Feasibility of Ultra-Short-Term Analysis of Heart Rate and Systolic Arterial Pressure Variability at Rest and during Stress via Time-Domain and Entropy-Based Measures
Abstract
1. Introduction
2. Materials and Methods
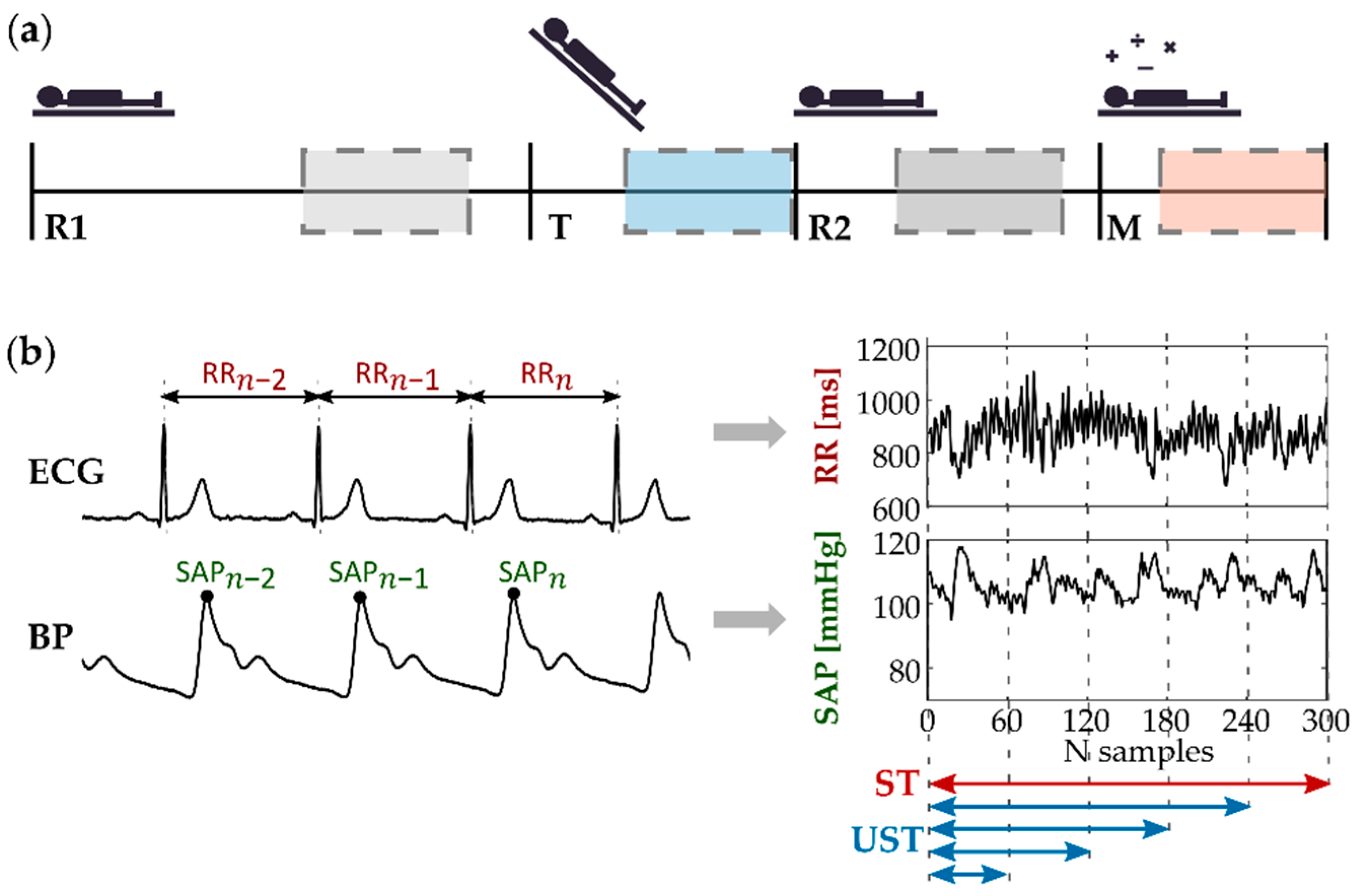
2.1. Experimental Protocol
- A resting condition (R1) with the subject laying in the supine position for 15 min, in order to stabilize the physiological signals on a baseline level;
- A head-up tilt (T) test aimed at evoking mild orthostatic stress by inclining the motorized table by 45 degrees for 8 min;
- Another resting condition (R2) with the subject laying in the supine position for 10 min, in order to restore the physiological parameters to their baseline values;
- A 6 min long mental arithmetic (M) task aimed to evoke cognitive load (i.e., mental stress), during which subjects were asked to mentally calculate the sum of three digits in the least possible time, indicating whether the result was an even or odd number.
2.2. Time Series Extraction
2.3. Time-Domain Analysis
2.4. Information Domain Analysis
2.5. Statistical Analysis
3. Results
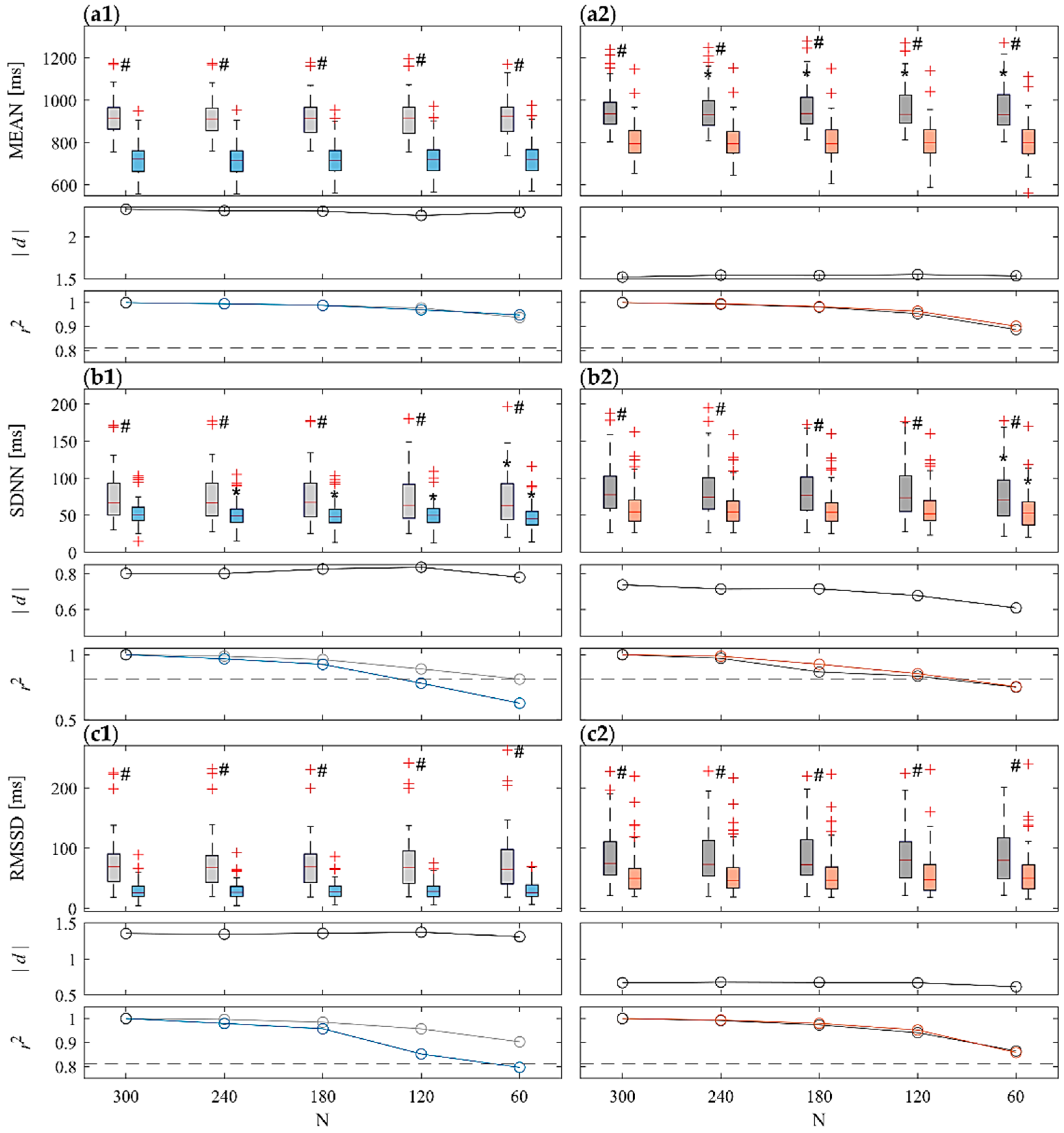
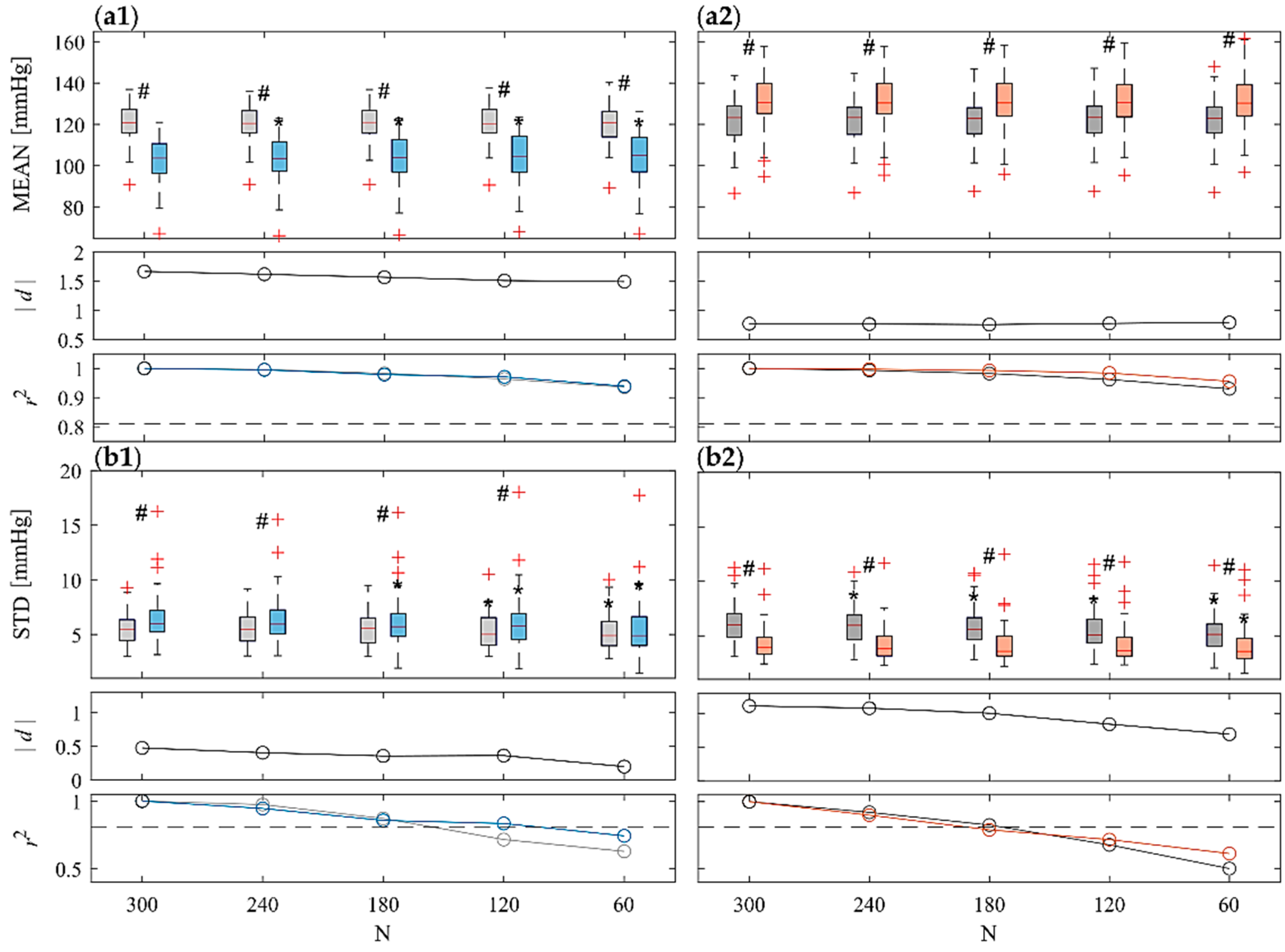
3.1. Time-Domain Analysis
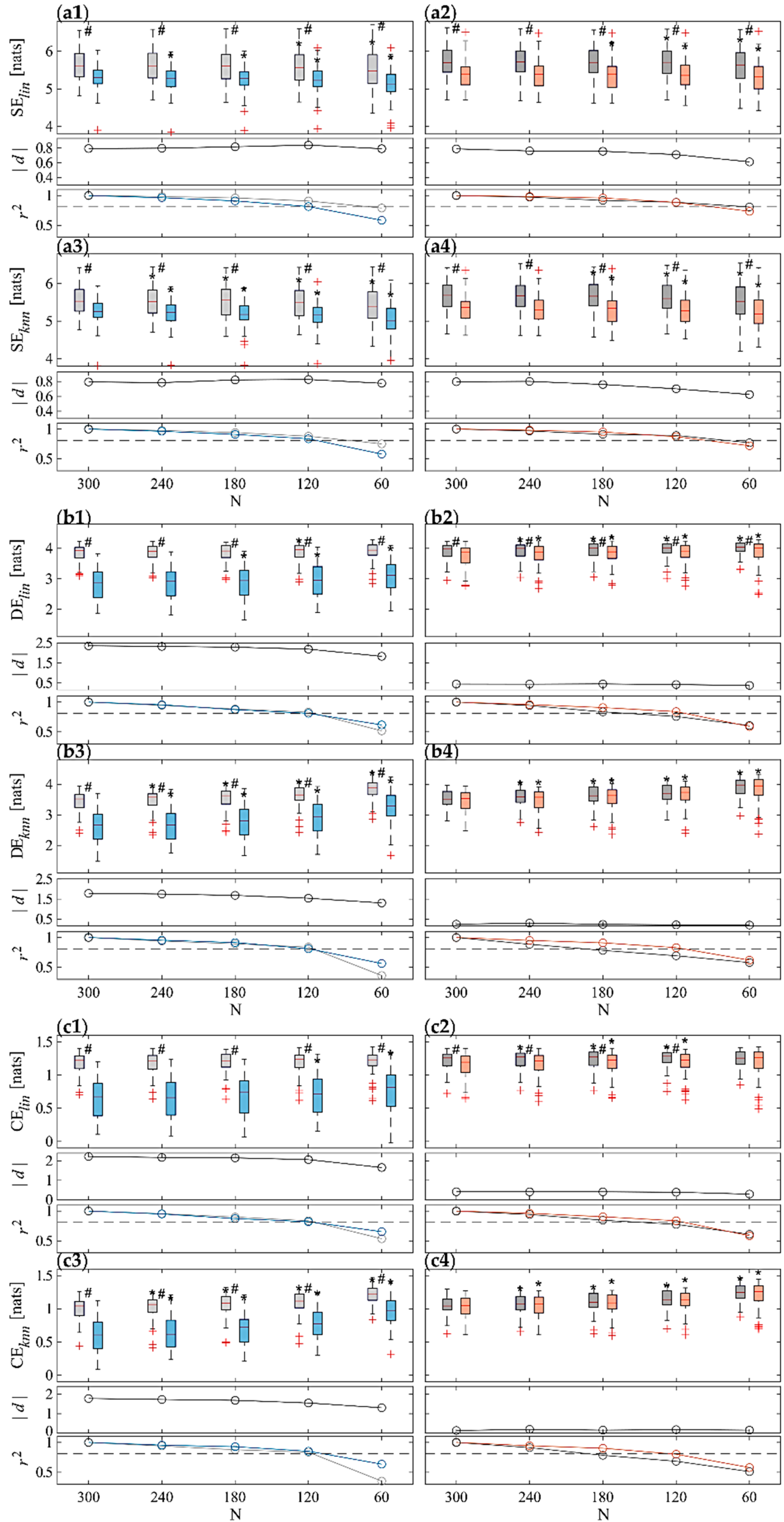
3.2. Information Domain Analyses
4. Discussion
4.1. Time-Domain Analyses on ST Series
4.2. Information Domain Analyses on ST Series
4.3. Ultra-Short-Term versus Short-Term Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaddha, A.; Robinson, E.A.; Kline-Rogers, E.; Alexandris-Souphis, T.; Rubenfire, M. Mental Health and Cardiovascular Disease. Am. J. Med. 2016, 129, 1145–1148. [Google Scholar] [CrossRef]
- Huang, C.-J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular Reactivity, Stress, and Physical Activity. Front. Physiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.M.; Gaughran, F. The Interface of Physical and Mental Health. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 673–682. [Google Scholar] [CrossRef]
- Freeman, J.V.; Dewey, F.E.; Hadley, D.M.; Myers, J.; Froelicher, V.F. Autonomic Nervous System Interaction with the Cardiovascular System during Exercise. Prog. Cardiovasc. Dis. 2006, 48, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Monti, A.; Medigue, C.; Nedelcoux, H.; Escourrou, P. Autonomic Control of the Cardiovascular System during Sleep in Normal Subjects. Eur. J. Appl. Physiol. 2002, 87, 174–181. [Google Scholar] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C.; Colonna, A.; Bozzini, S.; Matrone, B.; Guasti, L.; Paganini, E.M.; Falcone, R.; Pelissero, G. Cardiovascular Risk Factors and Sympatho-Vagal Balance: Importance of Time-Domain Heart Rate Variability. J. Clin. Exp. Cardiol. 2014, 5, 2. [Google Scholar]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Malpas, S.C. Neural Influences on Cardiovascular Variability: Possibilities and Pitfalls. Am. J. Physiol. Circ. Physiol. 2002, 282, H6–H20. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Adochiei, F.-C.; Edu, I.-R.; Schroeder, R.; Costin, H.; Bär, K.-J.; Voss, A. Cardiovascular and Cardiorespiratory Coupling Analyses: A Review. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120191. [Google Scholar] [CrossRef]
- Cohen, M.A.; Taylor, J.A. Short-Term Cardiovascular Oscillations in Man: Measuring and Modelling the Physiologies. J. Physiol. 2002, 542, 669–683. [Google Scholar] [CrossRef]
- Porta, A.; Baselli, G.; Cerutti, S. Implicit and Explicit Model-Based Signal Processing for the Analysis of Short-Term Cardiovascular Interactions. Proc. IEEE 2006, 94, 805–818. [Google Scholar] [CrossRef]
- Parati, G.; Di Rienzo, M.; Ulian, L.; Santucciu, C.; Girard, A.; Elghozi, J.-L.; Mancia, G. Clinical Relevance Blood Pressure Variability. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1998, 16, S25–S33. [Google Scholar] [CrossRef][Green Version]
- Mancia, G.; Parati, G.; Di Rienzo, M.; Zanchetti, A. Blood Pressure Variability. In Handbook of Hypertension, Vol 17: Pathophysiology of Hypertension; Zanchetti, A., Mancia, G., Eds.; Elsevier Science Publishing Co., Inc.: Amsterdam, The Netherlands, 1997; pp. 117–169. [Google Scholar]
- Pernice, R.; Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Busacca, A.; Faes, L. Comparison of Short-Term Heart Rate Variability Indexes Evaluated through Electrocardiographic and Continuous Blood Pressure Monitoring. Med. Biol. Eng. Comput. 2019, 57, 1247–1263. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Assessment and Management of Blood-Pressure Variability. Nat. Rev. Cardiol. 2013, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Castiglioni, P.; Parati, G.; Faini, A. Information-Domain Analysis of Cardiovascular Complexity: Night and Day Modulations of Entropy and the Effects of Hypertension. Entropy 2019, 21, 550. [Google Scholar] [CrossRef]
- Porta, A.; Castiglioni, P.; Bari, V.; Bassani, T.; Marchi, A.; Cividjian, A.; Quintin, L.; Di Rienzo, M. K-Nearest-Neighbor Conditional Entropy Approach for the Assessment of the Short-Term Complexity of Cardiovascular Control. Physiol. Meas. 2012, 34, 17. [Google Scholar] [CrossRef]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.-K.; Schmidt, G.; Yamamoto, Y.; Reviewers:, D.; Gorenek, B. Advances in Heart Rate Variability Signal Analysis: Joint Position Statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association Co-Endorsed by the Asia Pacific Heart Rhythm Society. Ep Eur. 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Guzzetti, S.; Furlan, R.; Gnecchi-Ruscone, T.; Montano, N.; Malliani, A. Complexity and Nonlinearity in Short-Term Heart Period Variability: Comparison of Methods Based on Local Nonlinear Prediction. IEEE Trans. Biomed. Eng. 2006, 54, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Anastasova, S.; Zhang, K.; Rosa, B.G.; Lo, B.P.L.; Assender, H.E.; Yang, G.Z. Towards Wearable and Flexible Sensors and Circuits Integration for Stress Monitoring. IEEE J. Biomed. Health Inform. 2020, 24, 2208–2215. [Google Scholar] [CrossRef]
- Dias, D.; Cunha, J.P.S. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Chalabianloo, N.; Sas, C.; Ersoy, C. HRV and Stress: A Mixed-Methods Approach for Comparison of Wearable Heart Rate Sensors for Biofeedback. IEEE Access 2021, 9, 14005–14024. [Google Scholar] [CrossRef]
- Hernando, D.; Roca, S.; Sancho, J.; Alesanco, Á.; Bailón, R. Validation of the Apple Watch for Heart Rate Variability Measurements during Relax and Mental Stress in Healthy Subjects. Sensors 2018, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Kos, A.; Umek, A. Wearable Sensor Devices for Prevention and Rehabilitation in Healthcare: Swimming Exercise with Real-Time Therapist Feedback. IEEE Internet Things J. 2019, 6, 1331–1341. [Google Scholar] [CrossRef]
- Kakria, P.; Tripathi, N.; Kitipawong, P. A Real-Time Health Monitoring System for Remote Cardiac Patients Using Smartphone and Wearable Sensors. Int. J. Telemed. Appl. 2015, 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Montesinos, L.; Melillo, P.; James, C.; Pecchia, L. Ultra-Short Term HRV Features as Surrogates of Short Term HRV: A Case Study on Mental Stress Detection in Real Life. BMC Med. Inform. Decis. Mak. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Seok, H.S.; Shin, H. Is Ultra-Short-Term Heart Rate Variability Valid in Non-Static Conditions? Front. Physiol. 2021, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Finžgar, M.; Podržaj, P. Feasibility of Assessing Ultra-Short-Term Pulse Rate Variability from Video Recordings. PeerJ 2020, 8, e8342. [Google Scholar] [CrossRef]
- Holmes, C.J.; Fedewa, M.V.; Winchester, L.J.; MacDonald, H.V.; Wind, S.A.; Esco, M.R. Validity of Smartphone Heart Rate Variability Pre-and Post-Resistance Exercise. Sensors 2020, 20, 5738. [Google Scholar] [CrossRef]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements. Biofeedback 2016, 44, 229–233. [Google Scholar] [CrossRef]
- Pecchia, L.; Castaldo, R.; Montesinos, L.; Melillo, P. Are Ultra-short Heart Rate Variability Features Good Surrogates of Short-term Ones? State-of-the-art Review and Recommendations. Healthc. Technol. Lett. 2018, 5, 94–100. [Google Scholar] [CrossRef]
- Sandercock, G.R.H.; Bromley, P.D.; Brodie, D.A. The Reliability of Short-Term Measurements of Heart Rate Variability. Int. J. Cardiol. 2005, 103, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Protheroe, C.L.; Ravensbergen, H.R.J.C.; Inskip, J.A.; Claydon, V.E. Tilt Testing with Combined Lower Body Negative Pressure: A “Gold Standard” for Measuring Orthostatic Tolerance. JoVE (J. Vis. Exp.) 2013, 73, e4315. [Google Scholar] [CrossRef]
- Malik, M.; Huikuri, H.; Lombardi, F.; Schmidt, G. The Purpose of Heart Rate Variability Measurements. Clin. Auton. Res. 2017, 27, 139–140. [Google Scholar] [CrossRef]
- Shaffer, F.; Meehan, Z.M.; Zerr, C.L. A Critical Review of Ultra-Short-Term Heart Rate Variability Norms Research. Front. Neurosci. 2020, 14, 594880. [Google Scholar] [CrossRef]
- Volpes, G.; Barà, C.; Valenti, S.; Javorka, M.; Busacca, A.; Faes, L.; Pernice, R. Feasibility of Ultra-Short Term Complexity Analysis of Heart Rate Variability in Resting State and During Orthostatic Stress. In Proceedings of the 2022 12th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Štrbské Pleso, Slovakia, 9–12 October 2022; pp. 1–2. [Google Scholar]
- Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Lazarova, Z.; Javorka, K.; Faes, L. Basic Cardiovascular Variability Signals: Mutual Directed Interactions Explored in the Information Domain. Physiol. Meas. 2017, 38, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Lazarova, Z.; Wiszt, R.; Faes, L. Towards Understanding the Complexity of Cardiovascular Oscillations: Insights from Information Theory. Comput. Biol. Med. 2018, 98, 48–57. [Google Scholar] [CrossRef]
- Vollmer, M. A Robust, Simple and Reliable Measure of Heart Rate Variability Using Relative RR Intervals. In Proceedings of the 2015 Computing in Cardiology Conference (CinC), Nice, France, 6–9 September 2015; pp. 609–612. [Google Scholar]
- Valente, M.; Javorka, M.; Porta, A.; Bari, V.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Nollo, G.; Faes, L. Univariate and Multivariate Conditional Entropy Measures for the Characterization of Short-Term Cardiovascular Complexity under Physiological Stress. Physiol. Meas. 2018, 39, 014002. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Faes, L.; Ivanov, P.C. Entropy Measures, Entropy Estimators, and Their Performance in Quantifying Complex Dynamics: Effects of Artifacts, Nonstationarity, and Long-Range Correlations. Phys. Rev. E 2017, 95, 062114. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Faes, L.; Escudero, J.; Humeau-Heurtier, A.; Silva, L.E.V. Entropy Analysis of Univariate Biomedical Signals: Review and Comparison of Methods. In Frontiers in Entropy across the Disciplines: Panorama of Entropy: Theory, Computation, and Applications; World Scientific Publishing: Singapore, 2020; pp. 233–286. [Google Scholar]
- Hutcheson, G.D. Ordinary Least-Squares Regression. In The SAGE Dictionary of Quantitative Management Research; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2011; pp. 224–228. [Google Scholar]
- Esco, M.R.; Flatt, A.A. Ultra-Short-Term Heart Rate Variability Indexes at Rest and Post-Exercise in Athletes: Evaluating the Agreement with Accepted Recommendations. J. Sports Sci. Med. 2014, 13, 535. [Google Scholar]
- Munoz, M.L.; van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; de Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M. Validity of (Ultra-) Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef] [PubMed]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Shaffer, F.; Shearman, S.; Meehan, Z.; Gravett, N.; Urban, H. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements: A Comparison of Pearson Product-Moment Correlation Coefficient and Limits of Agreement (LoA) Concurrent Validity Criteria. In Physiological Recording Technology and Applications in Biofeedback and Neurofeedback; Moss, D., Shaffer, F., Eds.; Association for Applied Psychophysiology and Biofeedback: Oakbrook Terrace, IL, USA, 2019; pp. 214–220. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, Ed.; Lawrence Earlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Baumert, M.; Czippelova, B.; Ganesan, A.; Schmidt, M.; Zaunseder, S.; Javorka, M. Entropy Analysis of RR and QT Interval Variability during Orthostatic and Mental Stress in Healthy Subjects. Entropy 2014, 16, 6384–6393. [Google Scholar] [CrossRef]
- Wasmund, W.L.; Westerholm, E.C.; Watenpaugh, D.E.; Wasmund, S.L.; Smith, M.L. Interactive Effects of Mental and Physical Stress on Cardiovascular Control. J. Appl. Physiol. 2002, 92, 1828–1834. [Google Scholar] [CrossRef]
- Hjortskov, N.; Rissén, D.; Blangsted, A.K.; Fallentin, N.; Lundberg, U.; Søgaard, K. The Effect of Mental Stress on Heart Rate Variability and Blood Pressure during Computer Work. Eur. J. Appl. Physiol. 2004, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.R.; Boscan, P.; Pickering, A.E.; Nalivaiko, E. The Yin and Yang of Cardiac Autonomic Control: Vago-Sympathetic Interactions Revisited. Brain Res. Rev. 2005, 49, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Cacioppo, J.T.; Binkley, P.F.; Uchino, B.N.; Quigley, K.S.; Fieldstone, A. Autonomic Cardiac Control. III. Psychological Stress and Cardiac Response in Autonomic Space as Revealed by Pharmacological Blockades. Psychophysiology 1994, 31, 599–608. [Google Scholar] [CrossRef]
- Faes, L.; Porta, A.; Cucino, R.; Cerutti, S.; Antolini, R.; Nollo, G. Causal Transfer Function Analysis to Describe Closed Loop Interactions between Cardiovascular and Cardiorespiratory Variability Signals. Biol. Cybern. 2004, 90, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Furlan, R.; Rimoldi, O.; Pagani, M.; Malliani, A.; Van De Borne, P. Quantifying the Strength of the Linear Causal Coupling in Closed Loop Interacting Cardiovascular Variability Signals. Biol. Cybern. 2002, 86, 241–251. [Google Scholar] [CrossRef]
- Porta, A.; Catai, A.M.; Takahashi, A.C.M.; Magagnin, V.; Bassani, T.; Tobaldini, E.; Van De Borne, P.; Montano, N. Causal Relationships between Heart Period and Systolic Arterial Pressure during Graded Head-up Tilt. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R378–R386. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Information Domain Approach to the Investigation of Cardio-Vascular, Cardio-Pulmonary, and Vasculo-Pulmonary Causal Couplings. Front. Physiol. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, B.E.; Gisolf, J.; Karemaker, J.M.; Wesseling, K.H.; Secher, N.H.; Van Lieshout, J.J. Time Course Analysis of Baroreflex Sensitivity during Postural Stress. Am. J. Physiol. Circ. Physiol. 2006, 291, H2864–H2874. [Google Scholar] [CrossRef]
- Mijatovic, G.; Pernice, R.; Perinelli, A.; Antonacci, Y.; Busacca, A.; Javorka, M.; Ricci, L.; Faes, L. Measuring the Rate of Information Exchange in Point-Process Data With Application to Cardiovascular Variability. Front. Netw. Physiol. 2022, 1, 765332. [Google Scholar] [CrossRef]
- Krohova, J.; Faes, L.; Czippelova, B.; Turianikova, Z.; Mazgutova, N.; Pernice, R.; Busacca, A.; Marinazzo, D.; Stramaglia, S.; Javorka, M. Multiscale Information Decomposition Dissects Control Mechanisms of Heart Rate Variability at Rest and During Physiological Stress. Entropy 2019, 21, 526. [Google Scholar] [CrossRef]
- Pinto, H.; Pernice, R.; Eduarda Silva, M.; Javorka, M.; Faes, L.; Rocha, A.P. Multiscale Partial Information Decomposition of Dynamic Processes with Short and Long-Range Correlations: Theory and Application to Cardiovascular Control. Physiol. Meas. 2022, 43, 85004. [Google Scholar] [CrossRef]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive Decrease of Heart Period Variability Entropy-Based Complexity during Graded Head-up Tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef]
- Pernice, R.; Volpes, G.; Krohova, J.C.; Javorka, M.; Busacca, A.; Faes, L. Feasibility of Linear Parametric Estimation of Dynamic Information Measures to Assess Physiological Stress from Short-Term Cardiovascular Variability. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 290–293. [Google Scholar]
- Fauvel, J.P.; Cerutti, C.; Quelin, P.; Laville, M.; Gustin, M.P.; Paultre, C.Z.; Ducher, M. Mental Stress–Induced Increase in Blood Pressure Is Not Related to Baroreflex Sensitivity in Middle-Aged Healthy Men. Hypertension 2000, 35, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Baselli, G.; Porta, A.; Pagani, M. Coupling Arterial Windkessel with Peripheral Vasomotion: Modeling the Effects on Low-Frequency Oscillations. IEEE Trans. Biomed. Eng. 2005, 53, 53–64. [Google Scholar] [CrossRef]
- Faes, L.; Kugiumtzis, D.; Nollo, G.; Jurysta, F.; Marinazzo, D. Estimating the Decomposition of Predictive Information in Multivariate Systems. Phys. Rev. E 2015, 91, 032904. [Google Scholar] [CrossRef]
- Clemson, P.T.; Hoag, J.B.; Cooke, W.H.; Eckberg, D.L.; Stefanovska, A. Beyond the Baroreflex: A New Measure of Autonomic Regulation Based on the Time-Frequency Assessment of Variability, Phase Coherence and Couplings. Front. Netw. Physiol. 2022, 2, 891604. [Google Scholar] [CrossRef]
- Faes, L.; Gómez-Extremera, M.; Pernice, R.; Carpena, P.; Nollo, G.; Porta, A.; Bernaola-Galván, P. Comparison of Methods for the Assessment of Nonlinearity in Short-Term Heart Rate Variability under Different Physiopathological States. Chaos Interdiscip. J. Nonlinear Sci. 2019, 29, 123114. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Schmitt, L.; Yazdani, S.; Vesin, J.-M.; Millet, G.P. Minimal Window Duration for Accurate HRV Recording in Athletes. Front. Neurosci. 2017, 11, 456. [Google Scholar] [CrossRef]
- Salahuddin, L.; Cho, J.; Jeong, M.G.; Kim, D. Ultra Short Term Analysis of Heart Rate Variability for Monitoring Mental Stress in Mobile Settings. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 4656–4659. [Google Scholar]
- Baek, H.J.; Cho, C.-H.; Cho, J.; Woo, J.-M. Reliability of Ultra-Short-Term Analysis as a Surrogate of Standard 5-Min Analysis of Heart Rate Variability. Telemed. e-Health 2015, 21, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Le, F.K.; Lahiri, M.; Kannankeril, P.J.; Ng, J.; Kadish, A.H. Assessment of Parasympathetic Reactivation after Exercise. Am. J. Physiol. Circ. Physiol. 2006, 290, H2446–H2452. [Google Scholar] [CrossRef]
- McNames, J.; Aboy, M. Reliability and Accuracy of Heart Rate Variability Metrics versus ECG Segment Duration. Med. Biol. Eng. Comput. 2006, 44, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Montesinos, L.; Pecchia, L. Ultra-Short Entropy for Mental Stress Detection. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2018; Springer: Berlin/Heidelberg, Germany, 2019; pp. 287–291. [Google Scholar]
- Lee, S.; Hwang, H.B.; Park, S.; Kim, S.; Ha, J.H.; Jang, Y.; Hwang, S.; Park, H.-K.; Lee, J.; Kim, I.Y. Mental Stress Assessment Using Ultra Short Term HRV Analysis Based on Non-Linear Method. Biosensors 2022, 12, 465. [Google Scholar] [CrossRef]
- Faes, L.; Porta, A.; Javorka, M.; Nollo, G. Efficient Computation of Multiscale Entropy over Short Biomedical Time Series Based on Linear State-Space Models. Complexity 2017, 2017, 1768264. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailon, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography Pulse Rate Variability as a Surrogate Measurement of Heart Rate Variability during Non-Stationary Conditions. Physiol. Meas. 2010, 31, 1271. [Google Scholar] [CrossRef]
- Mejía-Mejía, E.; May, J.M.; Torres, R.; Kyriacou, P.A. Pulse Rate Variability in Cardiovascular Health: A Review on Its Applications and Relationship with Heart Rate Variability. Physiol. Meas. 2020, 41, 07TR01. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, X.; Liu, C.; Du, G.; Zhao, L.; Dong, H.; Zhao, S.; Liu, Y. Feasibility Study for Detection of Mental Stress and Depression Using Pulse Rate Variability Metrics via Various Durations. Biomed. Signal Process. Control 2023, 79, 104145. [Google Scholar] [CrossRef]
- Wang, J.H.-S.; Yeh, M.-H.; Chao, P.C.-P.; Tu, T.-Y.; Kao, Y.-H.; Pandey, R. A Fast Digital Chip Implementing a Real-Time Noise-Resistant Algorithm for Estimating Blood Pressure Using a Non-Invasive, Cuffless PPG Sensor. Microsyst. Technol. 2020, 26, 3501–3516. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpes, G.; Barà, C.; Busacca, A.; Stivala, S.; Javorka, M.; Faes, L.; Pernice, R. Feasibility of Ultra-Short-Term Analysis of Heart Rate and Systolic Arterial Pressure Variability at Rest and during Stress via Time-Domain and Entropy-Based Measures. Sensors 2022, 22, 9149. https://doi.org/10.3390/s22239149
Volpes G, Barà C, Busacca A, Stivala S, Javorka M, Faes L, Pernice R. Feasibility of Ultra-Short-Term Analysis of Heart Rate and Systolic Arterial Pressure Variability at Rest and during Stress via Time-Domain and Entropy-Based Measures. Sensors. 2022; 22(23):9149. https://doi.org/10.3390/s22239149
Chicago/Turabian StyleVolpes, Gabriele, Chiara Barà, Alessandro Busacca, Salvatore Stivala, Michal Javorka, Luca Faes, and Riccardo Pernice. 2022. "Feasibility of Ultra-Short-Term Analysis of Heart Rate and Systolic Arterial Pressure Variability at Rest and during Stress via Time-Domain and Entropy-Based Measures" Sensors 22, no. 23: 9149. https://doi.org/10.3390/s22239149
APA StyleVolpes, G., Barà, C., Busacca, A., Stivala, S., Javorka, M., Faes, L., & Pernice, R. (2022). Feasibility of Ultra-Short-Term Analysis of Heart Rate and Systolic Arterial Pressure Variability at Rest and during Stress via Time-Domain and Entropy-Based Measures. Sensors, 22(23), 9149. https://doi.org/10.3390/s22239149







