Wearable Healthcare Monitoring Based on a Microfluidic Electrochemical Integrated Device for Sensing Glucose in Natural Sweat
Abstract
:1. Introduction
2. Materials
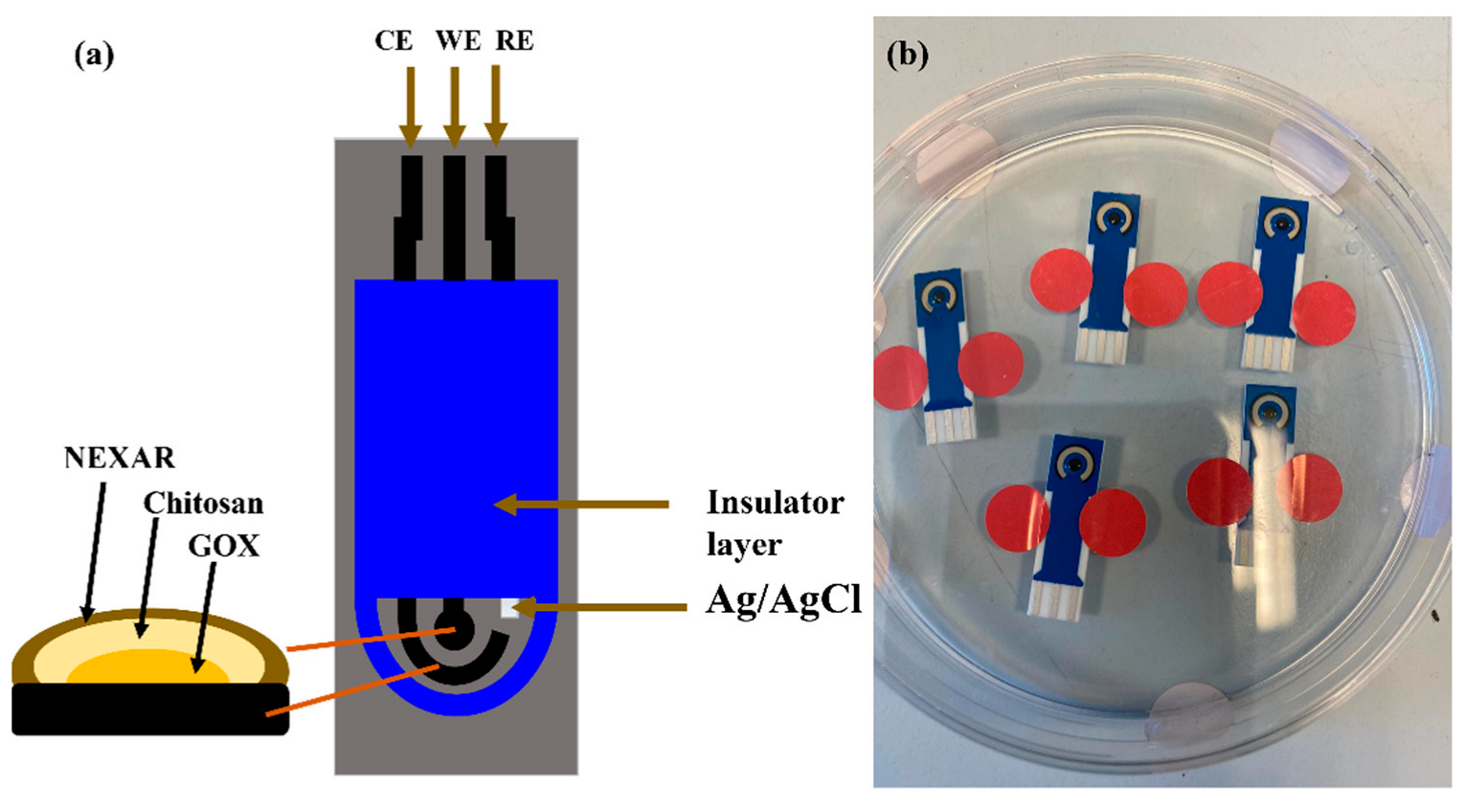
2.1. Electrode Surface Modification
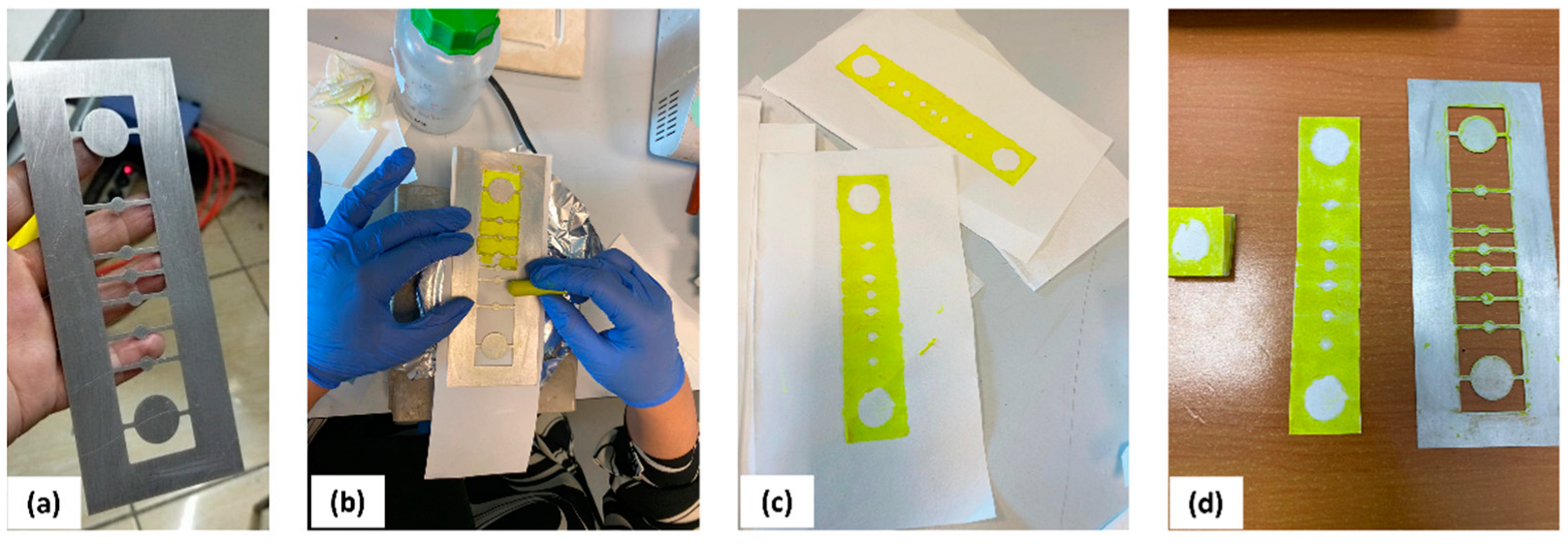
2.2. Device Fabrication
2.3. Off-Body and On-Body Characterization of the Glucose Sensor and the Paper-Based Microfluidic
3. Results
3.1. Field-Emission Scanning Electron Microscopy (FESEM) Analysis
3.2. Off-Body Testing of the Glucose Sensor
3.3. Integrating the Glucose Sensor with the Paper-Based Microfluidic
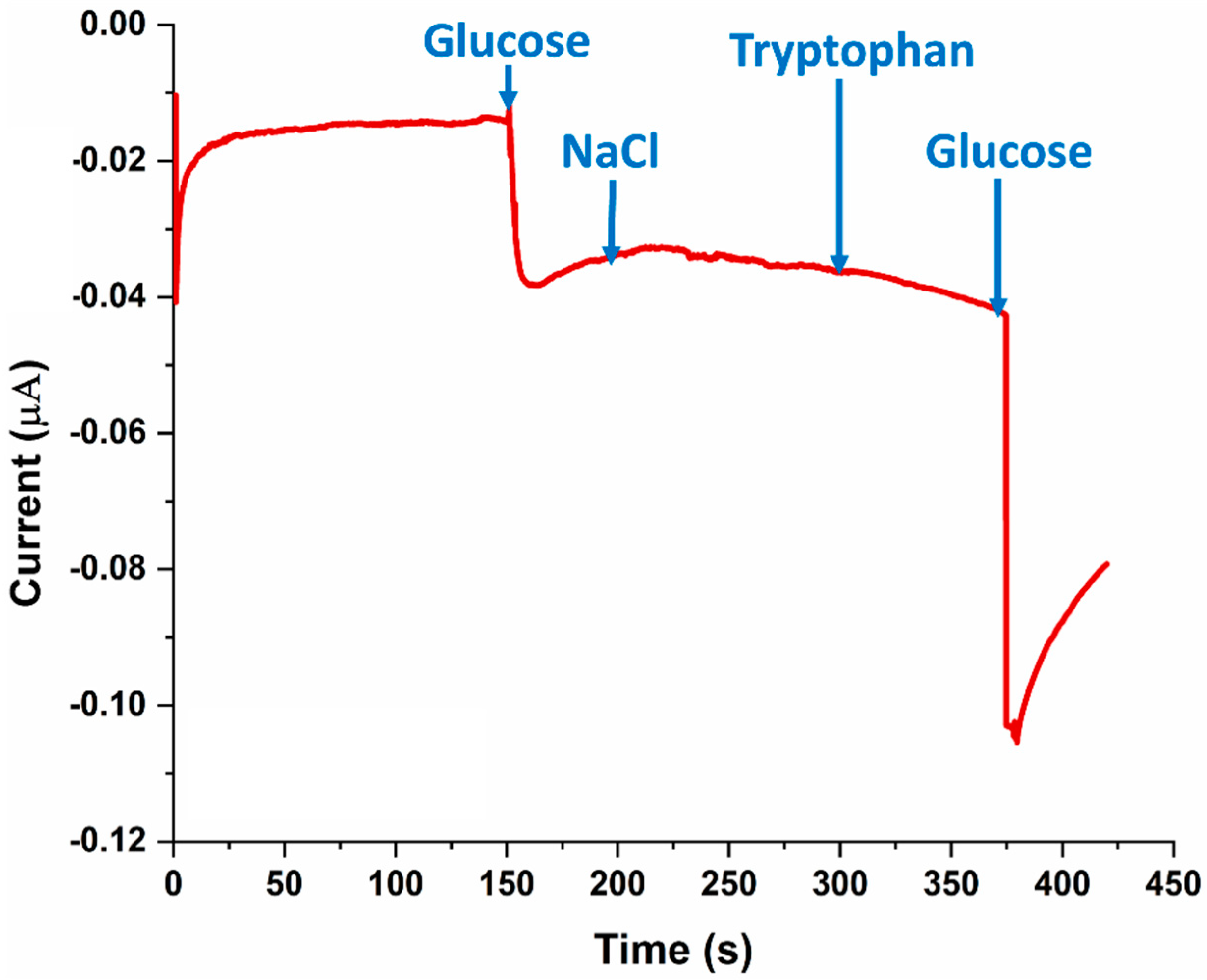
3.4. SPE Selectivity for Glucose
3.5. On-Body Sweat Glucose Measurements
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moonen, E.J.; Haakma, J.R.; Peri, E.; Pelssers, E.; Mischi, M.; Den Toonder, J.M. Wearable sweat sensing for prolonged, semicontinuous, and nonobtrusive health monitoring. View 2020, 1, 20200077. [Google Scholar] [CrossRef]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G. Wearable Sweat Biosensors Refresh Personalized Health/Medical Diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Serag, A.; Shakkour, Z.; Halboup, A.M.; Kobeissy, F.; Farag, M.A. Sweat metabolome and proteome: Recent trends in analytical advances and potential biological functions. J. Proteom. 2021, 246, 104310. [Google Scholar] [CrossRef]
- Karpova, E.V.; Shcherbacheva, E.V.; Galushin, A.A.; Vokhmyanina, D.V.; Karyakina, E.E.; Karyakin, A.A. Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal. Chem. 2019, 91, 3778–3783. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-based fingertip blood-free reliable glucose monitoring: Personalized data processing for predicting blood glucose concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B Chem. 2021, 343, 130131. [Google Scholar] [CrossRef]
- Zhou, W.; He, Q.; Ye, H.; Ye, C.; Wu, X.; Chu, J. Recent advances in flexible sweat glucose biosensors. J. Phys. D Appl. Phys. 2021, 54, 423001. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C. Porous enzymatic membrane for nanotextured glucose sweat sensors with high stability toward reliable noninvasive health monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef]
- Masawat, P.; Liawruangrath, S.; Slater, J. Flow injection measurement of lead using mercury-free disposable gold-sputtered screen-printed carbon electrodes (SPCE). Sens. Actuators B Chem. 2003, 91, 52–59. [Google Scholar] [CrossRef]
- Moulaee, K.; Neri, G. Electrochemical amino acid sensing: A review on challenges and achievements. Biosensors 2021, 11, 502. [Google Scholar] [CrossRef]
- Baker, L.B.; Wolfe, A.S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 2020, 120, 719–752. [Google Scholar] [CrossRef] [Green Version]
- Saran, T.; Turska, M.; Kocki, T.; Zawadka, M.; Zieliński, G.; Turski, W.A.; Gawda, P. Effect of 4-week physical exercises on tryptophan, kynurenine and kynurenic acid content in human sweat. Sci. Rep. 2021, 11, 11092. [Google Scholar] [CrossRef]
- Baba, D.; Nugraha, A.S.; Iqbal, M.; Bo, J.; Li, C.; Alshehri, A.A.; You, J.; Malgras, V.; Yamachi, Y.; Asahi, T. Nafion®-coated mesoporous Pd film toward remarkably enhanced detection of lactic acid. RSC Adv. 2018, 8, 10446–10449. [Google Scholar] [CrossRef]
- Izadyar, A.; Van, M.N.; Rodriguez, K.A.; Seok, I.; Hood, E.E. A bienzymatic amperometric glucose biosensor based on using a novel recombinant Mn peroxidase from corn and glucose oxidase with a Nafion membrane. J. Electroanal. Chem. 2021, 895, 115387. [Google Scholar] [CrossRef]
- Shi, G.M.; Zuo, J.; Tang, S.H.; Wei, S.; Chung, T.S. Layer-by-layer (LbL) polyelectrolyte membrane with Nexar™ polymer as a polyanion for pervaporation dehydration of ethanol. Sep. Purif. Technol. 2015, 140, 13–22. [Google Scholar] [CrossRef]
- Filice, S.; Sciuto, E.L.; Scalese, S.; Faro, G.; Libertino, S.; Corso, D.; Timpanaro, R.M.; Laganà, P.; Coniglio, M.A. Innovative Antibiofilm Smart Surface against Legionella for Water Systems. Microorganisms 2022, 10, 870. [Google Scholar] [CrossRef]
- Filice, S.; Urzì, G.; Milazzo, R.; Privitera, S.; Lombardo, S.; Compagnini, G.; Scalese, S. Applicability of a New Sulfonated Pentablock Copolymer Membrane and Modified Gas Diffusion Layers for Low-Cost Water Splitting Processes. Energies 2019, 12, 2064. [Google Scholar] [CrossRef] [Green Version]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Harshman, S.W.; Pitsch, R.L.; Schaeublin, N.M.; Smith, Z.K.; Strayer, K.E.; Phelps, M.S.; Qualley, A.V.; Cowan, D.W.; Rose, S.D.; O’connor, M.L. Metabolomic stability of exercise-induced sweat. J. Chromatogr. B 2019, 1126, 121763. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Havenith, G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur. J. Appl. Physiol. 2011, 111, 1391–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.; He, J.; Chen, X.; Qiao, Y.; Wang, F.; Xia, Q.; Yu, L.; Lu, Z. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.-E. Highly electrocatalytic, durable, and stretchable nanohybrid fiber for on-body sweat glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]


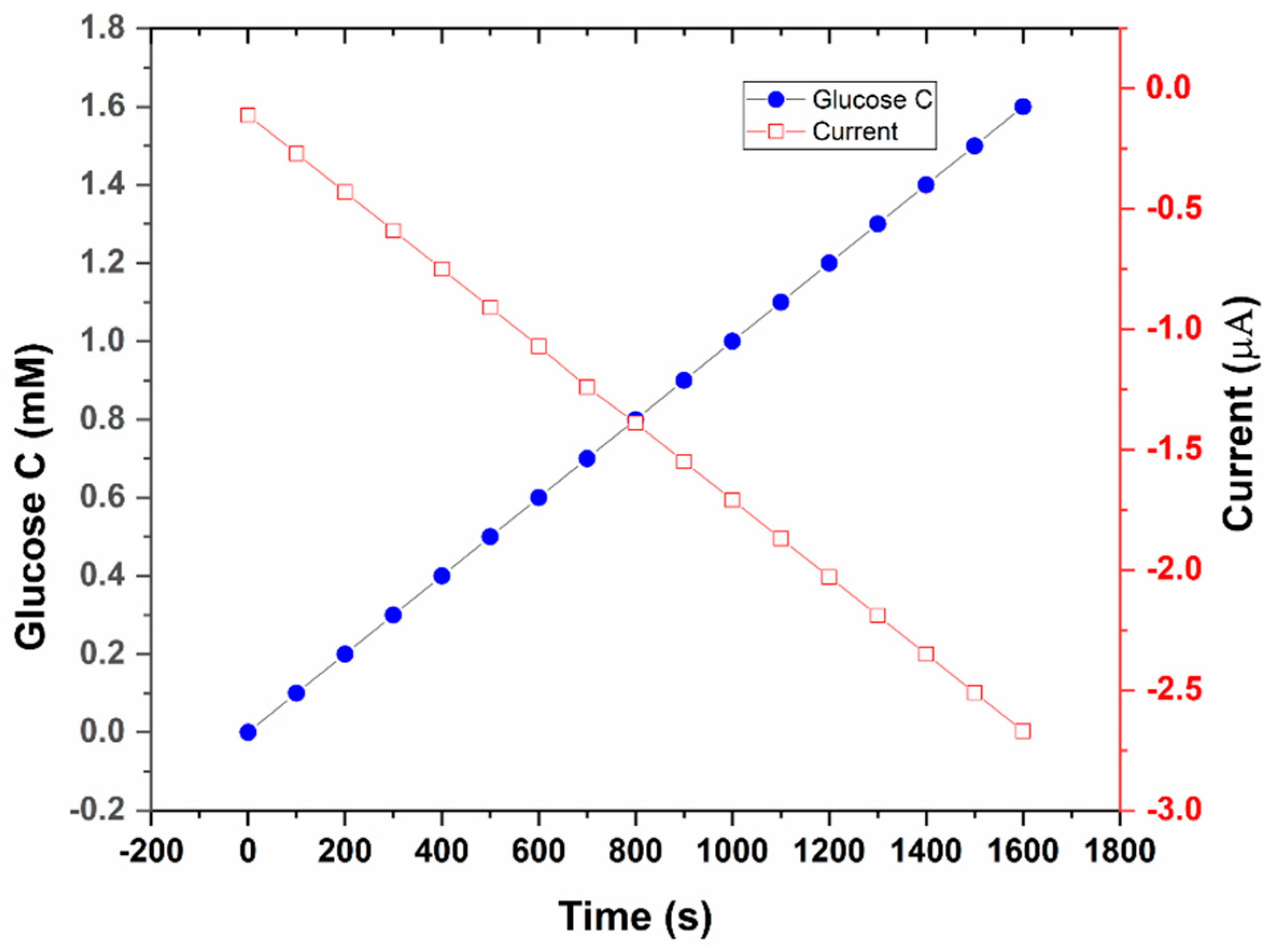



| Time (s) | Glucose Concentration (mM) | Current (µA) |
|---|---|---|
| 20 | 0.0 | −0.10 |
| 40 | 0.5 | −0.52 |
| 60 | 1.0 | −0.83 |
| 80 | 1.5 | −1.20 |
| 100 | 2.0 | −1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noura, Z.; Shah, I.; Aziz, S.; Ahmed, A.; Jung, D.-W.; Brahim, L.; ElMostafa, R. Wearable Healthcare Monitoring Based on a Microfluidic Electrochemical Integrated Device for Sensing Glucose in Natural Sweat. Sensors 2022, 22, 8971. https://doi.org/10.3390/s22228971
Noura Z, Shah I, Aziz S, Ahmed A, Jung D-W, Brahim L, ElMostafa R. Wearable Healthcare Monitoring Based on a Microfluidic Electrochemical Integrated Device for Sensing Glucose in Natural Sweat. Sensors. 2022; 22(22):8971. https://doi.org/10.3390/s22228971
Chicago/Turabian StyleNoura, Zouaghi, Imran Shah, Shahid Aziz, Aamouche Ahmed, Dong-Won Jung, Lakssir Brahim, and Ressami ElMostafa. 2022. "Wearable Healthcare Monitoring Based on a Microfluidic Electrochemical Integrated Device for Sensing Glucose in Natural Sweat" Sensors 22, no. 22: 8971. https://doi.org/10.3390/s22228971
APA StyleNoura, Z., Shah, I., Aziz, S., Ahmed, A., Jung, D.-W., Brahim, L., & ElMostafa, R. (2022). Wearable Healthcare Monitoring Based on a Microfluidic Electrochemical Integrated Device for Sensing Glucose in Natural Sweat. Sensors, 22(22), 8971. https://doi.org/10.3390/s22228971









