Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms
Abstract
1. Introduction
- Introducing the technological solution developed in the REHOME project, highlighting its main components, innovative features, and methodological approaches to meet the needs of patients and healthcare professionals and overcome the main weaknesses of telemedicine and eHealth services that emerge in the literature;
- Presenting three experimental protocols concerning the motor and cognitive platforms that involved groups of elderly patients affected by Parkinson’s disease (and forms of atypical parkinsonism) and mild cognitive impairment, target pathologies of the REHOME project;
- Presenting the preliminary results on the usability and user experience evaluation using questionnaires administered to the participants to get feedback on the strengths and weaknesses of the developed platforms.
2. Materials and Methods
2.1. The Architecture of the Solution
- HCP Platform (HCPP): to monitor patients remotely and to assess their progress;
- Cognitive Rehabilitation and Gaming Platform (CRGP): based on gaming to train five different cognitive domains and to improve memory and orientation skills;
- Motor Rehabilitation and Exergames Platform (MREP): for automatic assessment and rehabilitation of motor disabilities concerning limbs, posture, balance, and coordination;
- Sleep Evaluation Platform (SEP): to detect and evaluate sleep disorders.
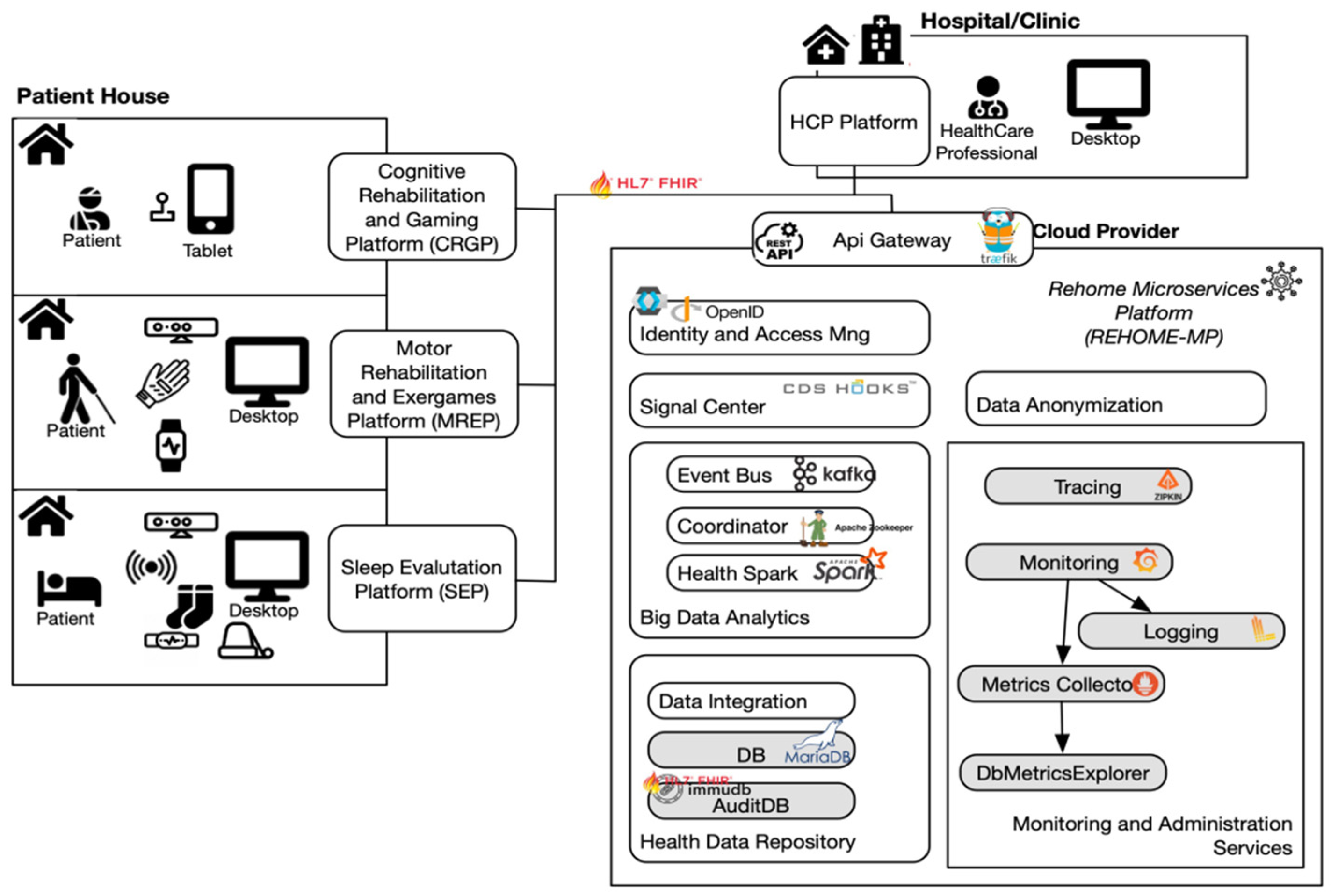
2.2. HCP Platform
- generic dashboards, common to the various diseases, summarizing the salient data in graphs and diagrams like scores, times, adherence, statistics, and variations over time of variables of interest;
- specific dashboards for each disease, tailored according to the needs and requirements of each platform (like views for specific data or domain specifics graphs, including video recording of training sessions).
2.3. Cognitive Rehabilitation and Gaming Platform
2.3.1. Spatial Memory Domain
2.4. Motor Rehabilitation and Exergames Platform
2.4.1. Assessment of the Motor Function through RGB-Depth Sensors
- Body motor tasks: traditional evaluative tasks derived from the UPDRS and balance scales, including leg agility (LA), sit-to-stand (S2S), gait (G), posture and postural stability (PoS), suitable for both parkinsonian and post-stroke hemiplegic subjects which belong to this category.
- Upper limb motor tasks: traditional evaluative tasks for the upper limbs derived from the UPDRS (i.e., finger tapping, hand movements, pronation and supination) and the MESUPES-Hand scale [53] (specifically, a subset of range-of-motion tasks) which belong to this category.
- Motor tasks in the virtual environment: to this category belong two exergames that stress motor control and coordination, specifically Lateral Weight Lifting (LWL) and Frontal Weight Lifting (FWL) of the upper limbs, and the exergame Bouncing Ball (BB), a gamified version of traditional leg agility.
2.4.2. Motor Rehabilitation with Exergames in a Virtual Environment
- Cross-country skiing (CCS): this exergame has been designed to stimulate synchronized and alternating movement of the upper limbs. Continuous and rhythmic movements of the upper limbs make a virtual skier (avatar) move on a cross-country track to the finish line. When the movement of the upper limbs is interrupted or is not rhythmic, the skier stops, thus stimulating the patient to resume the correct movement (Figure 5a). The scene reproduces a snowy scenario. Several gems are placed on the track, stimulating the patient to collect them by moving the avatar correctly. Total points and elapsed time are displayed in the upper right corner.
- Airplane (PLANE): this exergame has been designed to stimulate trunk movements and upper limb control. Trunk movements, while the arms are held in lateral extension at shoulder height, guide a virtual airplane (avatar) on a flight pathway consisting of a few rings and obstacles placed along the track. The goal is to guide the plane through the rings, avoiding the obstacles, to the end of the track. This game stimulates the user in moving the trunk correctly and continuously, while simultaneously keeping the arms in extension (Figure 5b). The scene reproduces a flight scenario. Colored circles on the flight path indicate the trajectory to follow, thus stimulating the patient to make trunk movements to pass only through the circles of the correct color, avoiding the others and obstacles. Total points and elapsed time are displayed in the upper right corner.
- Keyboard (KEY): this exergame has been designed to stimulate control of arm pointing and extension abilities. Arm movements move a virtual hand on a keyboard composed of colored keys, which light up in a predetermined sequence. Each key is associated with a sound. The goal is to repeat the proposed sequence by pressing the corresponding keys, while keeping the arm extended frontally, to compose a short “melody”. Pressing non-illuminated keys does not produce the associated sound, thus stimulating the user to correct the arm position (Figure 5c). The scene shows a piano with five colored keys. The keys light up one at a time in a random sequence, thus stimulating the patient to correctly point the extended arm at the lit key. Total points and elapsed time are displayed in the upper right corner.
2.5. Sleep Evaluation Platform
2.6. Usability Evaluation for CRGP and MREP Components
2.6.1. CRGP: Spatial Memory Domain on Elderly People with Mild Cognitive Disorders
2.6.2. MREP: Assessment of Motor Condition on PD Subjects
2.6.3. MREP: Motor Rehabilitation on Subjects with Movement Disorders
3. Results
3.1. CRGP: Usability of Spatial Memory Domain on Elderly People with Mild Cognitive Disorders
3.2. MREP: Usability of the Assessment of Motor Condition in PD Subjects
3.3. MREP: Usability of Motor Rehabilitation on Subjects with Movement Disorders
4. Discussion
- (a)
- The development and integration of various sensors and methodologies for multi-source signal collection, thus facilitating a more comprehensive analysis of patients’ overall condition and treatment effects. This novelty intends to overcome the limitations of many eHealth platforms and services that focus only on specific individual aspects without providing a comprehensive overview of the patient’s condition. Instead, this novelty could be particularly relevant from a clinical perspective, especially in complex and multi-system pathologies such as those considered by the REHOME project;
- (b)
- The use of recent, innovative, and promising methodologies in the context of motor and cognitive rehabilitation, such as gamification and exergaming in virtual environments, to favor patient condition assessment and motor–cognitive training in playful, fun, life-situation-inspired, and rewarding environments, with a focus on patients’ engagement, needs, motivations, and aesthetic preferences [101,102]. This novelty intends to foster greater patient involvement and satisfaction, thus enticing the patient to continue with the treatment according to the therapeutic plan. In this way, it could be possible to overcome the decline in interest that is one of the main issues of technological solutions as emerges in the literature;
- (c)
- Personalization of rehabilitation treatment and monitoring of different domains (motor, cognitive, sleep) through the definition of a customizable plan (e.g., in terms of type, difficulty, frequency, and duration of exercises) based on the patients’ needs, health conditions, and progress in achievement of new rehabilitation goals. This feature allows the patient’s current condition to be taken into account. On the one hand, this avoids discouraging him or her with exercises that are too difficult or stressful. This allows the therapist to easily set new therapeutic goals (e.g., increasing the difficulty or duration of exercises) that can be achieved by the patient. The lack of customization is another of the weaknesses highlighted in the literature, which does not allow a technological solution to easily and quickly adapt to different scenarios and specific needs;
- (d)
- High usability and interaction, through user interfaces specifically designed to facilitate the use of individual platforms, even in the home and unsupervised scenarios. This aspect allows the solution to be easily usable by the patient or with the support of a caregiver, but without specific technological expertise. The complexity, difficulty of use, and poor comfort of sensors and devices are known issues in the literature, which cause technological solutions to be gradually abandoned because they are often considered too complex and impractical;
- (e)
- The development of a scalable, flexible, easily extendible, cloud-based, and distributed microservices architecture based on standard HL7 FHIR protocol and models. This architectural style aims to reduce the costs of integrating additional or third-party home platforms and services to monitor and rehabilitate other pathological conditions. Moreover, using HL7 FHIR improves interoperability and data exchange with other healthcare infrastructures.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. The 2018 Ageing Report. Economic & Budgetary Projections for the 28 EU Member States (2016–2070); European Economy: Bruxelles, Belgium, 2018; ISBN 978-92-79-77460-7.
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; 267p, ISBN 978-92-4-156504-2.
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020—Highlights; United Nations: New York, NY, USA, 2020; 47p, ISBN 978-92-1-148347-5. [Google Scholar]
- Béjot, Y.; Yaffe, K. Ageing Population: A Neurological Challenge. Neuroepidemiology 2019, 52, 76–77. [Google Scholar] [CrossRef]
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Maestri, M.; Romigi, A.; Schirru, A.; Fabbrini, M.; Gori, S.; Bonuccelli, U.; Bonanni, E. Excessive daytime sleepiness and fatigue in neurological disorders. Sleep Breath. 2020, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; E Lang, C.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Park. Relat. Disord. 2016, 22 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef]
- Irazoki, E.; Contreras-Somoza, L.M.; Toribio-Guzmán, J.M.; Jenaro-Río, C.; Van der Roest, H.; Franco-Martín, M.A. Technologies for Cognitive Training and Cognitive Reha-bilitation for People with Mild Cognitive Impairment and Dementia. A Systematic Review. Front. Psychol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Steiner, B.; Saalfeld, B.; Elgert, L.; Haux, R.; Wolf, K.H. OnTARi: An ontology for factors influencing therapy adherence to rehabilitation. BMC Med. Inform. Decis. Mak. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Ogura, S.; Jakovljevic, M.M. Editorial: Global Population Aging—Health Care, Social and Economic Consequences. Front. Public Health 2018, 6, 335. [Google Scholar] [CrossRef]
- Tabish, S.A.; Nabil, S. Future of Healthcare Delivery: Strategies that will Reshape the Healthcare Industry Landscape. Int. J. Sci. Res. 2015, 4, 727–758. [Google Scholar]
- Fares, N.; Sherratt, R.; Elhajj, I. Directing and Orienting ICT Healthcare Solutions to Address the Needs of the Aging Population. Healthcare 2021, 9, 147. [Google Scholar] [CrossRef]
- Gallucci, A.; Trimarchi, P.D.; Abbate, C.; Tuena, C.; Pedroli, E.; Lattanzio, F.; Stramba-Badiale, M.; Cesari, M.; Giunco, F. ICT technologies as new promising tools for the managing of frailty: A systematic review. Aging Clin. Exp. Res. 2020, 33, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Schootemeijer, S.; van der Kolk, N.M.; Ellis, T.; Mirelman, A.; Nieuwboer, A.; Nieuwhof, F.; Schwarzschild, M.A.; de Vries, N.M.; Bloem, B.R. Barriers and Motivators to Engage in Exercise for Persons with Parkinson’s Disease. J. Park. Dis. 2020, 10, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.; Rukshan, A.; Marc, A. Designing user interfaces for the elderly: A systematic literature review. In Proceedings of the 2017 28th Australian Conference on Information Systems (ACIS 2017), Hobart, Tasmania, 3–6 December 2017; 61p. [Google Scholar]
- REHOME Project. Available online: https://progetto-rehome.it/ (accessed on 26 October 2022).
- Hosseiniravandi, M.; Kahlaee, A.H.; Karim, H.; Ghamkhar, L.; Safdari, R. Home-based telerehabilitation software systems for remote supervising: A systematic review. Int. J. Technol. Assess. Health Care 2020, 36, 113–125. [Google Scholar] [CrossRef]
- de Souza Miguel, G.F.; de Sá, A.A.R.; de Souza, J.T.; Naves, E.L.M. Home-based relerehabilitation: A review of remote therapy frameworks. Research, Society, and Development. Res. Soc. Dev. 2021, 10, e4910615489. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Tchero, H.; Tabue Teguo, M.; Lannuzel, A.; Rusch, E. Telerehabilitation for Stroke Survivors: Systematic Review and Me-ta-Analysis. J. Med. Internet Res. 2018, 20, e10867. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer’s disease and frontotemporal dementia: A systematic review. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Rabanifar, N.; Abdi, K. Barriers and Challenges of Implementing Telerehabilitation: A Systematic Review. Iran. Rehabil. J. 2021, 19, 121–128. [Google Scholar] [CrossRef]
- Ferraris, C.; Desideri, D.; Sacco, K.; Ronga, I.; Coppo, G.; Soprani, D.; Barbagallo, J.; Antinoro, S.; Ariano, P.; Privitera, L.; et al. Telerehabilitation of cognitive, motor and sleep disorders in neurological pathol-ogies: The REHOME project. In Proceedings of the 2022 IEEE Symposium on Computers and Communications (ISCC), Rhodes Island, Greece, 30 June–3 July 2022; pp. 1–6. [Google Scholar]
- Cockburn, A. Writing Effective Use Cases. Vol. 1; Addison-Wesley: Boston, MA, USA, 2001. [Google Scholar]
- FHIR HL7 R4. Available online: https://www.hl7.org/fhir/ (accessed on 26 October 2022).
- Laigner, R.; Kalinowski, M.; Diniz, P.; Barros, L.; Cassino, C.; Lemos, M.; Arruda, D.; Lifschitz, S.; Zhou, Y. From a monolithic big data system to a microservices event-driven architecture. In Proceedings of the 46th Euromicro Conference on Software Engineering and Advanced Applications (SEAA 2020), Portoroz, Slovenia, 26–28 August 2020; pp. 213–220. [Google Scholar]
- Apache Kafka. Available online: https://kafka.apache.org/ (accessed on 26 October 2022).
- FHIR CDS-HOOKS. Available online: https://cds-hooks.hl7.org/ (accessed on 26 October 2022).
- FHIR CQL. Available online: https://cql.hl7.org/ (accessed on 26 October 2022).
- Kohlmayer, F.; Prasser, F.; Eckert, C.; Kuhn, K.A. A flexible approach to distributed data anonimization. . Biomed. Inform. 2014, 50, 62–76. [Google Scholar] [CrossRef][Green Version]
- Grace, P.; Zigomitros, A.; Papageorgiou, A.; Patsakis, C.; Casino, F.; Pocs, M. Guidelines for Data Anonymization Report. OPERANDO. 2016. Available online: http://www.operando.eu/upload/operando/moduli/D4.3Guidelinesfordataanonymizationreportv1.0_77_326.pdf (accessed on 26 October 2022).
- Keycloak. Available online: https://www.keycloack.org (accessed on 26 October 2022).
- ImmuDB. Available online: https://immudb.io (accessed on 26 October 2022).
- Zipkin. Available online: https://zipkin.io (accessed on 7 October 2022).
- Grafana. Available online: https://grafana.com/ (accessed on 7 October 2022).
- Prometheus. Available online: https://prometheus.io (accessed on 7 October 2022).
- Grafana Loki. Available online: https://grafana.com/oss/loki (accessed on 7 October 2022).
- Kettner, F.; Schmidt, K.; Gaedke, M. Motivation Enhancement in mHealth via Gamification. In Proceedings of the 2019 4th International Conference on Informatics and Assistive Technologies for Healthcare, Medical Support and Wellbeing (Healthinfo 2019), Valencia, Spain, 24–28 November 2019; ISBN 978-1-61208-759-7. [Google Scholar]
- Clare, L.; Woods, B. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 2003, 4, CD003260. [Google Scholar]
- Iannizzi, P.; Bergamaschi, S.; Mondini, S.; Mapelli, D. Il Training Cognitivo Per le Demenze e le Cerebrolesioni Acquisite; Raffaello Cortina Editore: Milan, Italy, 2015; pp. 1–198. ISBN 9788860307538. [Google Scholar]
- Burgess, P.W.; Alderman, N.; Forbes, C.; Costello, A.; Laure, M.C.; Dawson, D.R.; Anderson, N.D.; Gilbert, S.J.; Dumontheil, I.; Channon, S. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Int. Neuropsychol. Soc. 2006, 12, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Angelini, M.; Calbi, M.; Ferrari, A.; Sbriscia-Fioretti, B.; Franca, M.; Gallese, V.; Umilta, M.A. Motor Inhibition during Overt and Covert Actions: An Electrical Neuroimaging Study. PLoS ONE 2015, 10, e0126800. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Moyle, J.J.; Fox, A. N2 and P3 modulation during partial inhibition in a modified go/nogo task. Int. J. Psychophysiol. 2016, 107, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, N.; MacKellar, C.; Čepukaitytė, G.; Patai, E.Z.; Nobre, A.C. Cognitive Training in the Elderly: Bottlenecks and New Avenues. J. Cogn. Neurosci. 2017, 29, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Ronga, I.; Perna, P.; Cicerale, A.; Del Fante, E.; Sarasso, P.; Geminiani, G.C. A Virtual Navigation Training Promotes the Remapping of Space in Allocentric Coordinates: Evidence from Behavioral and Neuroimaging Data. Front. Hum. Neurosci. 2022, 16, 693968. [Google Scholar] [CrossRef]
- Bellmund, J.L.S.; Gärdenfors, P.; Moser, E.I.; Doeller, C.F. Navigating cognition: Spatial codes for human thinking. Science 2018, 362, eaat6766. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Valentine, E.R.; Wilding, J.M.; Kapur, N. Routes to remembering: The brains behind superior memory. Nat. Neurosci. 2003, 6, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A. Virtual reality in episodic memory research: A review. Psychon. Bull. Rev. 2019, 26, 1213–1237. [Google Scholar] [CrossRef] [PubMed]
- Carelli, L.; Rusconi, M.L.; Scarabelli, C.; Stampatori, C.; Mattioli, F.; Riva, G. The transfer from survey (map-like) to route representations into Virtual Reality Mazes: Effect of age and cerebral lesion. J. Neuroeng. Rehabil. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Latini-Corazzini, L.; Nesa, M.P.; Ceccaldi, M.; Guedj, E.; Thinus-Blanc, C.; Cauda, F.; D’Agata, F.; Péruch, P. Route and survey processing of topographical memory during navigation. Psychol. Res. 2010, 74, 545–559. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Johansson, G.M.; Häger, C.K. Measurement properties of the Motor Evaluation Scale for Upper Extremity in Stroke patients (MESUPES). Disabil. Rehabil. 2012, 34, 288–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle Synergies in Parkinson’s Disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.E.; Adamczyk, P.G.; Ploeg, H.-L.; Pickett, K.A. Monitoring Motor Symptoms During Activities of Daily Living in Individuals with Parkinson’s Disease. Front. Neurol. 2018, 9, 1036. [Google Scholar] [CrossRef]
- Celadon, N.; Došen, S.; Binder, I.; Ariano, P.; Farina, D. Proportional estimation of finger movements from high-density surface electromyography. J. Neuroeng. Rehabil. 2016, 13, 73. [Google Scholar] [CrossRef]
- Microsoft Azure. Available online: https://azure.microsoft.com/it-it/services/kinect-dk/ (accessed on 26 October 2022).
- Liu, Z. 3D Skeletal Tracking on Azure Kinect. 2019. Available online: https://www.microsoft.com/en-us/research/uploads/prod/2020/01/AKBTSDK.pdf (accessed on 7 October 2022).
- Rosa, B.; Colombo Zefinetti, F.; Vitali, A.; Regazzoni, D. RGB-D Sensors as Marker-Less MOCAP Systems: A Comparison Between Microsoft Kinect V2 and the New Microsoft Kinect Azure. 2021. In Advances in Simulation and Digital Human Modeling; Wright, J.L., Barber, D., Scataglini, S., Rajulu, S.L., Eds.; AHFE 2021. Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2021; Volume 264, pp. 359–367. [Google Scholar]
- Albert, J.A.; Owolabi, V.; Gebel, A.; Brahms, C.M.; Granacher, U.; Arnrich, B. Evaluation of the Pose Tracking Performance of the Azure Kinect and Kinect v2 for Gait Analysis in Comparison with a Gold Standard: A Pilot Study. Sensors 2020, 20, 5104. [Google Scholar] [CrossRef]
- Romeo, L.; Marani, R.; Malosio, M.; Perri, A.G.; D’Orazio, T. Performance Analysis of Body Tracking with the Microsoft Azure Kinect. In Proceedings of the 2021 29th Mediterranean Conference on Control and Automation (MED), Puglia, Italy, 22–25 June 2021; pp. 572–577. [Google Scholar]
- Tölgyessy, M.; Dekan, M.; Chovanec, Ľ.; Hubinský, P. Evaluation of the Azure Kinect and Its Comparison to Kinect V1 and Kinect V2. Sensors 2021, 21, 413. [Google Scholar]
- Yeung, L.-F.; Yang, Z.; Cheng, K.C.-C.; Du, D.; Tong, R.K.-Y. Effects of camera viewing angles on tracking kinematic gait patterns using Azure Kinect, Kinect v2 and Orbbec Astra Pro v2. Gait Posture 2021, 87, 19–26. [Google Scholar] [CrossRef]
- Tölgyessy, M.; Dekan, M.; Chovanec, L. Skeleton Tracking Accuracy and Precision Evaluation of Kinect V1, Kinect V2, and the Azure Kinect. Appl. Sci. 2021, 11, 5756. [Google Scholar] [CrossRef]
- Clark, R.A.; Mentiplay, B.F.; Hough, E.; Pua, Y.H. Three-dimensional cameras and skeleton pose tracking for physical function assessment: A review of uses, validity, current developments and Kinect alternatives. Gait Posture 2019, 68, 193–200. [Google Scholar] [CrossRef]
- Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Cau, N.; Cimolin, V.; Azzaro, C.; Albani, G.; Priano, L.; Mauro, A. A Self-Managed System for Automated Assessment of UPDRS Upper Limb Tasks in Parkinson’s Disease. Sensors 2018, 18, 3523. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Cau, N.; Cimolin, V.; Azzaro, C.; Priano, L.; Mauro, A. Feasibility of Home-Based Automated Assessment of Postural Insta-bility and Lower Limb Impairments in Parkinson’s Disease. Sensors 2019, 19, 1129. [Google Scholar] [CrossRef] [PubMed]
- Ťupa, O.; Procházka, A.; Vyšata, O.; Schätz, M.; Mareš, J.; Vališ, M.; Mařík, V. Motion tracking and gait feature estimation for recognising Parkinson’s disease using MS Kinect. Biomed. Eng. Online 2015, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Vismara, L.; Ferraris, C.; Amprimo, G.; Pettiti, G.; Lopez, R.; Galli, M.; Cremascoli, R.; Sinagra, S.; Mauro, A.; et al. Computation of Gait Parameters in Post Stroke and Parkinson’s Disease: A Comparative Study Using RGB-D Sensors and Optoelectronic Systems. Sensors 2022, 22, 824. [Google Scholar] [CrossRef] [PubMed]
- Ospina, B.M.; Chaparro, J.A.V.; Paredes, J.D.A.; Pino, Y.J.C.; Navarro, A.; Orozco, J.L. Objective Arm Swing Analysis in Early-Stage Parkinson’s Disease Using an RGB-D Camera (Kinect®)1. J. Park. Dis. 2018, 8, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Eltoukhy, M.; Kuenze, C.; Oh, J.; Wooten, S.; Signorile, J. Kinect-based assessment of lower limb kinematics and dynamic postural control during the star excursion balance test. Gait Posture 2017, 58, 421–427. [Google Scholar] [CrossRef]
- Ayed, I.; Jaume-I-Capó, A.; Martínez-Bueso, P.; Mir, A.; Moyà-Alcover, G. Balance Measurement Using Microsoft Kinect v2: Towards Remote Evaluation of Patient with the Functional Reach Test. Appl. Sci. 2021, 11, 6073. [Google Scholar] [CrossRef]
- Ferraris, C.; Amprimo, G.; Masi, G.; Vismara, L.; Cremascoli, R.; Sinagra, S.; Pettiti, G.; Mauro, A.; Priano, L. Evaluation of Arm Swing Features and Asymmetry during Gait in Parkinson’s Disease Using the Azure Kinect Sensor. Sensors 2022, 22, 6282. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, B.; Hart, R.; Zhang, Y. The validity of a dual Azure Kinect-based motion capture system for gait analysis: A preliminary study. In Proceedings of the 2020 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC), Auckland, New Zealand, 7–10 December 2020; pp. 1201–1206. [Google Scholar]
- Antico, M.; Balletti, N.; Laudato, G.; Lazich, A.; Notarantonio, M.; Oliveto, R.; Ricciardi, S.; Scalabrino, S.; Simeone, J. Postural control assessment via Microsoft Azure Kinect DK: An evaluation study. Comput. Methods Programs Biomed. 2021, 209, 106324. [Google Scholar] [CrossRef]
- Amprimo, G.; Pettiti, G.; Priano, L.; Mauro, A.; Ferraris, C. Kinect-based Solution for the Home Monitoring of Gait and Balance in Elderly People with and without Neurological Diseases. In Proceedings of the 2021 2nd Italian Workshop on Artificial Intelligence for an Ageing Society (AI*IA 2021), Online, 29 November 2021; Available online: http://ceur-ws.org/Vol-3108/paper6.pdf (accessed on 26 October 2022).
- Lugaresi, C.; Tang, J.; Nash, H.; McClanahan, C.; Uboweja, E.; Hays, M.; Zhang, F.; Chang, C.; Yong, M.G.; Lee, J.; et al. MediaPipe: A framework for building perception pipelines. arXiv 2019, arXiv:1906.08172. [Google Scholar]
- Zhang, F.; Bazarevsky, V.; Vakunov, A.; Tkachenka, A.; Sung, G.; Chang, C.L.; Grundmann, M. Mediapipe hands: On-device real-time hand tracking. arXiv 2020, arXiv:1906.08172. [Google Scholar]
- Amprimo, G.; Ferraris, C.; Masi, G.; Pettiti, G.; Priano, L. GMH-D: Combining Google MediaPipe and RGB-Depth Cameras for Hand Motor Skills Remote Assessment. In Proceedings of the 2022 IEEE International Conference on Digital Health (ICDH), Barcelona, Spain, 10–16 July 2022; pp. 132–141. [Google Scholar]
- Technologies, U. Unity Real-Time Development Platform|3D, 2D VR & AR Visualizations. Available online: https://unity.com/ (accessed on 26 October 2022).
- Aşkın, A.; Atar, E.; Koçyiğit, H.; Tosun, A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somat. Mot. Res. 2018, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; Le, H.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Lee, H.C.; Huang, C.L.; Ho, S.H.; Sung, W.H. The Effect of a Virtual Reality Game Intervention on Balance for Patients with Stroke: A Ran-domized Controlled Trial. Games Health J. 2017, 6, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Pachoulakis, I.; Papadopoulos, N.; Analyti, A.; Pachoulakis, I.; Papadopoulos, N. Kinect-Based Exergames Tailored to Par-kinson Patients. Int. J. Comput. Games Technol. 2018, 2018, 2618271. [Google Scholar] [CrossRef]
- De Oliveira, L.C.; Mendes, L.C.; de Lopes, R.A.; Carneiro, J.A.; Cardoso, A.; Júnior, E.A.; de Oliveira Andrade, A. A systematic review of serious games used for rehabilitation of individuals with Parkinson’s disease. Res. Biomed. Eng. 2021, 37, 849–865. [Google Scholar] [CrossRef]
- Garcia-Agundez, A.; Folkerts, A.-K.; Konrad, R.; Caserman, P.; Tregel, T.; Goosses, M.; Göbel, S.; Kalbe, E. Recent advances in rehabilitation for Parkinson’s Disease with Exergames: A Systematic Review. J. Neuroeng. Rehabil. 2019, 16, 17. [Google Scholar] [CrossRef]
- Silva, K.; De Freitas, T.B.; Doná, F.; Ganança, F.F.; Ferraz, H.B.; Torriani-Pasin, C.; Pompeu, J.E. Effects of virtual rehabilitation versus conventional physical therapy on postural control, gait, and cognition of patients with Parkinson’s disease: Study protocol for a randomized controlled feasibility trial. Pilot Feasibility Stud. 2017, 3, 68. [Google Scholar] [CrossRef]
- Barry, G.; Galna, B.; Rochester, L. The role of exergaming in Parkinson’s disease rehabilitation: A systematic review of the evidence. J. Neuroeng. Rehabil. 2014, 11, 33. [Google Scholar] [CrossRef]
- Chiuchisan, I.; Geman, O.; Postolache, O. Future Trends in Exergaming using MS Kinect for Medical Rehabilitation. In Proceedings of the 2018 International Conference and Exposition on Electrical and Power Engineering (EPE 2018), Iasi, Romania, 18–19 October 2018; pp. 683–687. [Google Scholar]
- Fallmann, S.; Chen, L. Detecting chronic diseases from sleep-wake behaviour and clinical features. In Proceedings of the 2018 5th International Conference on System and Informatics (ICSAI 2018), Nanjing, China, 10–12 November 2018; pp. 1076–1084. [Google Scholar]
- Lee, J.; Hong, M.; Ryu, S. Sleep Monitoring System Using Kinect Sensor. Int. J. Distrib. Sens. Netw. 2015, 11, 875371. [Google Scholar] [CrossRef]
- Mendonca, F.; Mostafa, S.S.; Morgado-Dias, F.; Ravelo-Garcia, A.G.; Penzel, T. A Review of Approaches for Sleep Quality Analysis. IEEE Access 2019, 7, 24527–24546. [Google Scholar] [CrossRef]
- Faust, O.; Razaghi, H.; Barika, R.; Ciaccio, E.J.; Acharya, U.R. A review of automated sleep stage scoring based on physiological signals for the new millennia. Comput. Methods Programs Biomed. 2019, 176, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Surantha, N.; Lesmana, T.F.; Isa, S.M. Sleep stage classification using extreme learning machine and particle swarm optimi-zation for healthcare big data. J. Big Data 2021, 8, 14. [Google Scholar] [CrossRef]
- Eldele, E.; Chen, Z.; Liu, C.; Wu, M.; Kwoh, C.K.; Li, X.; Guan, C. An Attention-Based Deep Learning Approach for Sleep Stage Classification with Single-Channel EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Big-ECG: Cardiographic Predictive Cyber-Physical System for Stroke Management. IEEE Access 2021, 9, 123146–123164. [Google Scholar] [CrossRef]
- Maramba, I.; Chatterjee, A.; Newman, C. Methods of usability testing in the development of eHealth applications: A scoping review. Int. J. Med. Inform. 2019, 126, 95–104. [Google Scholar] [CrossRef]
- Lewis, J.R. The System Usability Scale: Past, Present, and Future. Int. J. Hum. Comput. Interact. 2018, 34, 577–590. [Google Scholar] [CrossRef]
- Sarasso, P.; Neppi-Modona, M.; Sacco, K.; Ronga, I. “Stopping for knowledge”: The sense of beauty in the perception-action cycle. Neurosci. Biobehav. Rev. 2020, 118, 723–738. [Google Scholar] [CrossRef]
- Amprimo, G.; Masi, G.; Priano, L.; Azzaro, C.; Galli, F.; Pettiti, G.; Mauro, A.; Ferraris, C. Assessment Tasks and Virtual Ex-ergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect. Sensors 2022, 22, 8173. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, P.; Neppi-Modona, M.; Rosaia, N.; Perna, P.; Barbieri, P.; Del Fante, E.; Ricci, R.; Sacco, K.; Ronga, I. Nice and Easy: Mismatch Negativity Responses Reveal a Significant Correlation Between Aesthetic Appreciation and Perceptual Learning. J. Exp. Psychol. Gen. 2022, 151, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Cros, M.; Sebastian-Romagosa, M.; Rodríguez-Serrano, J.; Opisso, E.; Ochoa, M.; Ortner, R.; Guger, C.; Tost, D. Effects of gamification in BCI functional rehabilitation. Front. Neurosci. 2020, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, P.; Ronga, I.; Neppi-Modona, M.; Sacco, K. The Role of Musical Aesthetic Emotions in Social Adaptation to the COVID-19 Pandemic. Front. Psychol. 2021, 12, 611639. [Google Scholar] [CrossRef]
- Sarasso, P.; Perna, P.; Barbieri, P.; Neppi-Modona, M.; Sacco, K.; Ronga, I. Memorisation and implicit perceptual learning are enhanced for preferred musical intervals and chords. Psychon. Bull. Rev. 2021, 28, 1623–1637. [Google Scholar] [CrossRef]
- Sarasso, P.; Barbieri, P.; Del Fante, E.; Bechis, L.; Neppi-Modona, M.; Sacco, K.; Ronga, I. Preferred music listening is associated with perceptual learning enhancement at the expense of self-focused attention. Psychon. Bull. Rev. 2022, 28, 1623–1637. [Google Scholar] [CrossRef]
- Da Gama, A.; Fallavollita, P.; Teichrieb, V.; Navab, N. Motor Rehabilitation Using Kinect: A Systematic Review. Games Health J. 2015, 4, 123–135. [Google Scholar] [CrossRef]
- Palacios-Navarro, G.; Garcia-Magarino, I.; Ramos-Lorente, P. A Kinect-Based System for Lower Limb Rehabilitation in Par-kinson’s Disease Patients: A Pilot Study. J. Med. Syst. 2015, 39, 103. [Google Scholar] [CrossRef]
- Shih, M.-C.; Wang, R.-Y.; Cheng, S.-J.; Yang, Y.-R. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 78. [Google Scholar] [CrossRef]
- De Melo Cerqueira, T.M.; de Moura, J.A.; de Lira, J.O.; Leal, J.C.; D’Amelio, M.; do Santos Mendes, F.A. Cognitive and motor effects of Kinect-based games training in people with and without Parkinson disease: A preliminary study. Physiother. Res. Int. 2020, 25, e1807. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, M.; Wang, X.; Fan, Y.; Chen, X.; Yao, L.; Zhang, H.; Ma, Z. Design and Evaluation of an Exergame System of Knee with the Azure Kinect. In Data Science ICPCSEE 2021. Communications in Computer and Information Science; Zeng, J., Qin, P., Jing, W., Song, X., Lu, Z., Eds.; Springer: Singapore, 2021; Volume 1452, pp. 331–342. [Google Scholar]







| Domain | Skills | Approach | Technology/Devices |
|---|---|---|---|
| Spatial memory domain |
| 3D Videogame spatial navigation in a virtual city (MindTheCity!) | Tablet, keyboard or joystick |
| Cognitive–motor rehabilitation domain |
| 3D Videogame—exploring a virtual desert island to activate specific mini-games (MindTheCraft!) | Tablet, keyboard or joystick |
| Single domain and ecological exercises |
| 360-degree exploration of environments and cognitive exercises in daily life virtual contexts | Tablet |
| Function | Skills | Approach | Technology/Devices |
|---|---|---|---|
| Assessment of motor condition |
| Standardized motor tasks and exercises in virtual environment using non-invasive body tracking algorithms | RGB-Depth camera (Azure Kinect DK, Microsoft®, Microsoft Corporation, Redmond, WA, USA) |
| Motor rehabilitation |
| 3D exergames in virtual environment | RGB-Depth camera (Azure Kinect DK, Microsoft®, Microsoft Corporation, Redmond, WA, USA) |
| Upper limb rehabilitation |
| Hand movement exercises using sEMG | sEMG Armband (REMO®, Morecognition s.r.l., Turin, Italy) |
| Function | Signals and Parameters | Technology/Devices |
|---|---|---|
| Evaluation of sleep disorders | Respiratory rate, hearth rate (ECG) | Chest strap (sensor prototype by Astel s.r.l., Pavone Canavese, Turin, Italy) |
| EEG, EOG, orinasal flux | Cap (sensor prototype by Astel s.r.l., Pavone Canavese, Turin, Italy) | |
| Periodic Leg Movements | Socks (sensor prototype by Astel s.r.l., Pavone Canavese, Turin, Italy) | |
| Room noise, temperature, humidity, illumination | Environmental sensor (Omron®, Kyoto, Japan) | |
| Presence in bed, quantity of movement, respiration and hearth rates | Pressure band (Emfit QS®, Emfit Ltd., Vaajakoski, Finland) | |
| Relevant body movement events | RGB-Depth camera (Azure Kinect DK, Microsoft®, Microsoft Corporation, Redmond, San Francisco, CA, USA)—only infrared streaming |
| Platform | # Subjects | Age | Target | Exclusion Criteria |
|---|---|---|---|---|
| CRGP—Spatial memory domain | 28 (14 M/14 F) | 73.0 ± 5.0 | Elderly people affected by mild neurocognitive disorder (DSM-5) |
|
| MREP—Assessment of motor condition | 27 (14 M/13 F) | 69.8 ± 9.1 | Subjects affected by Parkinson’s disease |
|
| MREP—Motor rehabilitation | 15 (8 M/7 F) | 71.4 ± 7.2 | Subjects affected by Parkinson’s disease, atypical parkinsonism |
|
| Questionnaire Categories (Reference Items) | % Positive Rating 1 (N = 27) |
|---|---|
| Usefulness (items 3,4) 2 | 87.1% |
| Usability (items 5,6,7) 2 | 90.1% |
| Easy-of-use (item 8) | 91.7% |
| Easy-of-use (item 9) 3 | 88.9% |
| User Engagement (item 10) 3 | 87.7% |
| User Perceived Status (items 11,12) 2 | 98.2% |
| Overall Satisfaction (item 2) | 96.3% |
| Questionnaire Categories (Reference Items) | % Positive Rating 1 (N = 15) |
|---|---|
| User satisfaction (item 1) | 70.0% |
| Easy-of-use (items 2, 3, 4) 2 | 53.0% |
| System coherence (items 5, 6) 2 | 85.0% |
| Usability (items 7, 8, 9, 10) 2 | 60.0% |
| Overall Satisfaction 3 | 50.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraris, C.; Ronga, I.; Pratola, R.; Coppo, G.; Bosso, T.; Falco, S.; Amprimo, G.; Pettiti, G.; Lo Priore, S.; Priano, L.; et al. Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms. Sensors 2022, 22, 9467. https://doi.org/10.3390/s22239467
Ferraris C, Ronga I, Pratola R, Coppo G, Bosso T, Falco S, Amprimo G, Pettiti G, Lo Priore S, Priano L, et al. Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms. Sensors. 2022; 22(23):9467. https://doi.org/10.3390/s22239467
Chicago/Turabian StyleFerraris, Claudia, Irene Ronga, Roberto Pratola, Guido Coppo, Tea Bosso, Sara Falco, Gianluca Amprimo, Giuseppe Pettiti, Simone Lo Priore, Lorenzo Priano, and et al. 2022. "Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms" Sensors 22, no. 23: 9467. https://doi.org/10.3390/s22239467
APA StyleFerraris, C., Ronga, I., Pratola, R., Coppo, G., Bosso, T., Falco, S., Amprimo, G., Pettiti, G., Lo Priore, S., Priano, L., Mauro, A., & Desideri, D. (2022). Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms. Sensors, 22(23), 9467. https://doi.org/10.3390/s22239467







