal and Preliminary Clinical Implementation. J. Neuroeng. Rehabil. 2018, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Mataki, Y.; Kamada, H.; Mutsuzaki, H.; Shimizu, Y.; Takeuchi, R.; Mizukami, M.; Yoshikawa, K.; Takahashi, K.; Matsuda, M.; Iwasaki, N.; et al. Use of Hybrid Assistive Limb (HAL®) for a Postoperative Patient with Cerebral Palsy: A Case Report. BMC Res. Notes 2018, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Mataki, Y.; Mutsuzaki, H.; Yoshikawa, K.; Takahashi, K.; Enomoto, K.; Sano, K.; Mizukami, M.; Tomita, K.; Ohguro, H.; et al. Immediate Effects of a Single Session of Robot-Assisted Gait Training Using Hybrid Assistive Limb (HAL) for Cerebral Palsy. J. Phys. Ther. Sci. 2018, 30, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Iwasaki, N.; Mataki, Y.; Mutsuzaki, H.; Yoshikawa, K.; Takahashi, K.; Enomoto, K.; Sano, K.; Kubota, A.; Nakayama, T.; et al. Robot-Assisted Training Using Hybrid Assistive Limb® for Cerebral Palsy. Brain Dev. 2018, 40, 642–648. [Google Scholar] [CrossRef]
- Takahashi, K.; Mutsuzaki, H.; Mataki, Y.; Yoshikawa, K.; Matsuda, M.; Enomoto, K.; Sano, K.; Kubota, A.; Mizukami, M.; Iwasaki, N.; et al. Safety and Immediate Effect of Gait Training Using a Hybrid Assistive Limb in Patients with Cerebral Palsy. J. Phys. Ther. Sci. 2018, 30, 1009–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sczesny-Kaiser, M.; Höffken, O.; Aach, M.; Cruciger, O.; Grasmücke, D.; Meindl, R.; Schildhauer, T.A.; Schwenkreis, P.; Tegenthoff, M. HAL® Exoskeleton Training Improves Walking Parameters and Normalizes Cortical Excitability in Primary Somatosensory Cortex in Spinal Cord Injury Patients. J. NeuroEng. Rehabil. 2015, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Bunge, L.R.; Davidson, A.J.; Helmore, B.R.; Mavrandonis, A.D.; Page, T.D.; Schuster-Bayly, T.R.; Kumar, S. Effectiveness of Powered Exoskeleton Use on Gait in Individuals with Cerebral Palsy: A Systematic Review. PLoS ONE 2021, 16, e0252193. [Google Scholar] [CrossRef]
- Van Gorp, M.; Hilberink, S.R.; Noten, S.; Benner, J.L.; Stam, H.J.; van der Slot, W.M.A.; Roebroeck, M.E. Epidemiology of Cerebral Palsy in Adulthood: A Systematic Review and Meta-Analysis of the Most Frequently Studied Outcomes. Arch. Phys. Med. Rehabil. 2020, 101, 1041–1052. [Google Scholar] [CrossRef]
- Wiart, L.; Rosychuk, R.J.; Wright, F.V. Evaluation of the Effectiveness of Robotic Gait Training and Gait-Focused Physical Therapy Programs for Children and Youth with Cerebral Palsy: A Mixed Methods RCT. BMC Neurol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Nakagawa, S.; Mutsuzaki, H.; Mataki, Y.; Endo, Y.; Matsuda, M.; Yoshikawa, K.; Kamada, H.; Iwasaki, N.; Yamazaki, M. Safety and Immediate Effects of Hybrid Assistive Limb in Children with Cerebral Palsy: A Pilot Study. Brain Dev. 2020, 42, 140–147. [Google Scholar] [CrossRef]
- Erfolgreiche Anwendung des HAL-Systems bei Kindern. 2020. Available online: https://youtu.be/xE3AMKz_dWw (accessed on 27 May 2022).
- Klinische Studie an der KMT Hamm. 2020. Available online: https://youtu.be/yOsAS93xg0A (accessed on 27 May 2022).
- Ammann-Reiffer, C.; Bastiaenen, C.H.G.; Meyer-Heim, A.D.; van Hedel, H.J.A. Effectiveness of Robot-Assisted Gait Training in Children with Cerebral Palsy: A Bicenter, Pragmatic, Randomized, Cross-over Trial (PeLoGAIT). BMC Pediatr. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Sankai, Y. Power Assist Method Based on Phase Sequence and Muscle Force Condition for HAL. Adv. Robot. 2005, 19, 717–734. [Google Scholar] [CrossRef]
- Watson, M.J. Refining the Ten-Metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.E. Clinical Significance Testing in Rehabilitation Research: What, Why, and How? Phys. Ther. Rev. 2007, 12, 287–296. [Google Scholar] [CrossRef]
- Storm, F.A.; Petrarca, M.; Beretta, E.; Strazzer, S.; Piccinini, L.; Maghini, C.; Panzeri, D.; Corbetta, C.; Morganti, R.; Reni, G.; et al. Minimum Clinically Important Difference of Gross Motor Function and Gait Endurance in Children with Motor Impairment: A Comparison of Distribution-Based Approaches. BioMed Res. Int. 2020, 2020, 2794036. [Google Scholar] [CrossRef]
- Lefmann, S.; Russo, R.; Hillier, S. The Effectiveness of Robotic-Assisted Gait Training for Paediatric Gait Disorders: Systematic Review. J. NeuroEng. Rehabil. 2017, 14, 1. [Google Scholar] [CrossRef]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
3 months |
|
6 months |
|
|
(CFCS level I–III) |
|
(CFCS level I–III) |
|
|
(for RAGT treatment) |
| Outcome | Assessment | Unit |
|---|---|---|
| Primary | ||
| Walking speed | 10MWT (SSW) | m/s |
| Secondary | ||
| Walking speed | 10MWT (max) | m/s |
| Endurance | 6MWT | m/6 min |
| Gross motor skills | GMFM-88 | |
| Total | % | |
| Dimension D (standing) and | % | |
| E (walking, running, jumping) | % | |
| Joint mobility | ||
| pROM | Degree (°) | |
| Balance | Pedoscan | mm |
| Total a-p COP-Movement | mm | |
| Total lateral COP-Movement | mm2 | |
| Area of sway | % | |
| Foot pressure (anterior-posterior) | % | |
| Foot pressure (left-right) |
| Subject | Age | Sex | Height | Weight | BMI | GMFCS | Paresis | Orthosis | Therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | F | 161 | 50 | 19.3 | 3 | Tetra | AFO | PT, OT |
| 2 | 12 | M | 140 | 25 | 12.8 | 3 | Tetra | AFO | PT, Petö |
| 3 | 14 | M | 150 | 45 | 20.0 | 3 | Tetra | None | PT, OT, LO |
| 4 | 11 | M | 146 | 38 | 17.8 | 3 | Tetra | None | PT |
| 5 | 17 | M | 165 | 68 | 25.0 | 3 | Di | AFO | PT |
| 6 | 16 | F | 53 | 40 | 17.1 | 3 | Tetra | AFO | PT, OT |
| 7 | 12 | M | 156 | 50 | 20.6 | 2 | Tetra | AFO | PT |
| 8 | 14 | M | 165 | 52 | 19.1 | 2 | Tetra | None | PT, OT, HIP |
| 9 | 11 | M | 153 | 42 | 17.9 | 3 | Tetra | FO | PT, OT |
| 10 DO | 11 | M | 150 | 43 | 19.1 | 2 | Tetra | AFO | PT |
| 11 DO | 16 | F | 155 | 56 | 23.3 | 2 | Tetra | AFO | PT, OT, LO |
| 12 | 15 | M | 160 | 62 | 24.2 | 3 | Di | None | PT, OT, LO |
| 13 | 9 | F | 125 | 24 | 15.4 | 2 | Tetra | FO | PT |
| 14 | 17 | M | 170 | 74 | 25.6 | 3 | Tetra | None | PT |
| 15 | 11 | M | 148 | 41 | 18.7 | 3 | Tetra | AFO | PT, OT, HIP, LO |
| 16 | 12 | M | 160 | 62 | 24.2 | 2 | Di | AFO | PT, OT, HIP |
| 17 | 14 | M | 172 | 54 | 18.3 | 3 | Tetra | AFO | PT, OT |
| 18 | 10 | M | 146 | 43 | 20.7 | 3 | Tetra | AFO | PT, HeP |
| 19 | 11 | M | 153 | 47 | 20.3 | 3 | Tetra | AFO | PT, OT |
| 20 | 11 | M | 146 | 36 | 16.9 | 2 | Tetra | AFO | PT |
| 21 | 15 | M | 160 | 65 | 25.4 | 3 | Tetra | None | PT |
| 22 DO | 17 | F | 155 | 55 | 22.9 | 3 | Tetra | None | PT |
| 23 | 14 | M | 170 | 71 | 24.6 | 3 | Tetra | Shoes | PT, LO |
| 24 | 12 | M | 150 | 41 | 18.2 | 3 | Di | AFO | PT, HIP |
| 25 DO | 12 | F | 160 | 55 | 21.5 | 2 | Di | AFO | None |
| 26 | 12 | M | 155 | 54 | 22.5 | 3 | Tetra | AFO | PT, HIP |
| 27 DO | 12 | M | 160 | 54 | 21.1 | 3 | Tetra | AFO | PT, OT |
| 28 DO | 9 | F | 140 | 38 | 19.4 | 3 | Di | None | PT, OT, HIP |
| 29 | 10 | M | 136 | 25 | 13.5 | 3 | Di | AFO | PT, OT |
| 30 | 15 | M | 175 | 68 | 22.2 | 2 | Tetra | AFO | PT, Petö |
| Measure (Time) | F-Value | p-Value | Partial η2 |
|---|---|---|---|
| 10MWT (SSW) | F = (1, 18) 0.85 | 0.378 | 0.045 |
| 10MWT (max) | F = (1, 18) 2.62 | 0.123 | 0.127 |
| 6MWT | F = (1, 18) 0.06 | 0.8 | 0.004 |
| GMFM (total) | F = (1, 18) 13.12 | 0.002 * | 0.422 |
| GMFM (D + E) | F = (1, 18) 4.59 | 0.046 * | 0.203 |
| Measure (Time * Group) | F-Value | p-Value | Partial η2 |
|---|---|---|---|
| Total a-p COP-Movement (mm) | F = (1,10) 0.376 | 0.554 | 0.036 |
| Total lateral COP-Movement (mm) | F = (1,10) 0.167 | 0.691 | 0.016 |
| Area of sway (mm2) | F = (1,10) 2.682 | 0.133 | 0.211 |
| Maximum pressure (%) L Maximum pressure (%) R Maximum pressure (%) anterior Maximum pressure (%) posterior | F = (1,10) 0.378 F = (1,10) 0.779 F = (1,10) 0.754 F = (1,10) 4.620 | 0.552 0.398 0.406 0.057 | 0.036 0.072 0.070 0.316 |
| Group | N | MV ± SD | MV ± SD | |
|---|---|---|---|---|
| pre | post | |||
| Total a-p COP-Movement (mm) | Intervention | 7 | 5.2 ± 6.6 | 6.0 ± 4.6 |
| Control | 9 | 7.3 ± 6.0 | 8.5 ± 9.0 | |
| Total lateral COP-Movement (mm) | Intervention | 7 | 4.8 ± 4.6 | 5.2 ± 3.9 |
| Control | 9 | 11.1 ± 7.9 | 11.8 ± 11.2 | |
| Area of sway (mm2) | Intervention | 7 | 6.9 ± 12.0 | 21.9 ± 45.6 |
| Control | 9 | 9.2 ± 14.1 | 22.3 ± 24.9 | |
| Maximum pressure (%) L | Intervention | 7 | 50.5 ± 9.7 | 53.1 ± 12.2 |
| Control | 9 | 48.8 ± 12.5 | 49.1 ± 11.0 | |
| Maximum pressure (%) R | Intervention | 7 | 49.5 ± 9.7 | 48.4 ± 11.4 |
| Control | 9 | 51.2 ± 12.5 | 49.9 ± 9.9 | |
| Maximum pressure (%) anterior | Intervention | 7 | 44.5 ± 4.3 | 52.3 ± 14.5 |
| Control | 9 | 51.1 ± 6.0 | 49.4 ± 7.4 | |
| Maximum pressure (%) posterior | Intervention | 7 | 55.5 ± 4.3 | 51.9 ± 6.4 |
| Control | 9 | 48.9 ± 6.0 | 50.6 ± 7.4 |
| Measure | F-Value | p-Value | Partial η2 |
|---|---|---|---|
| Hip L | F = (1,18) 0.527 | 0.477 | 0.028 |
| Flexion | F = (1,18) 3.176 | 0.092 | 0.15 |
| Extension | F = (1,18) 0.018 | 0.894 | 0.001 |
| Internal Rotation | F = (1,18) 1.045 | 0.32 | 0.055 |
| External Rotation | F = (1,18) 0.002 | 0.969 | 0 |
| Abduction | |||
| Knee L | F = (1,18) 0.004 | 0.95 | 0 |
| Flexion | F = (1,18) 0.004 | 0.951 | 0 |
| Extension | |||
| Hip R | F = (1,18) 4.423 | 0.05 | 0.197 |
| Flexion | F = (1,18) 3.425 | 0.081 | 0.16 |
| Extension | F = (1,18) 0.267 | 0.612 | 0.015 |
| Internal Rotation | F = (1,18) 1.539 | 0.231 | 0.079 |
| External Rotation | F = (1,18) 0.70 | 0.412 | 0.038 |
| Abduction | |||
| Knee R | F = (1,18) 1.299 | 0.269 | 0.067 |
| Flexion | F = (1,18) 0.485 | 0.495 | 0.026 |
| Extension |
| Group | N | MV ± SD | MV ± SD | MV ± SD | MV ± SD | |
|---|---|---|---|---|---|---|
| pre | post | pre | post | |||
| Hip left | Hip right | |||||
| Flexion | Intervention | 13 | 102.3 ± 17.5 | 105.8 ± 15.8 | 105.4 ± 16.8 | 106.5 ± 15.7 |
| Control | 12 | 100.0 ± 10.2 | 103.3 ± 11.1 | 101.3 ± 9.1 | 105.8 ± 8.7 | |
| Extension | Intervention | 13 | −3.5 ± 8.8 | 0.0 ± 7.4 | −3.8 ± 8.5 | 1.2 ± 5.8 |
| Control | 12 | −0.4 ± 8.9 | 0.8 ± 8.7 | −0.4 ± 5.4 | 1.7 ± 4.4 | |
| Internal Rotation | Intervention | 13 | 25.0 ± 18.1 | 26.9 ± 16.4 | 21.9 ± 13.5 | 26.5 ± 13.3 |
| Control | 12 | 35.8 ± 15.5 | 37.5 ± 18.8 | 36.3 ± 18.0 | 37.9 ± 18.4 | |
| External Rotation | Intervention | 13 | 41.5 ± 19.0 | 48.1 ± 16.9 | 41.5 ± 18.1 | 45.4 ± 16.6 |
| Control | 12 | 45.8 ± 20.5 | 44.6 ± 21.5 | 48.8 ± 21.1 | 47.1 ± 20.1 | |
| Abduction | Intervention | 13 | 19.6 ± 8.0 | 23.1 ± 9.0 | 15.4 ± 7.5 | 21.2 ± 9.4 |
| Control | 12 | 21.3 ± 7.1 | 23.8 ± 8.8 | 22.5 ± 10.1 | 24.2 ± 11.0 | |
| Knee left | Knee right | |||||
| Flexion | Intervention | 13 | 130.8 ± 17.7 | 134.6 ± 16.8 | 129.6 ± 18.8 | 131.9 ± 16.5 |
| Control | 12 | 135.4 ± 18.0 | 137.9 ± 15.1 | 136.7 ± 15.7 | 137.1 ± 15.9 | |
| Extension | Intervention | 13 | −6.2 ± 9.4 | −4.2 ± 8.6 | −6.5 ± 7.7 | −4.6 ± 8.8 |
| Control | 12 | −2.9 ± 4.0 | −1.3 ± 2.3 | −4.6 ± 6.2 | −0.8 ± 2.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moll, F.; Kessel, A.; Bonetto, A.; Stresow, J.; Herten, M.; Dudda, M.; Adermann, J. Use of Robot-Assisted Gait Training in Pediatric Patients with Cerebral Palsy in an Inpatient Setting—A Randomized Controlled Trial. Sensors 2022, 22, 9946. https://doi.org/10.3390/s22249946
Moll F, Kessel A, Bonetto A, Stresow J, Herten M, Dudda M, Adermann J. Use of Robot-Assisted Gait Training in Pediatric Patients with Cerebral Palsy in an Inpatient Setting—A Randomized Controlled Trial. Sensors. 2022; 22(24):9946. https://doi.org/10.3390/s22249946
Chicago/Turabian StyleMoll, Fabian, Axel Kessel, Anna Bonetto, Johanna Stresow, Monika Herten, Marcel Dudda, and Jens Adermann. 2022. "Use of Robot-Assisted Gait Training in Pediatric Patients with Cerebral Palsy in an Inpatient Setting—A Randomized Controlled Trial" Sensors 22, no. 24: 9946. https://doi.org/10.3390/s22249946
APA StyleMoll, F., Kessel, A., Bonetto, A., Stresow, J., Herten, M., Dudda, M., & Adermann, J. (2022). Use of Robot-Assisted Gait Training in Pediatric Patients with Cerebral Palsy in an Inpatient Setting—A Randomized Controlled Trial. Sensors, 22(24), 9946. https://doi.org/10.3390/s22249946







