Validation of Wearable Device Consisting of a Smart Shirt with Built-In Bioelectrodes and a Wireless Transmitter for Heart Rate Monitoring in Light to Moderate Physical Work
Abstract
1. Introduction
2. Materials and Methods
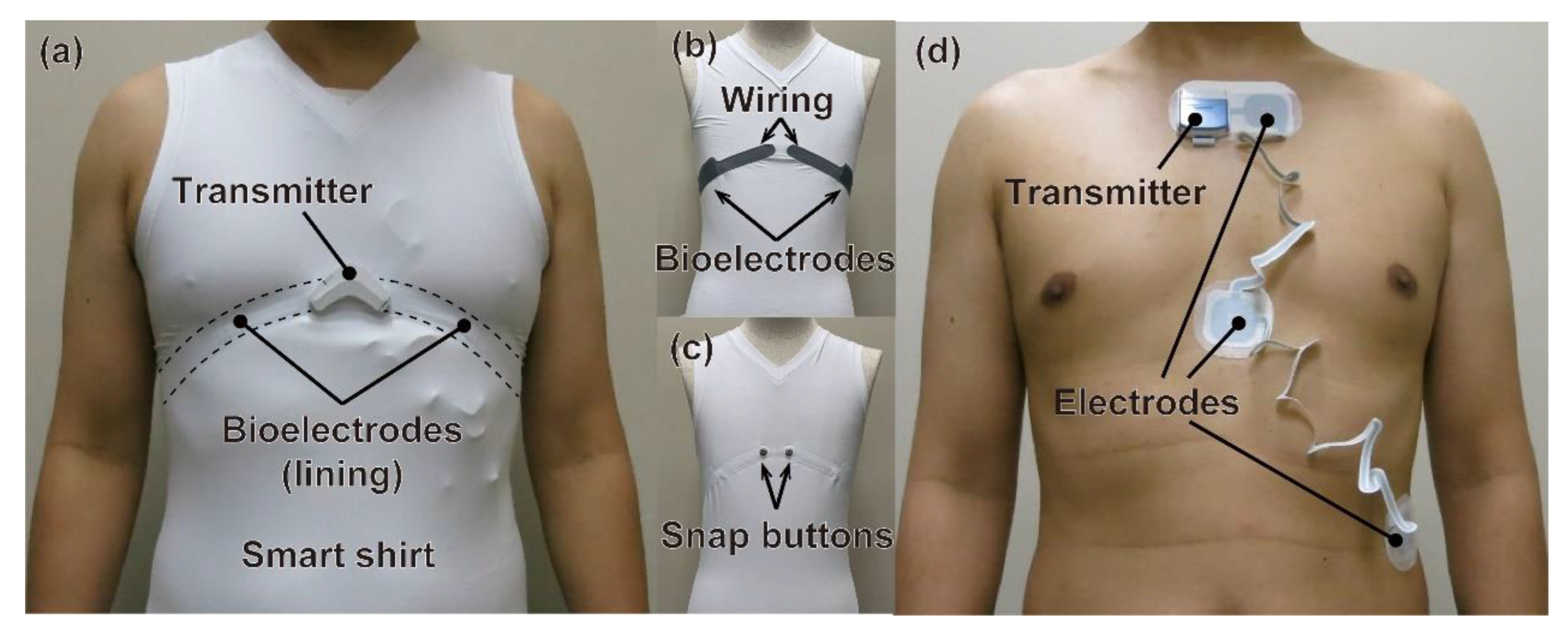
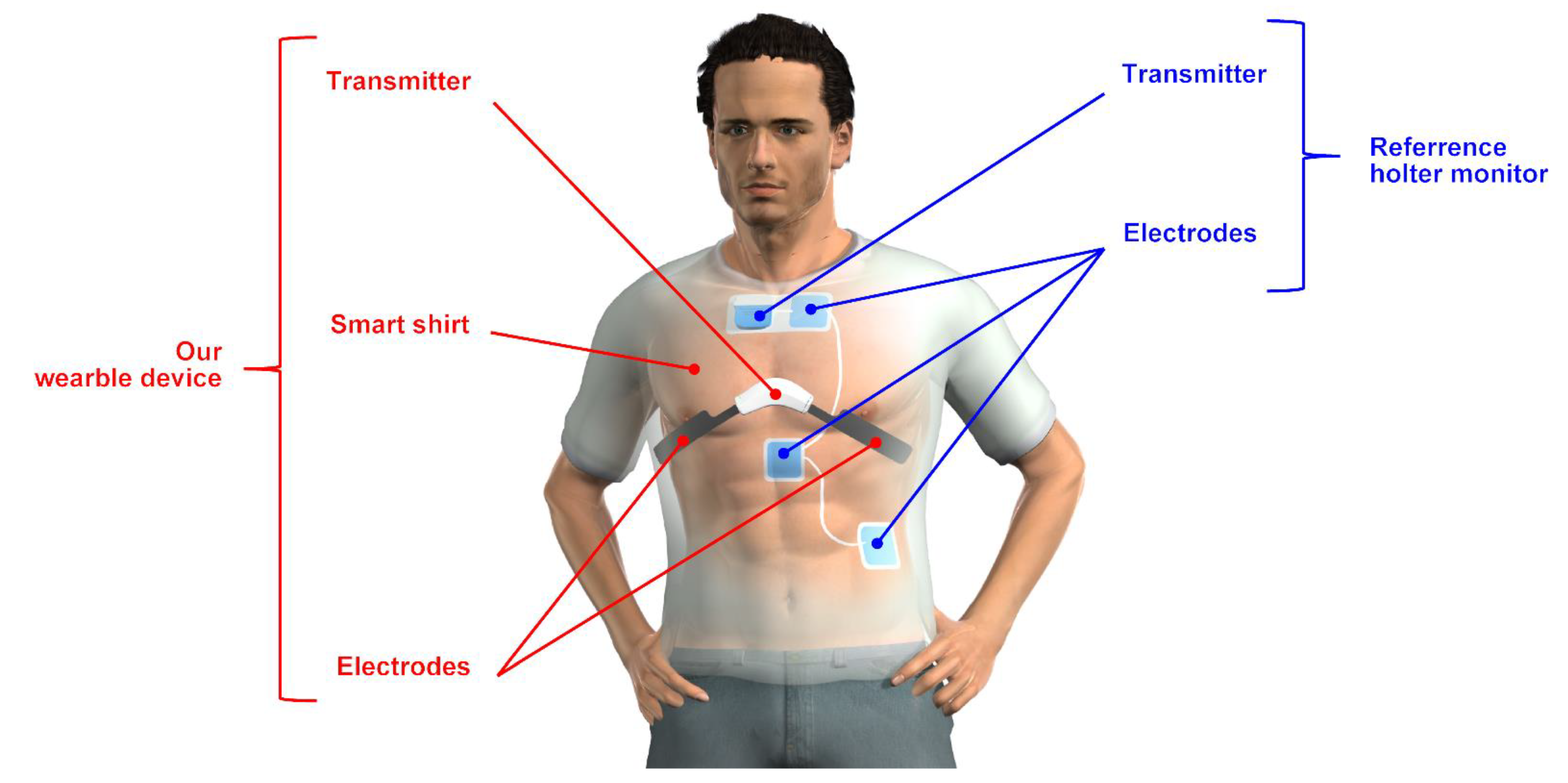
2.1. Device
2.1.1. Developed Wearable Device
2.1.2. Reference Holter Monitor
2.2. Experimental Conditions
2.2.1. Subjects and Ethical Approval
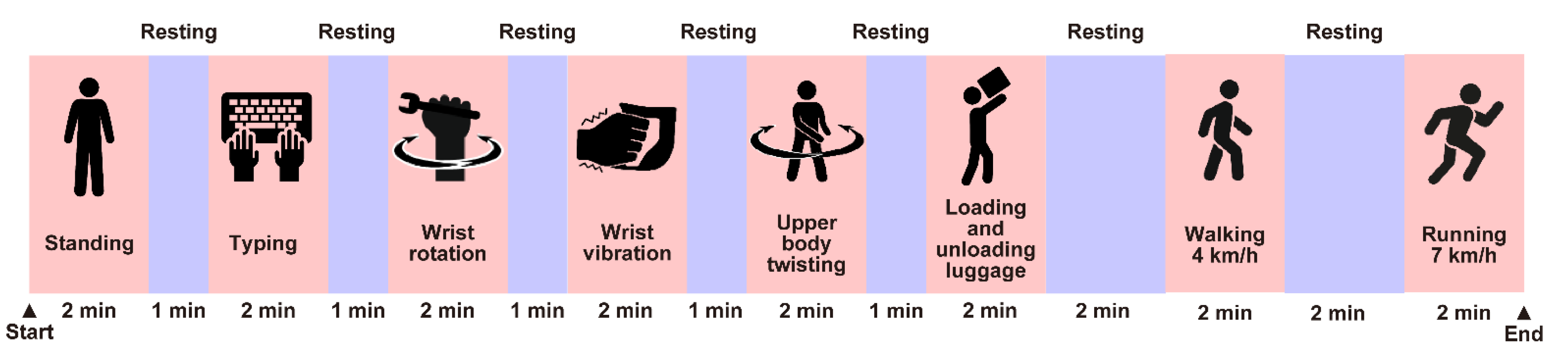
2.2.2. Experimental Design
2.3. Data Analysis
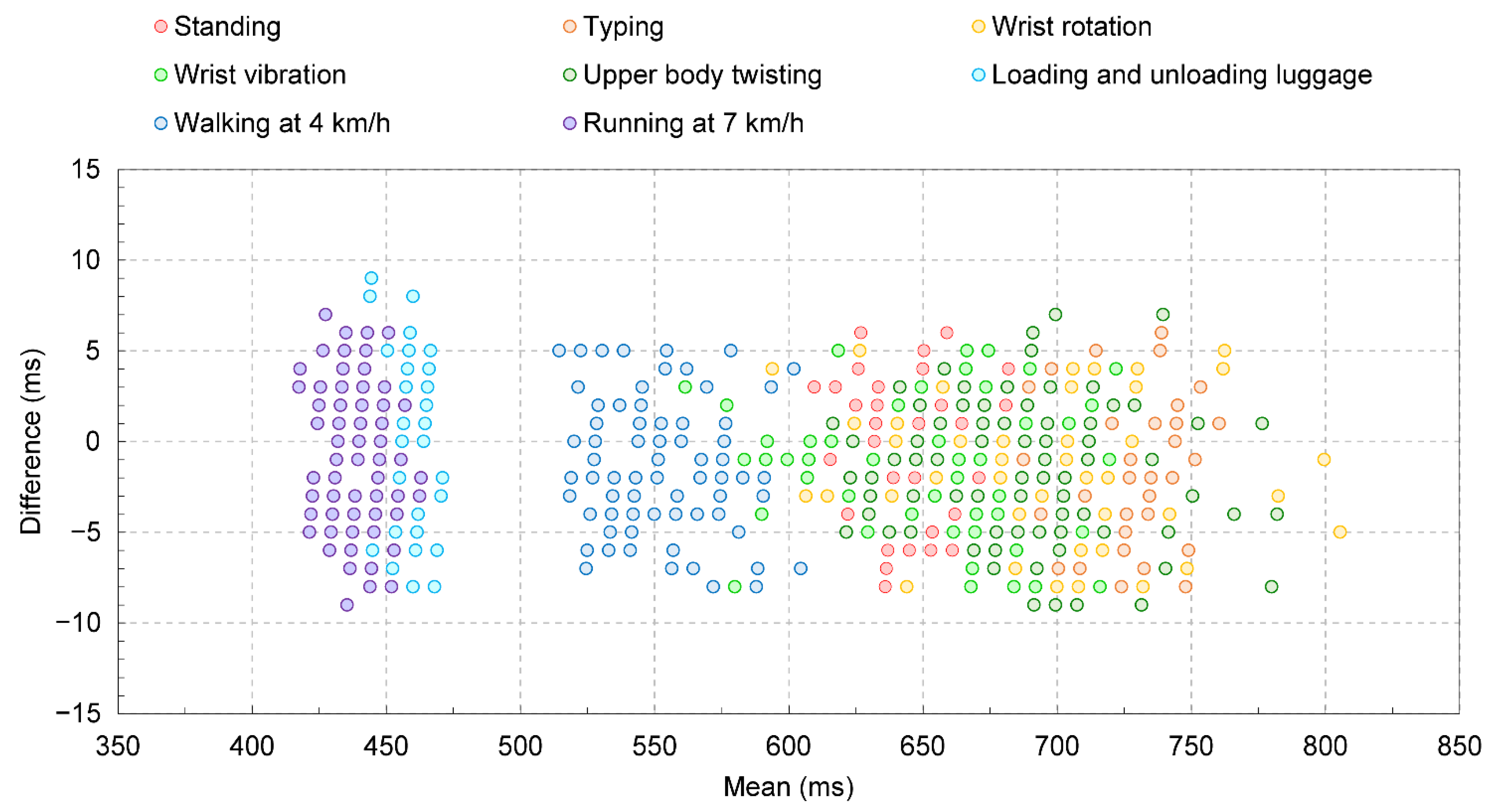
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vishal, P.; Austin, C.; Christopher, M.L.; Santosh, P. Trends in Workplace Wearable Technologies and Connected-Worker Solutions for Next-Generation Occupational Safety, Health, and Productivity. Adv. Intell. Syst. 2022, 4, 2100099. [Google Scholar]
- Aroganam, G.; Manivannan, N.; Harrison, D. Review on Wearable Technology Sensors Used in Consumer Sport Applications. Sensors 2019, 19, 1983. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Aydin, A.; Brunckhorst, O.; Dasgupta, P.; Ahmed, K. A Review of Wearable Technology in Medicine. J. R. Soc. Med. 2016, 109, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the in-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef] [PubMed]
- Yuki, H.; Takako, I.; Kei, K.; Tatsuro, A.; Hiroyoshi, T. Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations. Micromachines 2022, 13, 575. [Google Scholar]
- Cardinale, M.; Varley, M.C. Wearable Training-Monitoring Technology: Applications, Challenges, and Opportunities. Int. J. Sport. Physiol. Perform. 2017, 12 (Suppl. S2), S255–S262. [Google Scholar] [CrossRef]
- Schneider, C.; Hanakam, F.; Wiewelhove, T.; Doweling, A.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; Ferrauti, A. Heart Rate Monitoring in Team Sports-A Conceptual Framework for Contextualizing Heart Rate Measures for Training and Recovery Prescription. Front. Physiol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, Y.; Welk, G.J. Validity of Consumer-Based Physical Activity Monitors. Med. Sci. Sports Exerc. 2014, 46, 1840–1848. [Google Scholar] [CrossRef]
- Sushames, A.; Edwards, A.; Thompson, F.; McDermott, R.; Gebel, K. Validity and Reliability of Fitbit Flex for Step Count, Moderate to Vigorous Physical Activity and Activity Energy Expenditure. PLoS ONE 2016, 11, e0161224. [Google Scholar] [CrossRef]
- Kei, K.; Akio, T.; Yuki, H.; Yuichi, H.; Hiroyoshi, T. Wearable Biological/environmental Sensor and its application for smart healthcare services. NTT Tech. Rev. 2020, 18, 46–51. [Google Scholar]
- Lutz, J.; Memmert, D.; Raabe, D.; Dornberger, R.; Donath, L. Wearables for Integrative Performance and Tactic Analyses: Opportunities, Challenges, and Future Directions. Int. J. Environ. Res. Public Health 2020, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Alves, J.L.B.; Silva, J.H.D.C.; Costa, M.D.S.; Silva, A.S. Validity of a Smartphone Application and Chest Strap for Recording RR Intervals at Rest in Athletes. Int. J. Sport. Physiol. Perform. 2020, 15, 896–899. [Google Scholar] [CrossRef]
- Jerald, Y.; Long, Y.; Seulki, L.; Hyejung, K.; Hoi, J.Y. A Wearable ECG Acquisition System With Compact Planar-Fashionalble Circuit Board Shirt. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 897–902. [Google Scholar]
- Gilgen-Ammann, R.; Roos, L.; Wyss, T.; Veenstra, B.J.; Delves, S.K.; Beeler, N.; Buller, M.J.; Friedl, K.E. Validation of ambulatory monitoring devices to measure energy expenditure and heart rate in a military setting. Physiol. Meas. 2021, 42, 085008. [Google Scholar] [CrossRef] [PubMed]
- Marco, D.R.; Vittorio, R.; Francesco, R.; Bruno, B.; Gianfranco, P.; Paolo, C.; Paolo, M.; Maurizio, F. Evaluation of a textile-based wearable system for the electrocardiogram monitoring in cardiac patients. EP Eur. 2013, 15, 607–612. [Google Scholar]
- Katya, A.; Andrea, K.W.; Allison, P.A. Textile Electrocardiogram (ECG) Electrodes for Wearable Health Monitoring. Sensors 2020, 20, 1013. [Google Scholar]
- Constant, I.; Laude, D.; Murat, I.; Elghozi, J.L. Pulse rate variability is not a surrogate for heart rate variability. Clin. Sci. 1999, 97, 391–397. [Google Scholar] [CrossRef]
- Koichi, F.; Erika, A.; Keisuke, K.; Chikao, N.; Yoko, S.; Toshitaka, Y.; Toshihiro, H.; Manabu, K.; Yukiyoshi, S.; Fumi, M.; et al. Heart Rate Variability-Based Driver Drowsiness Detection and Its Validation With EEG. IEEE Trans. Biomed. Eng. 2019, 66, 1769–1778. [Google Scholar]
- Nobuaki, M.; Kei, K.; Takayuki, O. Lightweight heartbeat detection algorithm for consumer grade wearable ECG measurement devices and its implementation. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 4299–4302. [Google Scholar]
- Akimasa, H.; Taiki, M.; Ryota, U.; Sachiko, K.; Yuki, H.; Kazuhiko, T.; Yuichi, H.; Hiroyoshi, T.; Takashi, K.; Hideto, T. Body core temperature estimation using new compartment model with vital data from wearable devices. IEEE Access 2021, 9, 124452–124462. [Google Scholar]
- Yayoi, T.T.; Miwa, T.; Hiroshige, M.; Yasuhiro, H.; Kenji, Y.; Yuki, I.; Kuniya, A.; Wataru, S.; Nahoko, K.; Hiroshi, N.; et al. Validation of wearable textile electrodes for ECG monitoring. Heart Vessel. 2019, 34, 1203–1211. [Google Scholar]
- Kana, E.; Ryosuke, A.; Suehiro, S.; Kazuhiro, Y.; Tomohiro, Y. R-R Interval Outlier Processing for Heart Rate Variability Analysis using Wearable ECG Devices. Adv. Biomed. Eng. 2018, 7, 28–38. [Google Scholar]
- ISO8996; Ergonomics of the Thermal Environment—Determination of Metabolic Rate. ISO: Geneva, Switzerland, 2021.

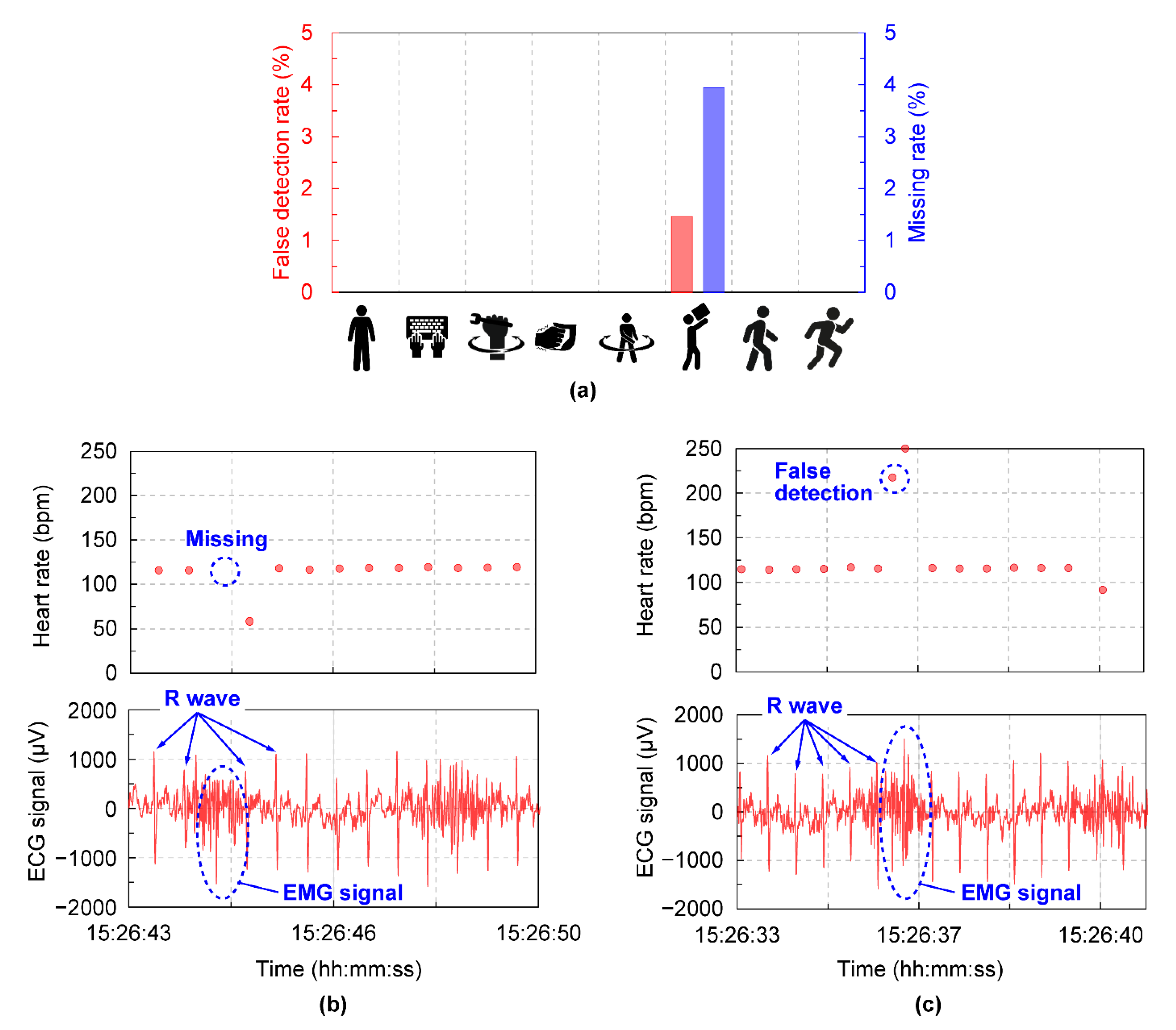
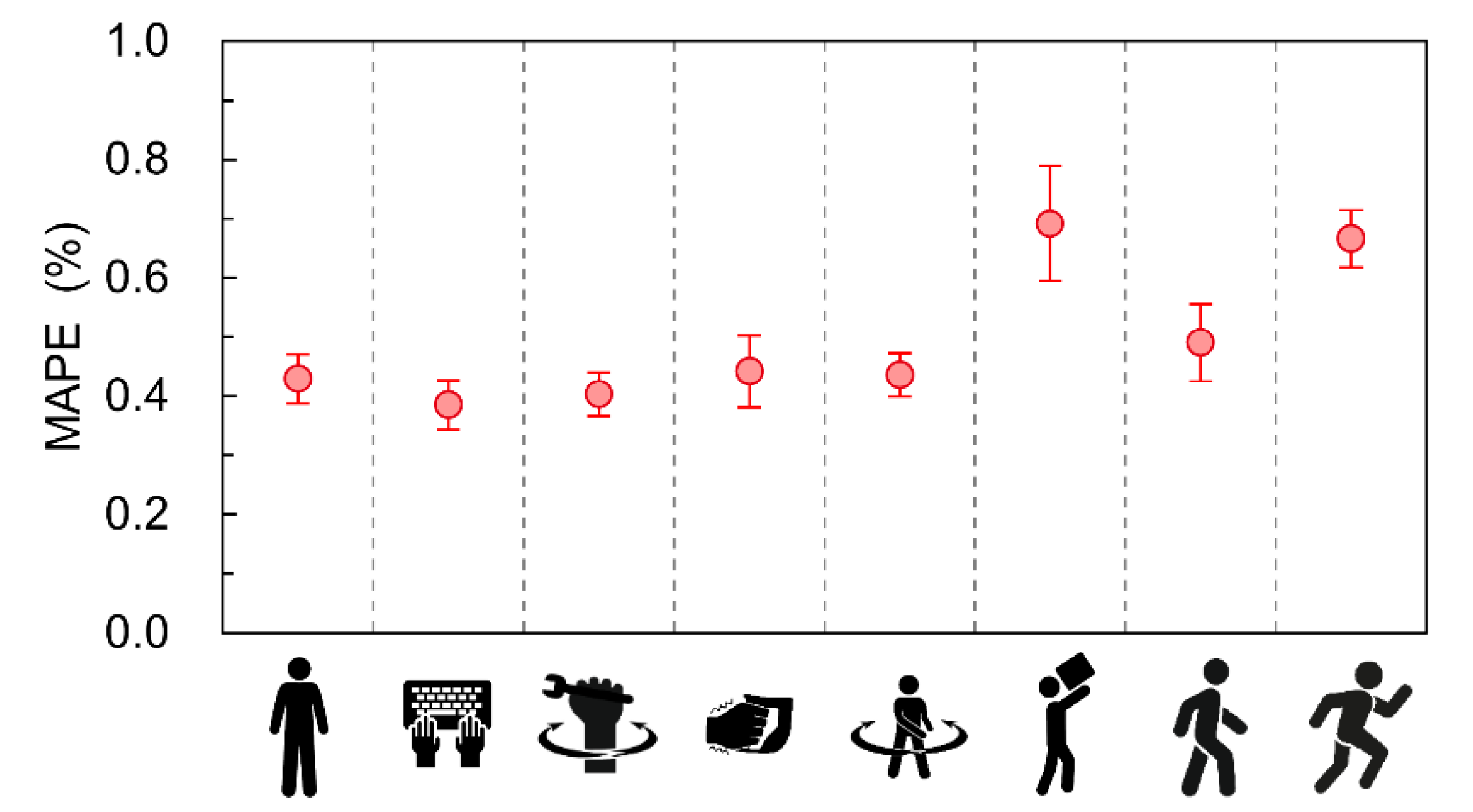
| Protocol | Average MAPE ± 95%CI (%) | Maximum MAPE (%) | Total Beats per Minute |
|---|---|---|---|
| Standing | 0.43 ± 0.045 | 0.48 | 84 |
| Typing | 0.39 ± 0.050 | 0.46 | 73 |
| Wrist rotation | 0.40 ± 0.044 | 0.50 | 77 |
| Wrist vibration | 0.44 ± 0.073 | 0.58 | 84 |
| Upper body twisting | 0.44 ± 0.044 | 0.50 | 80 |
| Loading and unloading luggage | 0.69 ± 0.116 | 0.92 | 110 |
| Walking at 4 km/h | 0.49 ± 0.078 | 0.64 | 98 |
| Running at 7 km/h | 0.67 ± 0.058 | 0.78 | 130 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Sato, R.; Takagahara, K.; Ishihara, T.; Watanabe, K.; Togo, H. Validation of Wearable Device Consisting of a Smart Shirt with Built-In Bioelectrodes and a Wireless Transmitter for Heart Rate Monitoring in Light to Moderate Physical Work. Sensors 2022, 22, 9241. https://doi.org/10.3390/s22239241
Hashimoto Y, Sato R, Takagahara K, Ishihara T, Watanabe K, Togo H. Validation of Wearable Device Consisting of a Smart Shirt with Built-In Bioelectrodes and a Wireless Transmitter for Heart Rate Monitoring in Light to Moderate Physical Work. Sensors. 2022; 22(23):9241. https://doi.org/10.3390/s22239241
Chicago/Turabian StyleHashimoto, Yuki, Rieko Sato, Kazuhiko Takagahara, Takako Ishihara, Kento Watanabe, and Hiroyoshi Togo. 2022. "Validation of Wearable Device Consisting of a Smart Shirt with Built-In Bioelectrodes and a Wireless Transmitter for Heart Rate Monitoring in Light to Moderate Physical Work" Sensors 22, no. 23: 9241. https://doi.org/10.3390/s22239241
APA StyleHashimoto, Y., Sato, R., Takagahara, K., Ishihara, T., Watanabe, K., & Togo, H. (2022). Validation of Wearable Device Consisting of a Smart Shirt with Built-In Bioelectrodes and a Wireless Transmitter for Heart Rate Monitoring in Light to Moderate Physical Work. Sensors, 22(23), 9241. https://doi.org/10.3390/s22239241







