Can Gait Features Help in Differentiating Parkinson’s Disease Medication States and Severity Levels? A Machine Learning Approach
Abstract
1. Introduction
2. Methodology
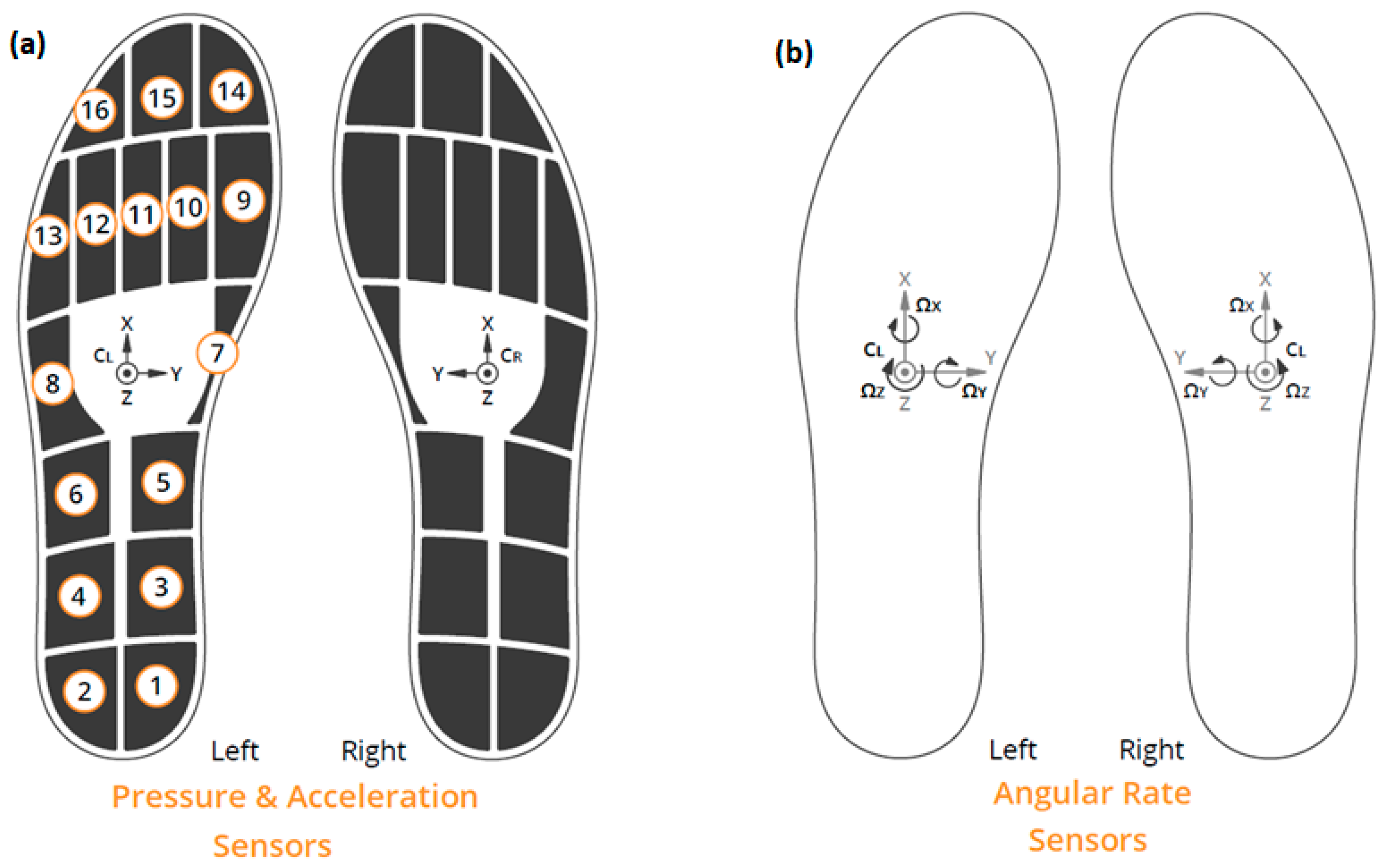
2.1. Materials and Setup
2.2. A Protocol for the Assessment of Gait of Parkinson’s Disease Patients Using Wearable Sensors
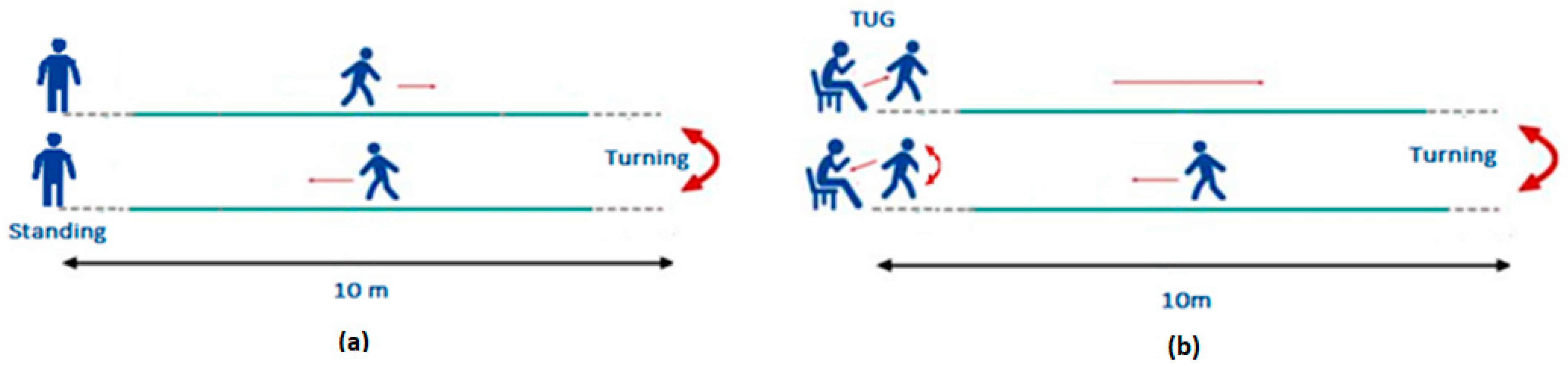
2.2.1. The Walk Straight and Turn Test
2.2.2. The Modified Timed Up and Go Test
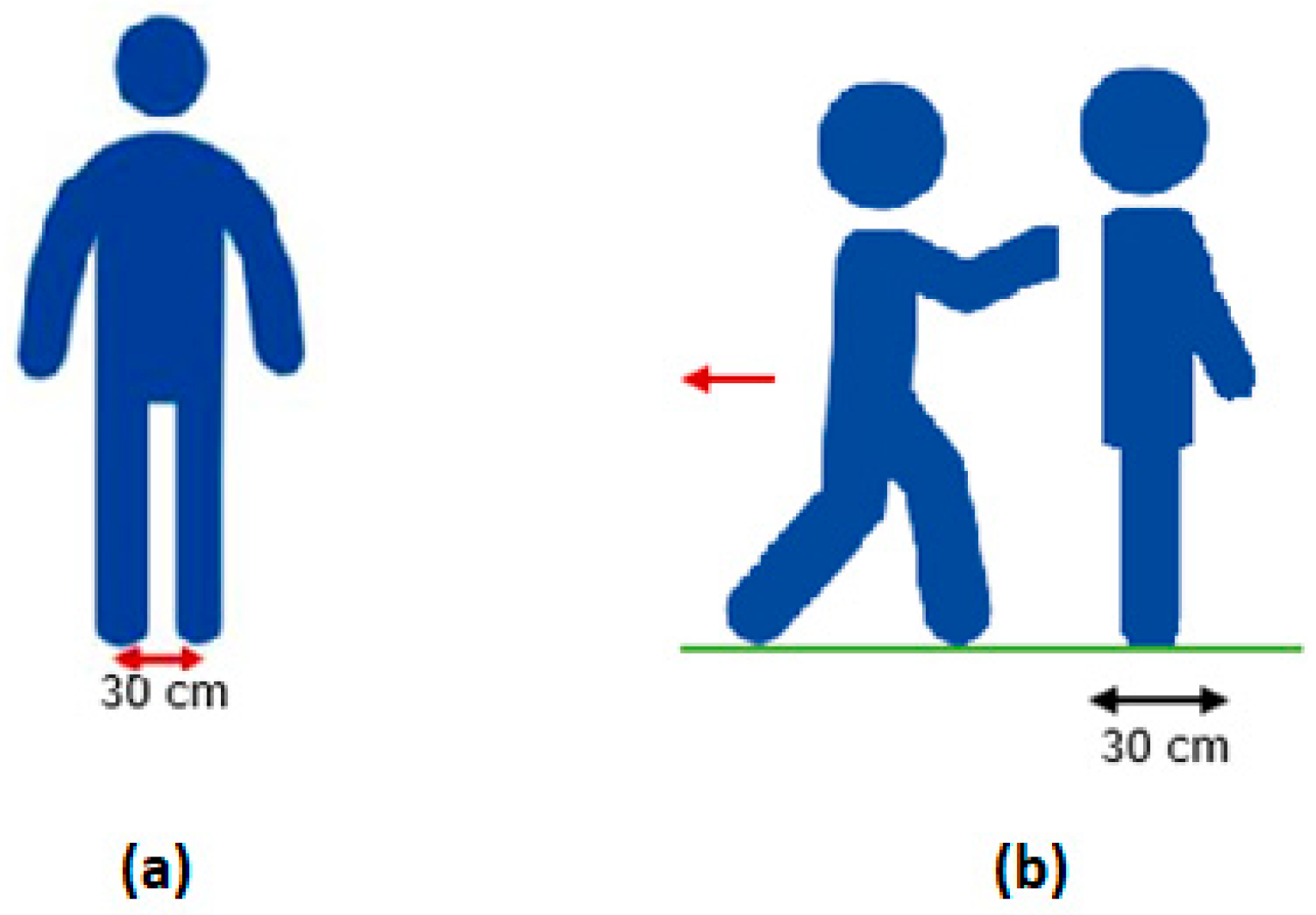
2.2.3. The Static Balance Test
2.2.4. The Retropulsion Test
2.2.5. The FoG and Dual Tasking Test
2.3. Participants
2.4. Parkinson’s Disease Ratings: MDS-UPDRS/Subsets and Severity Levels
3. Data Analysis
3.1. Gait Features Extraction
3.2. Feature Selection and Model Training
4. Results
4.1. Statistical Analysis
4.2. Machine Learning Analysis
4.2.1. Participant Groups Classification, PD/non-PD and PD/EL/S
4.2.2. Classification between Medication States ON/OFF
4.2.3. Parkinson’s Disease Severity Levels Based on MDS-UPDRS: Part III Ratings
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The emerging evidence of the parkinson pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018. [Google Scholar]
- Copas, A.N.M.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The pathogenesis of parkinson’s disease: A complex interplay between astrocytes, microglia, and T lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Chaudhuri, K.R.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021, 7, 1–21. [Google Scholar] [CrossRef]
- Moya-Galé, G.; Levy, E.S. Parkinson’s disease-associated dysarthria: Prevalence, impact and management strategies. Res. Rev. Park. 2019, 9, 9–16. [Google Scholar] [CrossRef]
- Hallett, M. Parkinson’s disease tremor: Pathophysiology. Park. Relat. Disord. 2012, 18, S85–S86. [Google Scholar] [CrossRef]
- Gandhi, K.R.; Saadabadi, A. Levodopa (L-Dopa). Available online: https://www.ncbi.nlm.nih.gov/books/NBK482140/ (accessed on 5 July 2022).
- Sharma, V.D.; Patel, M.; Miocinovic, S. Surgical treatment of parkinson’s disease: Devices and lesion approaches. Neurotherapeutics 2020, 17, 1525–1538. [Google Scholar] [CrossRef]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic criteria for Parkinson’s disease: From James Parkinson to the concept of prodromal disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P. The MDS-sponsored Revision of the Unified Parkinson’s Disease Rating Scale. J. Mov. Disord. 2008, 1, 1–33. [Google Scholar]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality 1967. Neurology 2001, 57, S11–S26. [Google Scholar] [PubMed]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Majumder, S.; Faisal, A.I.; Deen, M.J. Insole-based systems for health monitoring: Current solutions. Sensors 2022, 22, 438. [Google Scholar] [CrossRef] [PubMed]
- Chatzaki, C.; Skaramagkas, V.; Tachos, N.; Christodoulakis, G.; Maniadi, E.; Kefalopoulou, Z.; Fotiadis, D.; Tsiknakis, M. The smart-insole dataset: Gait analysis using wearable sensors with a focus on elderly and Parkinson’s patients. Sensors 2021, 21, 2821. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, Q.; Poston, K.L.; Sullivan, E.V.; Pfefferbaum, A.; Shahid, M.; Katz, M.; Kouhsari, L.M.; Schulman, K.; Milstein, A.; et al. Quantifying Parkinson’s disease motor severity under uncertainty using MDS-UPDRS videos. Med. Image Anal. 2021, 73, 102179. [Google Scholar] [CrossRef]
- Mandal, I.; Sairam, N.; Mandal, I.; Sairam, N. New machine-learning algorithms for prediction of Parkinson’s disease. Int. J. Syst. Sci. 2014, 45, 647–666. [Google Scholar] [CrossRef]
- Ahlrichs, C.; Lawo, M. Parkinson’s disease motor symptoms in machine learning: A review. Health Inform. Int. J. 2013, 2, 1–18. [Google Scholar] [CrossRef]
- Skaramagkas, V.; Andrikopoulos, G.; Kefalopoulou, Z.; Polychronopoulos, P. A study on the essential and parkinson’s arm tremor classification. Signals 2021, 2, 201–224. [Google Scholar] [CrossRef]
- Skaramagkas, V.; Andrikopoulos, G.; Kefalopoulou, Z.; Polychronopoulos, P. Towards differential diagnosis of essential and parkinson’s tremor via machine learning. In Proceedings of the 2020 28th Mediterranean Conference on Control and Automation (MED), Saint-Raphaël, Saint-Raphaël, France, 15–18 September 2020; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2020; pp. 782–787. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Kyritsis, K.; Klingelhoefer, L.; Bostanjopoulou, S.; Chaudhuri, K.R.; Delopoulos, A. Detecting parkinsonian tremor from IMU data collected in-the-wild using deep multiple-instance learning. Available online: https://zenodo.org/record/3519213 (accessed on 3 February 2022).
- Goschenhofer, J.; Pfister, F.M.J.; Yuksel, K.A.; Bischl, B.; Fietzek, U.; Thomas, J. Wearable-based parkinson’s disease severity monitoring using deep learning. Lect. Notes Comput. Sci. 2019, 11908, 400–415. [Google Scholar] [CrossRef]
- Ibrahim, A.; Zhou, Y.; Jenkins, M.E.; Trejos, A.L.; Naish, M.D. The design of a parkinson’s tremor predictor and estimator using a hybrid convolutional-multilayer perceptron neural network. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2020; Volume 2020, pp. 5996–6000. [Google Scholar] [CrossRef]
- Hobert, M.A.; Maetzler, W.; Aminian, K.; Chiari, L. Technical and clinical view on ambulatory assessment in Parkinson’s disease. Acta Neurol. Scand. 2014, 130, 139–147. [Google Scholar] [CrossRef]
- Dewey, D.C.; Miocinovic, S.; Bernstein, I.; Khemani, P.; Dewey, R.B.; Querry, R.; Chitnis, S.; Dewey, R.B., Jr. Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J. Neurol. Sci. 2014, 345, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kyrarini, M.; Wang, X.; Graser, A. Comparison of vision-based and sensor-based systems for joint angle gait analysis. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Turin, Italy, 7–9 May 2015; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2015; pp. 375–379. [Google Scholar] [CrossRef]
- Moro, M.; Marchesi, G.; Hesse, F.; Odone, F.; Casadio, M. Markerless vs. marker-based gait analysis: A proof of concept study. Sensors 2022, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Bachlin, M.; Plotnik, M.; Roggen, D.; Maidan, I.; Hausdorff, J.M.; Giladi, N.; Troster, G. Wearable assistant for Parkinsons disease patients with the freezing of gait symptom. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 436–446. [Google Scholar] [CrossRef]
- Zanardi, A.P.J.; da Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; dos Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Braun, B.; Veith, N.T.; Hell, R.; Döbele, S.; Roland, M.; Rollmann, M.; Holstein, J.H.; Pohlemann, T. Validation and reliability testing of a new, fully integrated gait analysis insole. J. Foot Ankle Res. 2015, 8, 54. [Google Scholar] [CrossRef]
- Stöggl, T.; Martiner, A. Validation of Moticon’s OpenGo sensor insoles during gait, jumps, balance and cross-country skiing specific imitation movements. J. Sports Sci. 2017, 35, 196–206. [Google Scholar] [CrossRef]
- Kakarla, T.P.; Varma, K.A.; Preejith, S.P.; Joseph, J.; Sivaprakasam, M. Accuracy Enhancement of Total Force by Capacitive Insoles. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Moticon-SCIENCE. Available online: https://www.moticon.de/ (accessed on 12 September 2022).
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of Gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Brognara, L.; Palumbo, P.; Grimm, B.; Palmerini, L. Assessing gait in Parkinson’s disease using wearable motion sensors: A systematic review. Diseases 2019, 7, 18. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed ‘Up & Go’: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘Timed Up and Go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.; Greene, B.R.; Doheny, E.P.; McKeown, D.J.; de Vito, G.; Caulfield, B. Reliability of quantitative TUG measures of mobility for use in falls risk assessment. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August 2011–3 September 2011; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2011; Volume 2011, pp. 466–469. [Google Scholar] [CrossRef]
- Mariani, B.; Jiménez, M.C.; Vingerhoets, F.J.G.; Aminian, K. On-shoe wearable sensors for gait and turning assessment of patients with Parkinson’s disease. IEEE Trans. Biomed. Eng. 2013, 60, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; Weerdesteyn, V.; Hagen, Y.J.; Duysens, J.; Giladi, N.; Bloem, B.R. Obstacle avoidance to elicit freezing of gait during treadmill walking. Mov. Disord. 2010, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B.; Tran, V.K.; Nutt, J.G. Multiple balance tests improve the assessment of postural stability in subjects with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Brauer, S.G.; Woollacott, M.H.; Lamont, R.; Clewett, S.; O’Sullivan, J.; Silburn, P.; Mellick, G.D.; Morris, M.E. Single and dual task gait training in people with Parkinson’s Disease: A protocol for a randomised controlled trial. BMC Neurol. 2011, 11, 90–96. [Google Scholar] [CrossRef]
- Ziegler, K.; Schroeteler, F.; Ceballos-Baumann, A.O.; Fietzek, U.M. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov. Disord. 2010, 25, 1012–1018. [Google Scholar] [CrossRef]
- Kluge, F.; Gaßner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards mobile gait analysis: Concurrent validity and test-retest reliability of an inertial measurement system for the assessment of spatio-temporal gait parameters. Sensors 2017, 17, 1522. [Google Scholar] [CrossRef]
- Combs, S.A.; Diehl, M.D.; Filip, J.; Long, E. Short-distance walking speed tests in people with Parkinson disease: Reliability, responsiveness, and validity. Gait Posture 2014, 39, 784–788. [Google Scholar] [CrossRef]
- Martínez-Martín, P.; Rodriguez-Blazquez, C.; Alvarez, M.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Castrillo, J.C.M.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Park. Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef]
- Kefalopoulou, Z.; Chatzaki, V.; Skaramagkas, C.; Chroni, E.; Tachos, N.; Fotiadis, D.I.; Tsiknakis, M. Pressure Sensor Insole Gait Assessment for Parkinson’s Disease Patients: A Pilot Study [Abstract]. Movement Disorder 2022 International Congress. 2022. Volume 37. Available online: https://www.mdsabstracts.org/abstract/pressure-sensor-insole-gait-assessment-for-parkinsons-disease-patients-a-pilot-study/ (accessed on 20 October 2022).
- Kasović, M.; Štefan, L.; Štefan, A. Normative data for gait speed and height norm speed in ≥ 60-year-old men and women. Clin. Interv. Aging 2021, 16, 225–230. [Google Scholar] [CrossRef]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Paker, N.; Bugdayci, D.; Goksenoglu, G.; Demircioğlu, D.T.; Kesiktas, N.; Ince, N. Gait speed and related factors in parkinson’s disease. J. Phys. Ther. Sci. 2015, 27, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Rota, V.; Perucca, L.; Simone, A.; Tesio, L. Walk ratio (step length/cadence) as a summary index of neuromotor control of gait: Application to multiple sclerosis. Int. J. Rehabil. Res. 2011, 34, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.R.; Simpson, C.S.; van Asseldonk, E.H.F.; van der Kooij, H.; Ijspeert, A.J. Mechanics of very slow human walking. Sci. Rep. 2019, 9, 18079. [Google Scholar] [CrossRef]
- Murakami, R.; Otaka, Y. Estimated lower speed boundary at which the walk ratio constancy is broken in healthy adults. J. Phys. Ther. Sci. 2017, 29, 722–725. [Google Scholar] [CrossRef]
- Vila, M.H.; Pérez, R.; Mollinedo, I.; Cancela, J.M. Analysis of gait for disease stage in patients with parkinson’s disease. Int. J. Environ. Res. Public Health 2021, 18, 720. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Lee, H.M.; Kang, S.H.; Pyo, S.J.; Kim, H.J.; Koh, S.B. Recuperation of slow walking in de novo Parkinson’s disease is more closely associated with increased cadence, rather than with expanded stride length. Gait Posture 2017, 58, 1–6. [Google Scholar] [CrossRef]
- Agrawal, P.; Abutarboush, H.F.; Ganesh, T.; Mohamed, A.W. Metaheuristic algorithms on feature selection: A survey of one decade of research (2009–2019). IEEE Access 2021, 9, 26766–26791. [Google Scholar] [CrossRef]
- Rácz, A.; Bajusz, D.; Héberger, K. Multi-level comparison of machine learning classifiers and their performance metrics. Molecules 2019, 24, 2811. [Google Scholar] [CrossRef]
- Gholamy, A.; Kreinovich, V.; Kosheleva, O. Why 70/30 or 80/20 Relation between Training and Testing Sets: A Pedagogical Explanation. Departmental Technical Reports (CS). Feburary. 2018. Available online: https://scholarworks.utep.edu/cs_techrep/1209/ (accessed on 30 November 2022).
- Kuhn, M.; Johnson, K. Over-fitting and model tuning. In Applied Predictive Modeling; Springer Nature: New York, NY, USA, 2013; pp. 1–600. [Google Scholar]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa is a double-edged sword for balance and gait in people with parkinson’s disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Cabeleira, M.E.P.; Pagnussat, A.S.; do Pinho, A.S.; Asquidamini, A.C.D.; Freire, A.B.; Pereira, B.T.; de Mello Rieder, C.R.; Schifino, G.P.; Fornari, L.H.T.; Junior, N.D.S.; et al. Impairments in gait kinematics and postural control may not correlate with dopamine transporter depletion in individuals with mild to moderate Parkinson’s disease. Eur. J. Neurosci. 2019, 49, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.; Bazán, P.R.; Oliveira, C.E.N.; Treza, R.D.C.; Hondo, S.M.; Angeles, E.L.; Bernardo, C.; Oliveira, L.D.S.; Carvalho, M.D.J.; Lima-Pardini, A.C.; et al. The effects of levodopa in the spatiotemporal gait parameters are mediated by self-selected gait speed in Parkinson’s disease. Eur. J. Neurosci. 2021, 54, 8020–8028. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.W.; Hong, W.; Ooi, C.P.; Chakraborty, S.; Barua, P.D.; Deo, R.C.; Soar, J.; Palmer, E.E.; Acharya, U.R. Application of Deep Learning Models for Automated Identification of Parkinson’s Disease: A Review (2011–2021). Sensors 2021, 21, 7034. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Correa, J.C.; Arias-Vergara, T.; Orozco-Arroyave, J.R.; Eskofier, B.M.; Klucken, J.; Noth, E. Multimodal Assessment of Parkinson’s Disease: A Deep Learning Approach. IEEE J. Biomed. Health Inform. 2019, 23, 1618–1630. [Google Scholar] [CrossRef] [PubMed]

| Details | Gait in Parkinson’s Disease Dataset | Daphnet Freezing of Gait Data Set | Smart-Insole Dataset |
|---|---|---|---|
| No. and Groups of participants | 93 PD patients/ 73 Healthy controls | 10 PD patients | 8 PD patients 9 Elderly 13 Adults |
| Types of tests | Walking for 2 min/ Dual tasking: a subset of participants | Walking straight with 180° turn/Random walking with stops and 360° turns/Simulating ADLS | Walking straight with 180° turn/Modified Timed Up and Go Test |
| Test for FoG | No | Yes | No |
| Walking pace | Normal-self-selected | Normal-self-selected | Slow, Normal, High—self-selected |
| Assessment with PD scales | H and Y staging and/or UPDRS | H and Y | 4 items of the MDS-UPDRS |
| ON and OFF medication states | Not addressed | Not addressed | Not addressed |
| Group | No. of Participants | Average Age [Years] | Age Span [Years] | Height [cm] | Weight [Kg] | Gender |
|---|---|---|---|---|---|---|
| Adults (S) | 18 | 50 | 34–59 | 171 | 76 | 8 Females, 10 Males |
| Elderly (EL) | 7 | 70 | 65–78 | 172 | 80 | 2 Females, 5 Males |
| PD patients (PD) | 19 | 63 | 29–74 | 171 | 78 | 5 Females, 14 Males |
| PD OFF State | PD ON State | PD DCIP | EL | S | |
|---|---|---|---|---|---|
| No. of Participants | 17 | 17 | 2 | 7 | 18 |
| Age [years] | 62 ± 11 | 62 ± 11 | 68 ± 8 | 70 ± 5 | 50 ± 6 |
| Disease Duration [years] | 10 ± 11 | 10 ± 11 | 17 ± 6 | N/A | N/A |
| LED * [mg] | N/A | 578 ± 174 | 1147 ± 671 | N/A | N/A |
| Total Score MDS-UPDRS-Part III | 42 ± 21 | 30 ± 20 | 33 ± 28 | N/A | N/A |
| Total Score Control Subset ** | 8 ± 7 | 5 ± 6 | 9 ± 6 | 2 ± 2 | 0 ± 1 |
| Type of Test | WST Slow | WST Normal | WST High | mTUG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group of Participants | S | EL | PD | S | EL | PD | S | EL | PD | S | EL | PD |
| Number of Recordings * | 68 | 28 | 124 | 68 | 28 | 128 | 68 | 28 | 128 | 68 | 28 | 124 |
| Left Step Duration (s) | 0.66 ± 0.09 | 0.69 ± 0.12 | 0.68 ± 0.13 | 0.60 ± 0.08 | 0.58 ± 0.07 | 0.60 ± 0.08 | 0.51 ± 0.07 | 0.49 ± 0.05 | 0.54 ± 0.09 | 0.56 ± 0.06 | 0.53 ± 0.07 | 0.57 ± 0.09 |
| Right Step Duration (s) | 0.66 ± 0.11 | 0.71 ± 0.12 | 0.69 ± 0.12 | 0.56 ± 0.06 | 0.57 ± 0.04 | 0.60 ± 0.08 | 0.52 ± 0.08 | 0.49 ± 0.04 | 0.55 ± 0.07 | 0.52 ± 0.06 | 0.54 ± 0.05 | 0.56 ± 0.07 |
| Step Duration (s) | 0.66 ± 0.09 | 0.70 ± 0.11 | 0.68 ± 0.12 | 0.58 ± 0.06 | 0.58 ± 0.05 | 0.60 ± 0.07 | 0.51 ± 0.08 | 0.49 ± 0.04 | 0.55 ± 0.06 | 0.54 ± 0.05 | 0.54 ± 0.05 | 0.57 ± 0.06 |
| Stride Duration (s) | 1.32 ± 0.18 | 1.40 ± 0.23 | 1.37 ± 0.24 | 1.16 ± 0.11 | 1.15 ± 0.09 | 1.20 ± 0.14 | 1.05 ± 0.14 | 0.97 ± 0.07 | 1.09 ± 0.12 | 1.08 ± 0.09 | 1.07 ± 0.10 | 1.14 ± 0.12 |
| Steps Number | 18.65 ± 1.40 | 18.64 ± 2.00 | 22.48 ± 6.52 | 16.00 ± 1.74 | 16.25 ± 1.46 | 19.52 ± 5.09 | 14.35 ± 1.52 | 14.39 ± 1.64 | 17.59 ± 5.13 | 15.91 ± 1.58 | 15.93 ± 1.76 | 19.54 ± 5.64 |
| Single Support Time (s) | 1.03 ± 0.24 | 1.21 ± 0.34 | 1.07 ± 0.31 | 0.87 ± 0.12 | 0.86 ± 0.08 | 0.90 ± 0.16 | 0.83 ± 0.19 | 0.78 ± 0.31 | 0.82 ± 0.10 | 1.14 ± 2.38 | 0.81 ± 0.10 | 0.90 ± 0.17 |
| Double Support Time (s) | 0.36 ± 0.15 | 0.39 ± 0.17 | 0.40 ± 0.16 | 0.35 ± 0.31 | 0.28 ± 0.08 | 0.32 ± 0.10 | 0.28 ± 0.10 | 0.23 ± 0.14 | 0.27 ± 0.08 | 0.27 ± 0.05 | 0.26 ± 0.06 | 0.28 ± 0.09 |
| Stance Time (s) | 0.84 ± 0.14 | 0.89 ± 0.17 | 0.87 ± 0.19 | 0.73 ± 0.10 | 0.71 ± 0.07 | 0.75 ± 0.11 | 0.64 ± 0.12 | 0.59 ± 0.06 | 0.66 ± 0.10 | 0.67 ± 0.07 | 0.66 ± 0.10 | 0.71 ± 0.10 |
| Swing Time (s) | 0.49 ± 0.06 | 0.52 ± 0.07 | 0.50 ± 0.08 | 0.45 ± 0.06 | 0.46 ± 0.05 | 0.47 ± 0.06 | 0.41 ± 0.06 | 0.39 ± 0.06 | 0.44 ± 0.04 | 0.43 ± 0.04 | 0.44 ± 0.05 | 0.46 ± 0.05 |
| Single Support (%) | 76.26 ± 11.64 | 84.63 ± 13.40 | 76.90 ± 12.04 | 72.49 ± 6.33 | 73.59 ± 7.26 | 73.80 ± 7.82 | 72.83 ± 6.23 | 72.80 ± 9.65 | 74.70 ± 7.12 | 74.96 ± 4.07 | 74.21 ± 5.57 | 73.45 ± 5.92 |
| Double Support (%) | 26.85 ± 8.65 | 27.37 ± 9.50 | 28.30 ± 7.35 | 26.44 ± 5.94 | 23.77 ± 5.70 | 25.85 ± 5.79 | 25.80 ± 6.63 | 23.76 ± 9.45 | 24.26 ± 5.20 | 24.15 ± 4.14 | 23.56 ± 4.13 | 23.96 ± 6.11 |
| Stance Phase (%) | 62.74 ± 3.08 | 62.74 ± 3.03 | 63.13 ± 3.53 | 61.84 ± 3.80 | 60.52 ± 3.14 | 61.06 ± 2.69 | 60.87 ± 4.01 | 60.17 ± 5.80 | 59.86 ± 3.15 | 60.65 ± 2.31 | 60.25 ± 3.28 | 60.39 ± 3.47 |
| Swing Phase (%) | 37.26 ± 3.08 | 37.26 ± 3.03 | 36.87 ± 3.53 | 38.16 ± 3.80 | 39.48 ± 3.14 | 38.94 ± 2.69 | 39.13 ± 4.01 | 39.72 ± 5.96 | 40.14 ± 3.15 | 39.35 ± 2.31 | 39.75 ± 3.28 | 39.61 ± 3.47 |
| Gait Velocity (m/s) | 0.91 ± 0.15 | 0.86 ± 0.19 | 0.78 ± 0.26 | 1.23 ± 0.21 | 1.22 ± 0.16 | 0.99 ± 0.25 | 1.52 ± 0.21 | 1.64 ± 0.25 | 1.24 ± 0.29 | 1.30 ± 0.18 | 1.34 ± 0.23 | 1.06 ± 0.28 |
| Step Length (m) | 0.57 ± 0.06 | 0.57 ± 0.06 | 0.50 ± 0.13 | 0.69 ± 0.16 | 0.66 ± 0.07 | 0.58 ± 0.14 | 0.75 ± 0.09 | 0.76 ± 0.09 | 0.65 ± 0.17 | 0.67 ± 0.07 | 0.67 ± 0.07 | 0.58 ± 0.13 |
| Stride Length (m) | 1.18 ± 0.13 | 1.17 ± 0.14 | 1.03 ± 0.29 | 1.39 ± 0.19 | 1.39 ± 0.15 | 1.18 ± 0.30 | 1.55 ± 0.18 | 1.58 ± 0.21 | 1.34 ± 0.35 | 1.39 ± 0.15 | 1.42 ± 0.18 | 1.19 ± 0.27 |
| Step Frequency (steps/min) | 88.67 ± 12.31 | 83.17 ± 12.99 | 85.84 ± 12.31 | 97.52 ± 12.26 | 98.75 ± 7.78 | 96.74 ± 10.68 | 110.95 ± 13.40 | 115.51 ± 8.26 | 105.30 ± 10.41 | 106.22 ± 10.02 | 105.73 ± 10.70 | 101.67 ± 9.56 |
| Walk Ratio (mm/step/min) | 6.62 ± 1.30 | 6.98 ± 1.05 | 5.89 ± 1.62 | 6.94 ± 1.32 | 6.74 ± 0.89 | 6.05 ± 1.78 | 6.93 ± 1.38 | 6.60 ± 1.08 | 6.14 ± 1.57 | 6.40 ± 0.99 | 6.41 ± 0.95 | 5.70 ± 1.31 |
| Type of Test | WST Slow | WST Normal | WST High | mTUG | ||||
|---|---|---|---|---|---|---|---|---|
| PD Medication State | OFF | ON | OFF | ON | OFF | ON | OFF | ON |
| Number of Recordings * | 60 | 64 | 60 | 68 | 60 | 68 | 56 | 68 |
| Left Step Duration (s) | 0.70 ± 0.14 | 0.66 ± 0.11 | 0.61 ± 0.08 | 0.59 ± 0.08 | 0.55 ± 0.09 | 0.53 ± 0.08 | 0.58 ± 0.08 | 0.57 ± 0.10 |
| Right Step Duration (s) | 0.71 ± 0.13 | 0.66 ± 0.10 | 0.61 ± 0.09 | 0.59 ± 0.07 | 0.55 ± 0.07 | 0.55 ± 0.07 | 0.56 ± 0.06 | 0.56 ± 0.08 |
| Step Duration (s) | 0.71 ± 0.13 | 0.66 ± 0.10 | 0.61 ± 0.07 | 0.59 ± 0.06 | 0.55 ± 0.06 | 0.54 ± 0.06 | 0.57 ± 0.05 | 0.57 ± 0.06 |
| Stride Duration (s) | 1.41 ± 0.26 | 1.33 ± 0.20 | 1.22 ± 0.15 | 1.18 ± 0.12 | 1.10 ± 0.13 | 1.08 ± 0.11 | 1.14 ± 0.10 | 1.13 ± 0.13 |
| Steps Number | 22.38 ± 5.27 | 22.69 ± 7.40 | 19.10 ± 4.47 | 19.88 ± 5.58 | 16.83 ± 3.77 | 18.26 ± 6.04 | 19.14 ± 4.22 | 19.87 ± 6.60 |
| Single Support Time (s) | 1.10 ± 0.32 | 1.03 ± 0.30 | 0.91 ± 0.17 | 0.88 ± 0.15 | 0.84 ± 0.12 | 0.80 ± 0.08 | 0.92 ± 0.17 | 0.87 ± 0.16 |
| Double Support Time (s) | 0.41 ± 0.19 | 0.39 ± 0.12 | 0.32 ± 0.11 | 0.31 ± 0.09 | 0.26 ± 0.09 | 0.28 ± 0.07 | 0.28 ± 0.07 | 0.29 ± 0.10 |
| Stance Time (s) | 0.91 ± 0.22 | 0.84 ± 0.15 | 0.76 ± 0.12 | 0.73 ± 0.09 | 0.66 ± 0.12 | 0.66 ± 0.08 | 0.71 ± 0.09 | 0.71 ± 0.11 |
| Swing Time (s) | 0.52 ± 0.08 | 0.49 ± 0.08 | 0.48 ± 0.05 | 0.47 ± 0.07 | 0.45 ± 0.05 | 0.43 ± 0.04 | 0.48 ± 0.05 | 0.45 ± 0.05 |
| Single Support (%) | 77.55 ± 12.52 | 76.29 ± 11.64 | 73.78 ± 8.04 | 73.81 ± 7.68 | 75.70 ± 7.97 | 73.82 ± 6.20 | 73.49 ± 4.01 | 73.42 ± 7.16 |
| Double Support (%) | 27.69 ± 8.70 | 28.88 ± 5.83 | 25.59 ± 6.11 | 26.07 ± 5.54 | 23.34 ± 5.26 | 25.08 ± 5.05 | 23.30 ± 4.80 | 24.50 ± 7.00 |
| Stance Phase (%) | 63.43 ± 4.05 | 62.85 ± 2.98 | 61.03 ± 2.66 | 61.09 ± 2.73 | 59.42 ± 3.57 | 60.24 ± 2.69 | 59.76 ± 3.17 | 60.90 ± 3.63 |
| Swing Phase (%) | 36.57 ± 4.05 | 37.15 ± 2.98 | 38.97 ± 2.66 | 38.91 ± 2.73 | 40.58 ± 3.57 | 39.76 ± 2.69 | 40.24 ± 3.17 | 39.10 ± 3.63 |
| Gait Velocity (m/s) | 0.76 ± 0.27 | 0.80 ± 0.25 | 1.00 ± 0.26 | 0.99 ± 0.24 | 1.27 ± 0.30 | 1.21 ± 0.28 | 1.06 ± 0.26 | 1.07 ± 0.30 |
| Step Length (m) | 0.50 ± 0.14 | 0.50 ± 0.13 | 0.59 ± 0.16 | 0.56 ± 0.12 | 0.67 ± 0.19 | 0.63 ± 0.15 | 0.58 ± 0.11 | 0.58 ± 0.14 |
| Stride Length (m) | 1.03 ± 0.32 | 1.03 ± 0.27 | 1.21 ± 0.34 | 1.16 ± 0.26 | 1.38 ± 0.39 | 1.31 ± 0.32 | 1.19 ± 0.24 | 1.19 ± 0.30 |
| Step Frequency (steps/min) | 83.62 ± 12.91 | 87.92 ± 11.43 | 95.25 ± 10.82 | 98.05 ± 10.46 | 104.61 ± 10.76 | 105.91 ± 10.14 | 100.81 ± 7.84 | 102.38 ± 10.78 |
| Walk Ratio (mm/step/min) | 6.04 ± 1.80 | 5.74 ± 1.41 | 6.33 ± 2.14 | 5.81 ± 1.35 | 6.26 ± 1.38 | 6.02 ± 1.72 | 5.73 ± 1.15 | 5.68 ± 1.44 |
| Type of Test | WST-Slow | WST-Normal | WST-High | mTUG | ||||
|---|---|---|---|---|---|---|---|---|
| Severity Level * | Mild | Moderate | Mild | Moderate | Mild | Moderate | Mild | Moderate |
| Number of Recordings ** | 64 | 60 | 68 | 60 | 68 | 60 | 64 | 60 |
| Left Step Duration (s) | 0.67 ± 0.1 | 0.69 ± 0.15 | 0.59 ± 0.07 | 0.61 ± 0.09 | 0.53 ± 0.1 | 0.55 ± 0.06 | 0.56 ± 0.09 | 0.59 ± 0.09 |
| Right Step Duration (s) | 0.67 ± 0.11 | 0.70 ± 0.14 | 0.59 ± 0.07 | 0.61 ± 0.09 | 0.56 ± 0.08 | 0.54 ± 0.06 | 0.56 ± 0.06 | 0.57 ± 0.08 |
| Step Duration (s) | 0.67 ± 0.09 | 0.70 ± 0.14 | 0.59 ± 0.05 | 0.61 ± 0.08 | 0.55 ± 0.06 | 0.55 ± 0.05 | 0.56 ± 0.05 | 0.58 ± 0.06 |
| Stride Duration (s) | 1.34 ± 0.19 | 1.40 ± 0.28 | 1.18 ± 0.11 | 1.22 ± 0.16 | 1.09 ± 0.13 | 1.09 ± 0.1 | 1.12 ± 0.1 | 1.15 ± 0.13 |
| Steps Number | 19.00 ± 2.95 | 26.20 ± 7.22 | 17.12 ± 2.37 | 22.23 ± 5.93 | 15.32 ± 2.11 | 20.17 ± 6.24 | 16.98 ± 2.38 | 22.27 ± 6.75 |
| Single Support Time (s) | 1.06 ± 0.26 | 1.07 ± 0.37 | 0.88 ± 0.12 | 0.92 ± 0.20 | 0.80 ± 0.07 | 0.83 ± 0.13 | 0.88 ± 0.13 | 0.91 ± 0.2 |
| Double Support Time (s) | 0.36 ± 0.09 | 0.44 ± 0.2 | 0.30 ± 0.08 | 0.33 ± 0.11 | 0.28 ± 0.09 | 0.26 ± 0.07 | 0.27 ± 0.09 | 0.29 ± 0.09 |
| Stance Time (s) | 0.84 ± 0.14 | 0.91 ± 0.22 | 0.73 ± 0.08 | 0.77 ± 0.13 | 0.66 ± 0.11 | 0.66 ± 0.08 | 0.70 ± 0.09 | 0.73 ± 0.11 |
| Swing Time (s) | 0.52 ± 0.08 | 0.49 ± 0.08 | 0.47 ± 0.05 | 0.48 ± 0.07 | 0.43 ± 0.03 | 0.45 ± 0.05 | 0.46 ± 0.05 | 0.47 ± 0.05 |
| Single Support (%) | 77.83 ± 11.15 | 75.91 ± 12.95 | 74.64 ± 7.49 | 72.85 ± 8.14 | 74.19 ± 6.61 | 75.27 ± 7.67 | 73.62 ± 5.58 | 73.28 ± 6.32 |
| Double Support (%) | 26.52 ± 4.72 | 30.21 ± 9.04 | 25.30 ± 4.71 | 26.47 ± 6.80 | 24.91 ± 4.96 | 23.53 ± 5.41 | 23.45 ± 5.72 | 24.49 ± 6.51 |
| Stance Phase (%) | 61.77 ± 2.64 | 64.58 ± 3.8 | 60.76 ± 2.40 | 61.41 ± 2.96 | 60.24 ± 3.05 | 59.42 ± 3.22 | 59.96 ± 3.37 | 60.84 ± 3.54 |
| Swing Phase (%) | 38.23 ± 2.64 | 35.42 ± 3.8 | 39.24 ± 2.40 | 38.59 ± 2.96 | 39.76 ± 3.05 | 40.58 ± 3.22 | 40.04 ± 3.37 | 39.16 ± 3.54 |
| Gait Velocity (m/s) | 0.90 ± 0.23 | 0.65 ± 0.22 | 1.11 ± 0.18 | 0.86 ± 0.24 | 1.38 ± 0.2 | 1.07 ± 0.29 | 1.20 ± 0.21 | 0.92 ± 0.28 |
| Step Length (m) | 0.57 ± 0.11 | 0.43 ± 0.11 | 0.64 ± 0.12 | 0.51 ± 0.13 | 0.72 ± 0.16 | 0.56 ± 0.14 | 0.64 ± 0.09 | 0.51 ± 0.13 |
| Stride Length (m) | 1.18 ± 0.27 | 0.87 ± 0.23 | 1.31 ± 0.27 | 1.04 ± 0.27 | 1.50 ± 0.33 | 1.16 ± 0.29 | 1.32 ± 0.2 | 1.04 ± 0.27 |
| Step Frequency (steps/min) | 87.12 ± 11.74 | 84.48 ± 12.84 | 97.70 ± 9.02 | 95.65 ± 12.27 | 105.00 ± 10.59 | 105.64 ± 10.29 | 102.52 ± 8.4 | 100.76 ± 10.66 |
| Walk Ratio (mm/step/min) | 6.63 ± 1.62 | 5.09 ± 1.17 | 6.63 ± 1.80 | 5.39 ± 1.51 | 6.78 ± 1.43 | 5.41 ± 1.39 | 6.27 ± 1.08 | 5.10 ± 1.27 |
| Statistical Significance | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Related Class | Medication State ON/OFF | MDS-UPDRS-Part III/ Severity Levels | ||||||
| Type of Test | WST Slow | WST Normal | WST High | mTUG | WST Slow | WST Normal | WST High | mTUG |
| Left Step Duration (s) | 0.010 | 0.923 | 0.665 | 0.257 | 0.154 | 0.276 | 0.300 | 0.679 |
| Right Step Duration (s) | 0.006 | 0.923 | 0.302 | 0.929 | 0.049 | 0.016 | 0.783 | 0.614 |
| Step Duration (s) | 0.003 | 0.274 | 0.216 | 0.477 | 0.055 | 0.110 | 0.349 | 0.959 |
| Stride Duration (s) | 0.004 | 0.823 | 0.213 | 0.486 | 0.055 | 0.043 | 0.415 | 0.891 |
| Steps Number | 0.018 | 0.073 | 0.000 | 0.021 | 0.018 | 0.001 | 0.434 | 0.132 |
| Single Support Time (s) | 0.010 | 0.562 | 0.000 | 0.158 | 0.011 | 0.004 | 0.114 | 0.193 |
| Double Support Time (s) | 0.454 | 0.677 | 0.023 | 0.651 | 0.504 | 0.753 | 0.124 | 0.915 |
| Stance Time (s) | 0.007 | 0.829 | 0.894 | 0.943 | 0.048 | 0.149 | 0.711 | 0.660 |
| Swing Time (s) | 0.000 | 0.003 | 0.001 | 0.040 | 0.005 | 0.000 | 0.003 | 0.217 |
| Single Support (%) | 0.074 | 0.757 | 0.003 | 0.328 | 0.061 | 0.447 | 0.593 | 0.254 |
| Double Support (%) | 0.139 | 0.247 | 0.000 | 0.809 | 0.693 | 0.169 | 0.005 | 0.871 |
| Stance Phase (%) | 0.458 | 0.289 | 0.013 | 0.290 | 0.611 | 0.006 | 0.041 | 0.811 |
| Swing Phase (%) | 0.683 | 0.222 | 0.010 | 0.345 | 0.611 | 0.006 | 0.041 | 0.811 |
| Gait Velocity (m/s) | 0.792 | 0.026 | 0.152 | 0.003 | 0.010 | 0.004 | 0.334 | 0.036 |
| Step Length (m) | 0.136 | 0.043 | 0.056 | 0.009 | 0.031 | 0.026 | 0.679 | 0.082 |
| Stride Length (m) | 0.179 | 0.037 | 0.091 | 0.012 | 0.055 | 0.042 | 0.630 | 0.060 |
| Step Frequency (steps/min) | 0.020 | 0.000 | 0.053 | 0.926 | 0.020 | 0.543 | 0.068 | 0.906 |
| Walk Ratio (mm/step/min) | 0.008 | 0.042 | 0.150 | 0.032 | 0.337 | 0.382 | 0.846 | 0.262 |
| Classification | PD-nonPD | PD-EL-S | Medication State ON/OFF | MDS-UPDRS: Part III Severity Levels | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Test | WST Slow | WST Normal | WST High | mTUG | WST Slow | WST Normal | WST High | mTUG | WST Slow | WST Normal | WST High | mTUG | WST Slow | WST Normal | WST High | mTUG |
| AdaBoost | 0.82 | 0.73 | 0.83 | 0.85 | 0.70 | 0.62 | 0.50 | 0.66 | 0.58 | 0.54 | 0.69 | 0.65 | 0.77 | 0.68 | 0.58 | 0.81 |
| Extra Trees | 0.85 | 0.73 | 0.76 | 0.80 | 0.74 | 0.64 | 0.64 | 0.57 | 0.62 | 0.64 | 0.58 | 0.73 | 0.65 | 0.68 | 0.62 | 0.69 |
| Random Forest | 0.88 | 0.73 | 0.71 | 0.75 | 0.77 | 0.60 | 0.70 | 0.57 | 0.62 | 0.61 | 0.62 | 0.62 | 0.73 | 0.57 | 0.62 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzaki, C.; Skaramagkas, V.; Kefalopoulou, Z.; Tachos, N.; Kostikis, N.; Kanellos, F.; Triantafyllou, E.; Chroni, E.; Fotiadis, D.I.; Tsiknakis, M. Can Gait Features Help in Differentiating Parkinson’s Disease Medication States and Severity Levels? A Machine Learning Approach. Sensors 2022, 22, 9937. https://doi.org/10.3390/s22249937
Chatzaki C, Skaramagkas V, Kefalopoulou Z, Tachos N, Kostikis N, Kanellos F, Triantafyllou E, Chroni E, Fotiadis DI, Tsiknakis M. Can Gait Features Help in Differentiating Parkinson’s Disease Medication States and Severity Levels? A Machine Learning Approach. Sensors. 2022; 22(24):9937. https://doi.org/10.3390/s22249937
Chicago/Turabian StyleChatzaki, Chariklia, Vasileios Skaramagkas, Zinovia Kefalopoulou, Nikolaos Tachos, Nicholas Kostikis, Foivos Kanellos, Eleftherios Triantafyllou, Elisabeth Chroni, Dimitrios I. Fotiadis, and Manolis Tsiknakis. 2022. "Can Gait Features Help in Differentiating Parkinson’s Disease Medication States and Severity Levels? A Machine Learning Approach" Sensors 22, no. 24: 9937. https://doi.org/10.3390/s22249937
APA StyleChatzaki, C., Skaramagkas, V., Kefalopoulou, Z., Tachos, N., Kostikis, N., Kanellos, F., Triantafyllou, E., Chroni, E., Fotiadis, D. I., & Tsiknakis, M. (2022). Can Gait Features Help in Differentiating Parkinson’s Disease Medication States and Severity Levels? A Machine Learning Approach. Sensors, 22(24), 9937. https://doi.org/10.3390/s22249937










