Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect
Abstract
1. Introduction
2. Materials and Methods
2.1. System Design and Human–Computer Interaction
2.2. Assessment of the Motor Condition: The Evaluative Motor Tasks
- In general, the evaluative motor tasks aim to characterize the patient’s motor condition through quantitative functional parameters. The subset implemented in our system is suitable for safely assessing lower limb mobility, gait, and postural stability even in home and minimally supervised settings. The following tasks have been considered:
- Leg Agility (LA): UPDRS task to assess the impairment of motor control and coordination in the lower limbs, typically affected by PD symptoms, through repetitive leg movements performed separately with the left and right leg.
- Postural Stability (PoS): a 30-s balance task to assess stability in the standing position through the swaying of the body’s center of mass (COM) estimated from the skeletal model, as in [54]. This task is indeed less risky than the traditional UPDRS pull-test, especially in home settings. However, the strong correlation between COM sways and postural instability and gait difficulty (PIGD) score has been previously verified [54].
2.3. Rehabilitation/Training of the Motor Condition: The Virtual Exergames
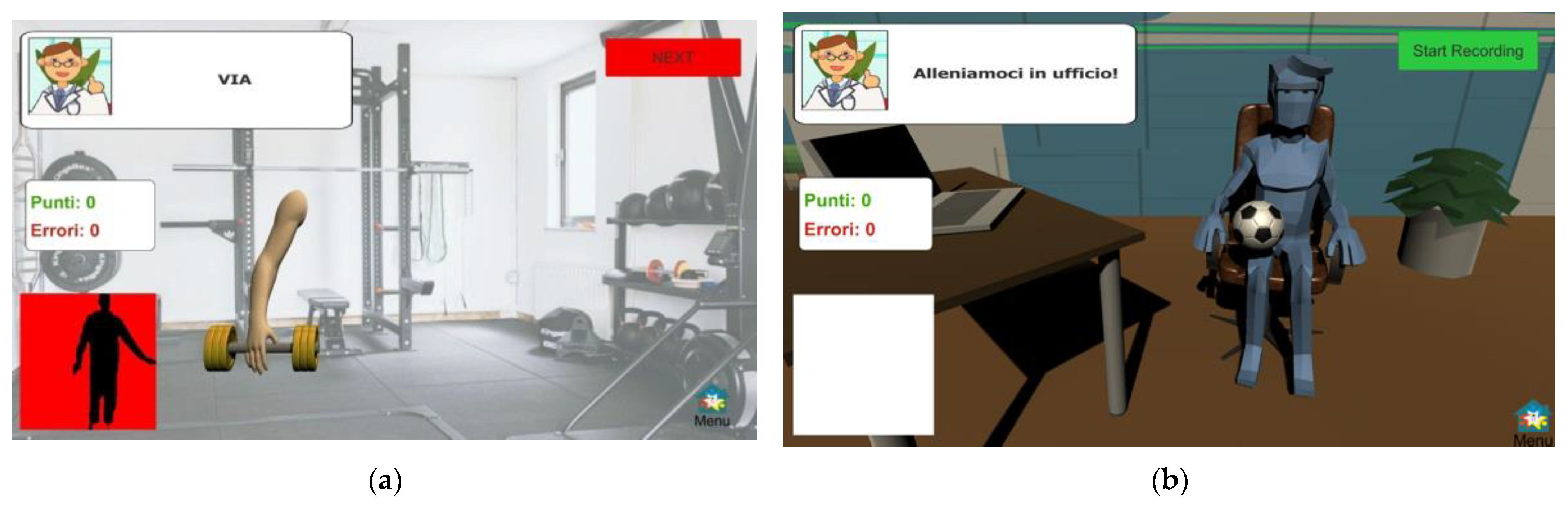
- Lateral Weightlifting (LWL): this exergame is performed in a standing position and consists of a sequence of lateral arm lifts. The exercise aims to strain the flexibility, agility, and mobility of the upper limbs. The exergame is set in a gymnasium scenario and mimics weightlifting to engage patients in pseudo-real physical activities.
- Frontal Weightlifting (FWL): this exergame is similar to the LWL since it stimulates motor control, coordination, and muscle tone by promoting trunk and arm extensions in the same scenario. In contrast to LWL, the exercise consists of a sequence of frontal arm lifts.
- Bouncing ball (BB): this exergame relies on repetitive movements of the lower limbs to stimulate motor control and coordination through leg mobility. The exercise aims to stress lower limb agility, thus counteracting balance dysfunctions and gait disorders. The exergame is set in an office scenario and consists of dribbling a ball with the legs (thighs), mimicking the movements of the LA task. The exercise is performed in a sitting position only.
2.4. Participants and Experimental Protocol
2.5. Objective Characterization of the Motor Performance
2.6. Statistical Analysis
3. Results
3.1. Group Characteristics and Data Collection
3.2. Statistical Analysis of the Evaluative Motor Tasks
3.3. Qualitative and Statistical Analysis of Virtual Exergames
3.4. Exergaming as Alternative or Complementary Evaluation
3.5. Exergaming as Mobility Training and Rehabilitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Church, F. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Proud, E.L.; Morris, M. Skilled Hand Dexterity in Parkinson’s Disease: Effects of Adding a Concurrent Task. Arch. Phys. Med. Rehabil. 2010, 91, 794–799, Erratum in Arch. Phys. Med. Rehabil. 2011, 92, 2098.. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Toset, S.; Cabrera-Martos, I.; Torres-Sánchez, I.; Ortiz-Rubio, A.; González-Jiménez, E.; Valenza, M.C. Effects of a Single Hand–Exercise Session on Manual Dexterity and Strength in Persons with Parkinson Disease: A Randomized Controlled Trial. PMR 2016, 8, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Debû, B.; Godeiro, C.D.O.; Lino, J.C.; Moro, E. Managing Gait, Balance, and Posture in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 23. [Google Scholar] [CrossRef]
- Contreras, A.; Grandas, F. Risk of Falls in Parkinson’s Disease: A Cross-Sectional Study of 160 Patients. Park. Dis. 2012, 2012, 362572. [Google Scholar] [CrossRef]
- Speelman, A.D.; van de Warrenburg, B.P.; van Nimwegen, M.; Petzinger, G.M.; Munneke, M.; Bloem, B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011, 7, 528–534. [Google Scholar] [CrossRef]
- Cusso, M.E.; Donald, K.J.; Khoo, T.K. The Impact of Physical Activity on Non-Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front. Med. 2016, 3, 35. [Google Scholar] [CrossRef]
- Mak, M.; Wong-Yu, I.S.; Shen, X.; Chung, C.L.-H. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Rafferty, M.R.; Nettnin, E.; Goldman, J.G.; MacDonald, J. Frameworks for Parkinson’s Disease Rehabilitation Addressing When, What, and How. Curr. Neurol. Neurosci. Rep. 2021, 21, 12. [Google Scholar] [CrossRef]
- Ellis, T.; Boudreau, J.K.; DeAngelis, T.R.; Brown, L.E.; Cavanaugh, J.T.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Barriers to Exercise in People with Parkinson Disease. Phys. Ther. 2013, 93, 628–636. [Google Scholar] [CrossRef]
- Schootemeijer, S.; van der Kolk, N.M.; Ellis, T.; Mirelman, A.; Nieuwboer, A.; Nieuwhof, F.; Schwarzschild, M.A.; de Vries, N.M.; Bloem, B.R. Barriers and Motivators to Engage in Exercise for Persons with Parkinson’s Disease. J. Park. Dis. 2020, 10, 1293–1299. [Google Scholar] [CrossRef]
- Isernia, S.; Di Tella, S.; Pagliari, C.; Jonsdottir, J.; Castiglioni, C.; Gindri, P.; Salza, M.; Gramigna, C.; Palumbo, G.; Molteni, F.; et al. Effects of an Innovative Telerehabilitation Intervention for People With Parkinson’s Disease on Quality of Life, Motor, and Non-motor Abilities. Front. Neurol. 2020, 11, 846. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- Pachoulakis, I.; Papadopoulos, N.; Analyti, A.; Pachoulakis, I.; Papadopoulos, N. Kinect-Based Exergames Tailored to Parkinson Patients. Int. J. Comput. Games Technol. 2018, 2018, 2618271. [Google Scholar] [CrossRef]
- Garcia-Agundez, A.; Folkerts, A.-K.; Konrad, R.; Caserman, P.; Tregel, T.; Goosses, M.; Göbel, S.; Kalbe, E. Recent advances in rehabilitation for Parkinson’s Disease with Exergames: A Systematic Review. J. Neuroeng. Rehabil. 2019, 16, 17. [Google Scholar] [CrossRef]
- De Oliveira, L.C.; Mendes, L.C.; de Lopes, R.A.; Carneiro, J.A.S.; Cardoso, A.; Júnior, E.A.L.; Andrade, A.D.O. A systematic review of serious games used for rehabilitation of individuals with Parkinson’s disease. Res. Biomed. Eng. 2021, 37, 849–865. [Google Scholar] [CrossRef]
- Ferraz, D.D.; Trippo, K.V.; Duarte, G.P.; Neto, M.G.; Santos, K.O.B.; Filho, J.O. The Effects of Functional Training, Bicycle Exercise, and Exergaming on Walking Capacity of Elderly Patients With Parkinson Disease: A Pilot Randomized Controlled Single-blinded Trial. Arch. Phys. Med. Rehabil. 2018, 99, 826–833. [Google Scholar] [CrossRef]
- Brachman, A.; Marszałek, W.; Kamieniarz, A.; Michalska, J.; Pawłowski, M.; Juras, G. Biomechanical measures of balance after balance-based exergaming training dedicated for patients with Parkinson’s disease. Gait Posture 2021, 87, 170–176. [Google Scholar] [CrossRef]
- Harris, D.M.; Rantalainen, T.; Muthalib, M.; Johnson, L.; Teo, W.-P. Exergaming as a Viable Therapeutic Tool to Improve Static and Dynamic Balance among Older Adults and People with Idiopathic Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2015, 7, 167. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, M.; Wang, Y.-X.; He, Z.-W.; Chi, S.-Q.; Yang, Z.-H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabilit. 2019, 33, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Lee, D.-K.; Song, H.-S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.F.; You, T.; Leveille, S.G. Potential Benefits of Exergaming for Cognition and Dual-Task Function in Older Adults: A Systematic Review. J. Aging Phys. Act. 2016, 24, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Busch, J.-H.; Roeben, B.; Otterbein, S.; Saraykin, P.; Leks, E.; Liepelt-Scarfone, I.; Synofzik, M.; Elshehabi, M.; Maetzler, W.; et al. Effects of Exergaming on Attentional Deficits and Dual-Tasking in Parkinson’s Disease. Front. Neurol. 2019, 10, 646. [Google Scholar] [CrossRef]
- Ballesteros, S.; Voelcker-Rehage, C.; Bherer, L. Cognitive and Brain Plasticity Induced by Physical Exercise, Cognitive Training, Video Games, and Combined Interventions. Front. Hum. Neurosci. 2018, 12, 169. [Google Scholar] [CrossRef]
- Silva, K.G.; De Freitas, T.B.; Doná, F.; Ganança, F.F.; Ferraz, H.B.; Torriani-Pasin, C.; Pompeu, J.E. Effects of virtual rehabilitation versus conventional physical therapy on postural control, gait, and cognition of patients with Parkinson’s disease: Study protocol for a randomized controlled feasibility trial. Pilot Feasibility Stud. 2017, 3, 68. [Google Scholar] [CrossRef]
- Cerqueira, T.M.D.M.; De Moura, J.A.; De Lira, J.O.; Leal, J.; D’Amelio, M.; Mendes, F.A.D.S. Cognitive and motor effects of Kinect-based games training in people with and without Parkinson disease: A preliminary study. Physiother. Res. Int. 2019, 25, e1807. [Google Scholar] [CrossRef]
- Perez-Marcos, D.; Bieler-Aeschlimann, M.; Serino, A. Virtual Reality as a Vehicle to Empower Motor-Cognitive Neurorehabilitation. Front. Psychol. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Walton, C.C.; Shine, J.M.; Mowszowski, L.; Naismith, S.L.; Lewis, S.J.G. Freezing of gait in Parkinson’s disease: Current treatments and the potential role for cognitive training. Restor. Neurol. Neurosci. 2014, 32, 411–422. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- AlMahadin, G.; Lotfi, A.; Zysk, E.; Siena, F.L.; Mc Carthy, M.; Breedon, P. Parkinson’s disease: Current assessment methods and wearable devices for evaluation of movement disorder motor symptoms—A patient and healthcare professional perspective. BMC Neurol. 2020, 20, 419. [Google Scholar] [CrossRef]
- Rahman, W.; Lee, S.; Islam, S.; Antony, V.N.; Ratnu, H.; Ali, M.R.; Al Mamun, A.; Wagner, E.; Jensen-Roberts, S.; Waddell, E.; et al. Detecting Parkinson Disease Using a Web-Based Speech Task: Observational Study. J. Med. Internet Res. 2021, 23, e26305. [Google Scholar] [CrossRef]
- Borzì, L.; Varrecchia, M.; Olmo, G.; Artusi, C.A.; Fabbri, M.; Rizzone, M.G.; Romagnolo, A.; Zibetti, M.; Lopiano, L. Home monitoring of motor fluctuations in Parkinson’s disease patients. J. Reliab. Intell. Environ. 2019, 5, 145–162. [Google Scholar] [CrossRef]
- Nyholm, D.; Kowalski, J.; Aquilonius, S.-M. Wireless real-time electronic data capture for self-assessment of motor function and quality of life in Parkinson’s disease. Mov. Disord. 2004, 19, 446–451. [Google Scholar] [CrossRef]
- Erb, M.K.; Karlin, D.R.; Ho, B.K.; Thomas, K.C.; Parisi, F.; Vergara-Diaz, G.P.; Daneault, J.-F.; Wacnik, P.W.; Zhang, H.; Kangarloo, T.; et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit. Med. 2020, 3, 6. [Google Scholar] [CrossRef]
- Cikajlo, I.; Hukić, A.; Dolinšek, I.; Zajc, D.; Vesel, M.; Krizmanič, T.; Blažica, B.; Biasizzo, A.; Novak, F.; Potisk, K.P. Can telerehabilitation games lead to functional improvement of upper extremities in individuals with Parkinson’s disease? Int. J. Rehabil. Res. 2018, 41, 230–238. [Google Scholar] [CrossRef]
- Cikajlo, I.; Hukić, A.; Zajc, D. Exergaming as Part of the Telerehabilitation Can Be Adequate to the Outpatient Training: Preliminary Findings of a Non-randomized Pilot Study in Parkinson’s Disease. Front. Neurol. 2021, 12, 625225. [Google Scholar] [CrossRef]
- Vellata, C.; Belli, S.; Balsamo, F.; Giordano, A.; Colombo, R.; Maggioni, G. Effectiveness of Telerehabilitation on Motor Impairments, Non-motor Symptoms and Compliance in Patients with Parkinson’s Disease: A Systematic Review. Front. Neurol. 2021, 12, 627999. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Levin, O.S.; Iakovleva, O.V.; Coloman, I.I.; Kuzmina, A.V. Could New Generations of Sensors Reshape the Management of Parkinson’s Disease? Clin. Transl. Neurosci. 2021, 5, 18. [Google Scholar] [CrossRef]
- Mangal, N.K.; Tiwari, A.K. A review of the evolution of scientific literature on technology-assisted approaches using RGB-D sensors for musculoskeletal health monitoring. Comput. Biol. Med. 2021, 132, 104316. [Google Scholar] [CrossRef] [PubMed]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef] [PubMed]
- Capecci, M.; Pepa, L.; Verdini, F.; Ceravolo, M.G. A smartphone-based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait Posture 2016, 50, 28–33. [Google Scholar] [CrossRef]
- Ossig, A.A.C.; Antonini, A.; Buhmann, C.; Classen, J.; Csoti, J.A.; Falkenburger, B.; Schwarz, M.; Winkler, J.; Storch, A. Wearable sensor-based objective assessment of motor symptoms in Parkinson’s disease. J. Neural Transm. 2015, 123, 57–64. [Google Scholar] [CrossRef]
- Perumal, S.V.; Sankar, R. Gait and tremor assessment for patients with Parkinson’s disease using wearable sensors. ICT Express 2016, 2, 168–174. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, W.-W.; Park, H.; Lee, H.J.; Kim, S.K.; Kim, H.B.; Jeon, B.; Park, K.S. Automatic Classification of Tremor Severity in Parkinson’s Disease Using a Wearable Device. Sensors 2017, 17, 2067. [Google Scholar] [CrossRef]
- Di Biase, L.; Summa, S.; Tosi, J.; Taffoni, F.; Marano, M.; Cascio Rizzo, A.; Vecchio, F.; Formica, D.; Di Lazzaro, V.; Di Pino, G.; et al. Quantitative Analysis of Bradykinesia and Rigidity in Parkinson’s Disease. Front. Neurol. 2018, 9, 121. [Google Scholar] [CrossRef]
- Pham, M.H.; Warmerdam, E.; Elshehabi, M.; Schlenstedt, C.; Bergeest, L.-M.; Heller, M.; Haertner, L.; Ferreira, J.J.; Berg, D.; Schmidt, G.; et al. Validation of a Lower Back “Wearable”-Based Sit-to-Stand and Stand-to-Sit Algorithm for Patients With Parkinson’s Disease and Older Adults in a Home-Like Environment. Front. Neurol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Ribeiro, N.F.; Santos, C.P. Inertial measurement units: A brief state of the art on gait analysis. In Proceedings of the 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG), Coimbra, Portugal, 16–18 February 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Cau, N.; Cimolin, V.; Azzaro, C.; Albani, G.; Priano, L.; Mauro, A. A Self-Managed System for Automated Assessment of UPDRS Upper Limb Tasks in Parkinson’s Disease. Sensors 2018, 18, 3523. [Google Scholar] [CrossRef]
- Amprimo, G.; Ferraris, C.; Masi, G.; Pettiti, G.; Priano, L. GMH-D: Combining Google MediaPipe and RGB-Depth Cameras for Hand Motor Skills Remote Assessment. In Proceedings of the IEEE International Conference on Digital Health (ICDH), Barcelona, Spain, 10–16 July 2022; pp. 132–141. [Google Scholar] [CrossRef]
- Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Cau, N.; Cimolin, V.; Azzaro, C.; Priano, L.; Mauro, A. Feasibility of Home-Based Automated Assessment of Postural Instability and Lower Limb Impairments in Parkinson’s Disease. Sensors 2019, 19, 1129. [Google Scholar] [CrossRef]
- Ťupa, O.; Procházka, A.; Vyšata, O.; Schätz, M.; Mareš, J.; Vališ, M.; Mařík, V. Motion tracking and gait feature estimation for recognising Parkinson’s disease using MS Kinect. Biomed. Eng. Online 2015, 14, 97. [Google Scholar] [CrossRef]
- Cimolin, V.; Vismara, L.; Ferraris, C.; Amprimo, G.; Pettiti, G.; Lopez, R.; Galli, M.; Cremascoli, R.; Sinagra, S.; Mauro, A.; et al. Computation of Gait Parameters in Post Stroke and Parkinson’s Disease: A Comparative Study Using RGB-D Sensors and Optoelectronic Systems. Sensors 2022, 22, 824. [Google Scholar] [CrossRef]
- Ospina, B.M.; Chaparro, J.A.V.; Paredes, J.D.A.; Pino, Y.J.C.; Navarro, A.; Orozco, J.L. Objective Arm Swing Analysis in Early-Stage Parkinson’s Disease Using an RGB-D Camera (Kinect®)1. J. Park. Dis. 2018, 8, 563–570. [Google Scholar] [CrossRef]
- Ferraris, C.; Amprimo, G.; Masi, G.; Vismara, L.; Cremascoli, R.; Sinagra, S.; Pettiti, G.; Mauro, A.; Priano, L. Evaluation of Arm Swing Features and Asymmetry during Gait in Parkinson’s Disease Using the Azure Kinect Sensor. Sensors 2022, 22, 6282. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Kuenze, C.; Oh, J.; Wooten, S.; Signorile, J. Kinect-based assessment of lower limb kinematics and dynamic postural control during the star excursion balance test. Gait Posture 2017, 58, 421–427. [Google Scholar] [CrossRef]
- Ayed, I.; Jaume-i-Capó, A.; Martínez-Bueso, P.; Mir, A.; Moyà-Alcover, G. Balance Measurement Using Microsoft Kinect v2: Towards Remote Evaluation of Patient with the Functional Reach Test. Appl. Sci. 2021, 11, 6073. [Google Scholar] [CrossRef]
- Da Gama, A.; Fallavollita, P.; Teichrieb, V.; Navab, N. Motor Rehabilitation Using Kinect: A Systematic Review. Games Health J. 2015, 4, 123–135. [Google Scholar] [CrossRef]
- Chiuchisan, I.; Geman, O.; Postolache, O. Future Trends in Exergaming using MS Kinect for Medical Rehabilitation. In Proceedings of the International Conference and Exposition on Electrical And Power Engineering (EPE), Iasi, Romania, 18–19 October 2018. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Palacios-Navarro, G.; García-Magariño, I.; Ramos-Lorente, P. A Kinect-Based System for Lower Limb Rehabilitation in Parkinson’s Disease Patients: A Pilot Study. J. Med. Syst. 2015, 39, 103. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.H.; Rovini, E.; Dolciotti, C.; Bongioanni, P.; De Petris, G.; Cavallo, F. Leap motion evaluation for assessment of upper limb motor skills in Parkinson’s disease. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 116–121. [Google Scholar] [CrossRef]
- Shih, M.-C.; Wang, R.-Y.; Cheng, S.-J.; Yang, Y.-R. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Guyot, M.; Nuic, D.; Mandigout, S.; Compagnat, M.; Welter, M.L.; Daviet, J.C.; Perrochon, A. Effectiveness of home-based rehabilitation using active video games on quality of life, cognitive and motor functions in people with Parkinson’s disease: A systematic review. Disabil. Rehabil. 2022, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tölgyessy, M.; Dekan, M.; Chovanec, Ľ.; Hubinský, P. Evaluation of the Azure Kinect and Its Comparison to Kinect V1 and Kinect V2. Sensors 2021, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.-F.; Yang, Z.; Cheng, K.C.-C.; Du, D.; Tong, R.K.-Y. Effects of camera viewing angles on tracking kinematic gait patterns using Azure Kinect, Kinect v2 and Orbbec Astra Pro v2. Gait Posture 2021, 87, 19–26. [Google Scholar] [CrossRef]
- Rosa, B.; Colombo Zefinetti, F.; Vitali, A.; Regazzoni, D. RGB-D Sensors as Marker-Less MOCAP Systems: A Comparison Between Microsoft Kinect V2 and the New Microsoft Kinect Azure. In Advances in Simulation and Digital Human Modeling; AHFE 2021. Lecture Notes in Networks and Systems; Wright, J.L., Barber, D., Scataglini, S., Rajulu, S.L., Eds.; Springer: Cham, Switzerland, 2021; Volume 264. [Google Scholar] [CrossRef]
- Albert, J.A.; Owolabi, V.; Gebel, A.; Brahms, C.M.; Granacher, U.; Arnrich, B. Evaluation of the Pose Tracking Performance of the Azure Kinect and Kinect v2 for Gait Analysis in Comparison with a Gold Standard: A Pilot Study. Sensors 2020, 20, 5104. [Google Scholar] [CrossRef]
- Romeo, L.; Marani, R.; Malosio, M.; Perri, A.G.; D’Orazio, T. Performance Analysis of Body Tracking with the Microsoft Azure Kinect. In Proceedings of the 29th Mediterranean Conference on Control and Automation (MED), Puglia, Italy, 22–25 June 2021; pp. 572–577. [Google Scholar] [CrossRef]
- Tölgyessy, M.; Dekan, M.; Chovanec, Ľ. Skeleton Tracking Accuracy and Precision Evaluation of Kinect V1, Kinect V2, and the Azure Kinect. Appl. Sci. 2021, 11, 5756. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, B.; Hart, R.; Zhang, Y. The validity of a dual Azure Kinect-based motion capture system for gait analysis: A preliminary study. In Proceedings of the Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC), Auckland, New Zealand, 7–10 December 2020; pp. 1201–1206. [Google Scholar]
- Antico, M.; Balletti, N.; Laudato, G.; Lazich, A.; Notarantonio, M.; Oliveto, R.; Ricciardi, S.; Scalabrino, S.; Simeone, J. Postural control assessment via Microsoft Azure Kinect DK: An evaluation study. Comput. Methods Programs Biomed. 2021, 209, 106324. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, M. Design and Evaluation of an Exergame System of Knee with the Azure Kinect. In Data Science. ICPCSEE 2021. Communications in Computer and Information Science; Zeng, J., Qin, P., Jing, W., Song, X., Lu, Z., Eds.; Springer: Singapore, 2021; Volume 1452. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Yang, S.; Tan, Z.-X.; Wang, M.-M.; Xing, Y.; Dong, F.; Zhang, F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020, 245, 117345. [Google Scholar] [CrossRef]
- Liu, Z. 3D Skeletal Tracking on Azure Kinect. 2019. Available online: https://www.microsoft.com/en-us/research/uploads/prod/2020/01/AKBTSDK.pdf (accessed on 28 September 2021).
- Amprimo, G.; Pettiti, G.; Priano, L.; Mauro, A.; Ferraris, C. Kinect-based Solution for the Home Monitoring of Gait and Balance in Elderly People with and without Neurological Diseases. In Proceedings of the 2nd Italian Workshop on Artificial Intelligence for an Ageing Society (AI*IA 2021), Online, 29 November 2021; Available online: http://ceur-ws.org/Vol-3108/paper6.pdf (accessed on 7 July 2022).
- Azure Kinect DK. Available online: https://azure.microsoft.com/it-it/services/kinect-dk/ (accessed on 20 September 2022).
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.E.; Sheikh, Y. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 43, 172–186. [Google Scholar] [CrossRef]
- Dodd, C.; Rukshan, A.; Marc, A. Designing user interfaces for the elderly: A systematic literature review. In Proceedings of the 28th Australasian Conference on Information System (ACIS 2017), Hobart, Tasmania, 5–6 December 2017. [Google Scholar]
- Gianaria, E.; Grangetto, M. Robust gait identification using Kinect dynamic skeleton data. Multimed. Tools Appl. 2018, 78, 13925–13948. [Google Scholar] [CrossRef]
- Ferraris, C.; Cimolin, V.; Vismara, L.; Votta, V.; Amprimo, G.; Cremascoli, R.; Galli, M.; Nerino, R.; Mauro, A.; Priano, L. Monitoring of Gait Parameters in Post-Stroke Individuals: A Feasibility Study Using RGB-D Sensors. Sensors 2021, 21, 5945. [Google Scholar] [CrossRef]
- Lafond, D.; Duarte, M.; Prince, F. Comparison of three methods to estimate the center of mass during balance assessment. J. Biomech. 2004, 37, 1421–1426. [Google Scholar] [CrossRef]
- González, A.; Hayashibe, M.; Bonnet, V.; Fraisse, P. Whole Body Center of Mass Estimation with Portable Sensors: Using the Statically Equivalent Serial Chain and a Kinect. Sensors 2014, 14, 16955–16971. [Google Scholar] [CrossRef]
- Clauser, C.E.; McConville, J.T.; Young, J.W. Weight, Volume, and Center of Mass Segments of the Human Body. J. Occup. Med. 1971, 13, 270. [Google Scholar]
- The Jamovi Project. 2021. Available online: https://www.jamovi.org/about.html (accessed on 7 July 2022).


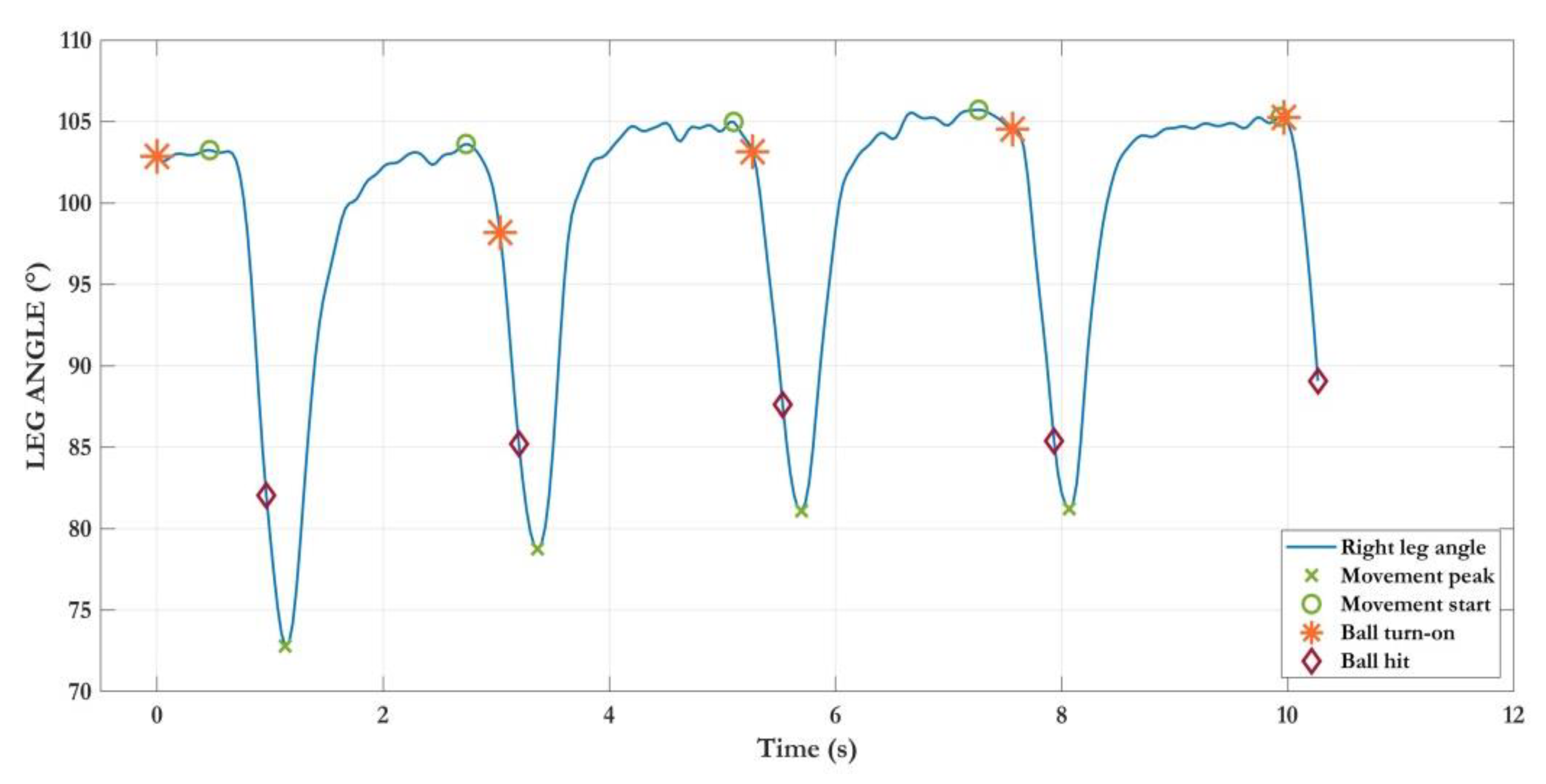
| Task | Parameter | Meaning | Unit |
|---|---|---|---|
| LA/BB | EXCM | Mean of ANGLEG excursions | deg |
| KNEEM | Mean ANGKNEE during motion | deg | |
| ANKLESA_YZ | ANKLE sway area (Frontal) | cm2 | |
| KNEESA_YZ | KNEE sway area (Frontal) | cm2 | |
| ANKLESA_XY | ANKLE sway area (Transverse) | cm2 | |
| KNEESA_XY | KNEE Sway Area (Transverse) | cm2 | |
| ANKLESV | ANKLE joint sway volume | cm3 | |
| KNEESV | KNEE joint sway volume | cm3 | |
| SPEEDM | Mean of leg movements speed | deg/s | |
| FMAX | Frequency at the maximum power | Hz | |
| B90 | Frequency at 90% of the power | Hz | |
| PoS | APR | Range of antero-posterior (AP) sway | cm |
| APT | Total antero-posterior sway | cm | |
| APS | Maximum antero-posterior sway speed | cm/s | |
| MLR | Range of medio-lateral (ML) sway | cm | |
| MLT | Total medio-lateral sway | cm | |
| MLS | Maximum medio-lateral sway speed | cm/s | |
| AREA | Sway area (AP-ML) | cm2 | |
| G | CADENCE | Number of steps per minute | step/min |
| DSUP | Duration of double support | s | |
| STANCE | Stance duration (%gait cycle) | % | |
| STEPL | Mean of step length | m | |
| SPEEDWALK | Mean gait speed | m/s | |
| SPEEDARM | Maximum speed on AP | cm/s | |
| APARM_R | Range of antero-posterior arm sway | cm | |
| MLARM_R | Range of medio-lateral arm sway | cm | |
| UDARM_R | Range of up-down arm sway | cm | |
| AREAARM_S | Sway area of arm (AP-ML) | cm2 | |
| MAXARM_AR | Maximum arm angle range | deg | |
| ASAAP_S | Asymmetry of antero-posterior arm sway | % | |
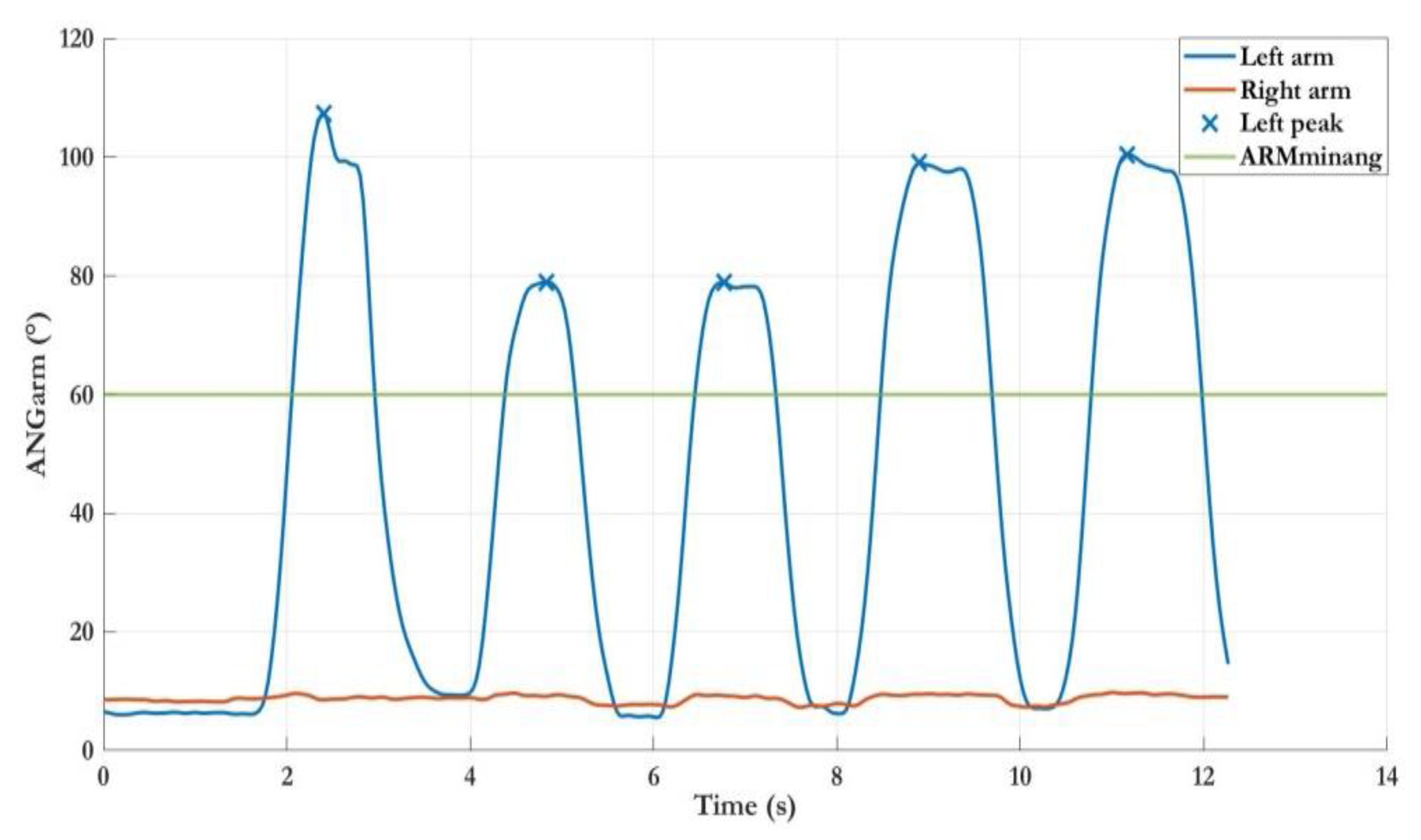
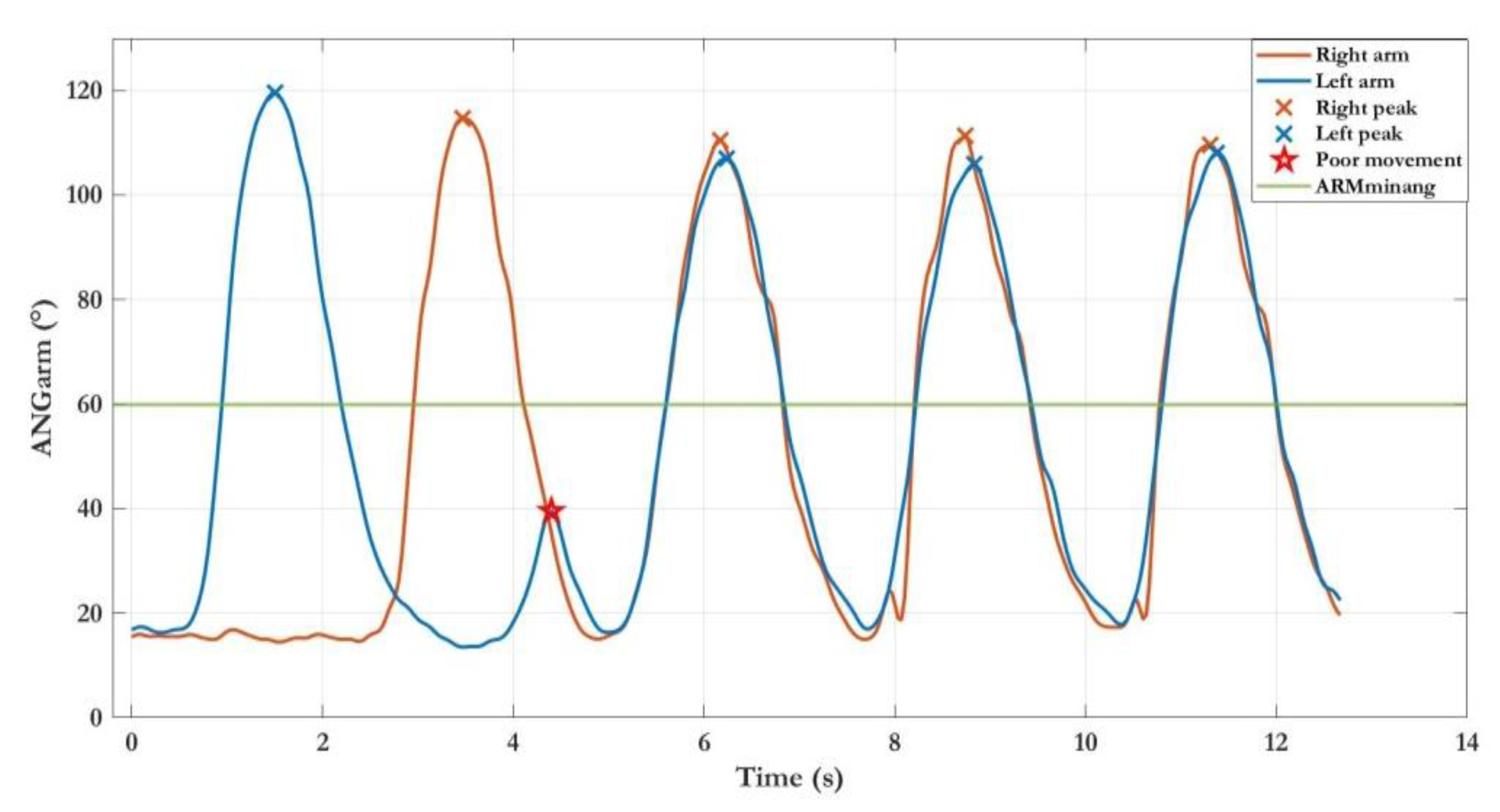
| LWL/FWL | ARMM | Mean of maximum angle peaks of ANGARM | deg |
| ELBOWM | Mean of ANGELBOW | deg | |
| DURM | Mean of arm movements duration | s | |
| SPEEDM | Mean of arm movements speed | deg/s | |
| INDEXSIM | Index of simultaneity (only last level) | - | |
| EXTIME | Time to complete the exercise | s |
| Exercise | PD Executions | HC Executions |
|---|---|---|
| LA | 75 (5) | 59 (1) |
| PoS | 40 (0) | 30 (0) |
| Gait | 40 (0) | 30 (0) |
| LWL | 53 (7) | 42 (3) |
| FWL | 55 (5) | 42 (3) |
| BB | 36 (4) | 28 (2) |
| Median (1° and 3° Percentiles) | Mann–Whitney | ||||
|---|---|---|---|---|---|
| Task | Parameter [Unit] | PD Group | HC Group | Statistic | p-Value |
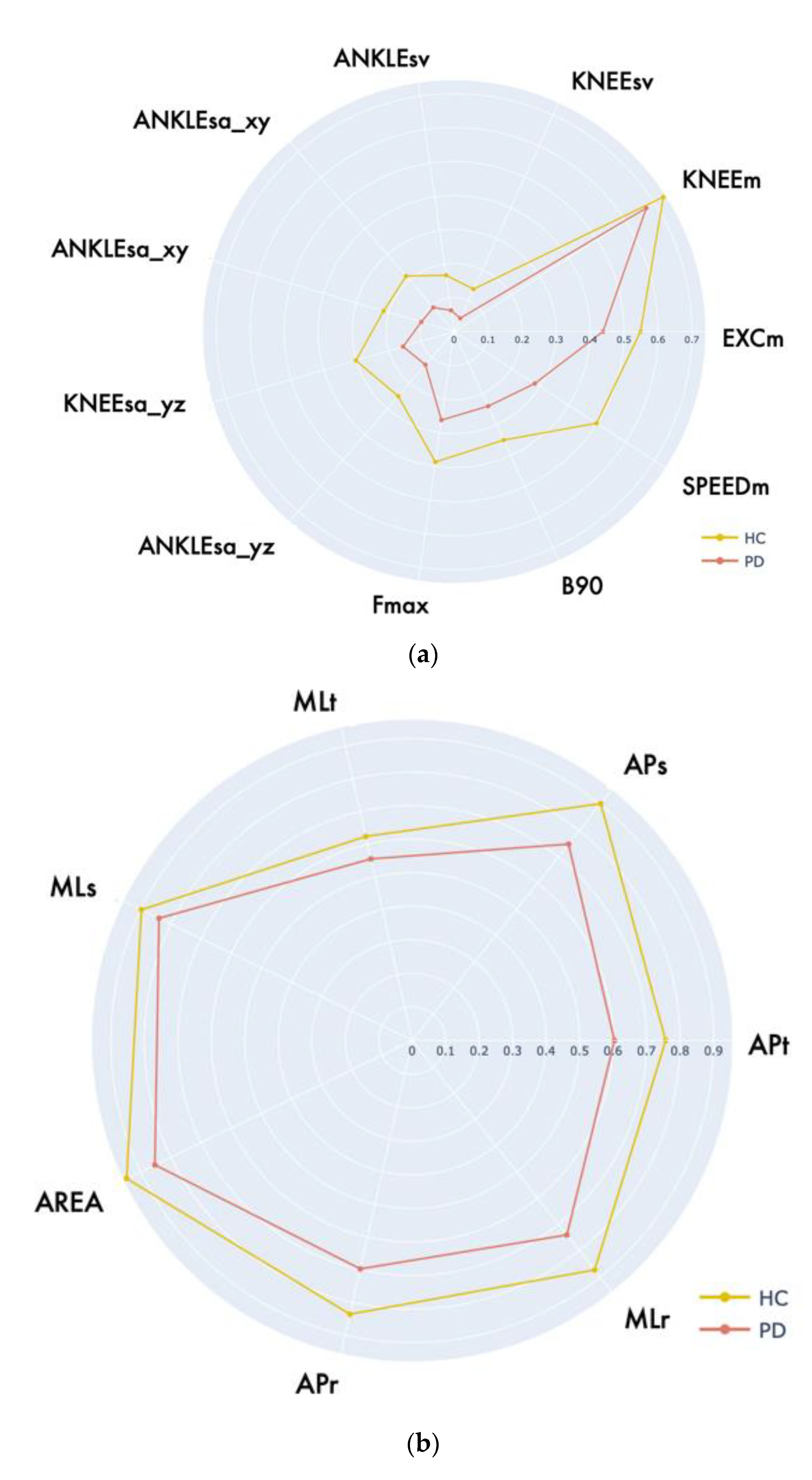
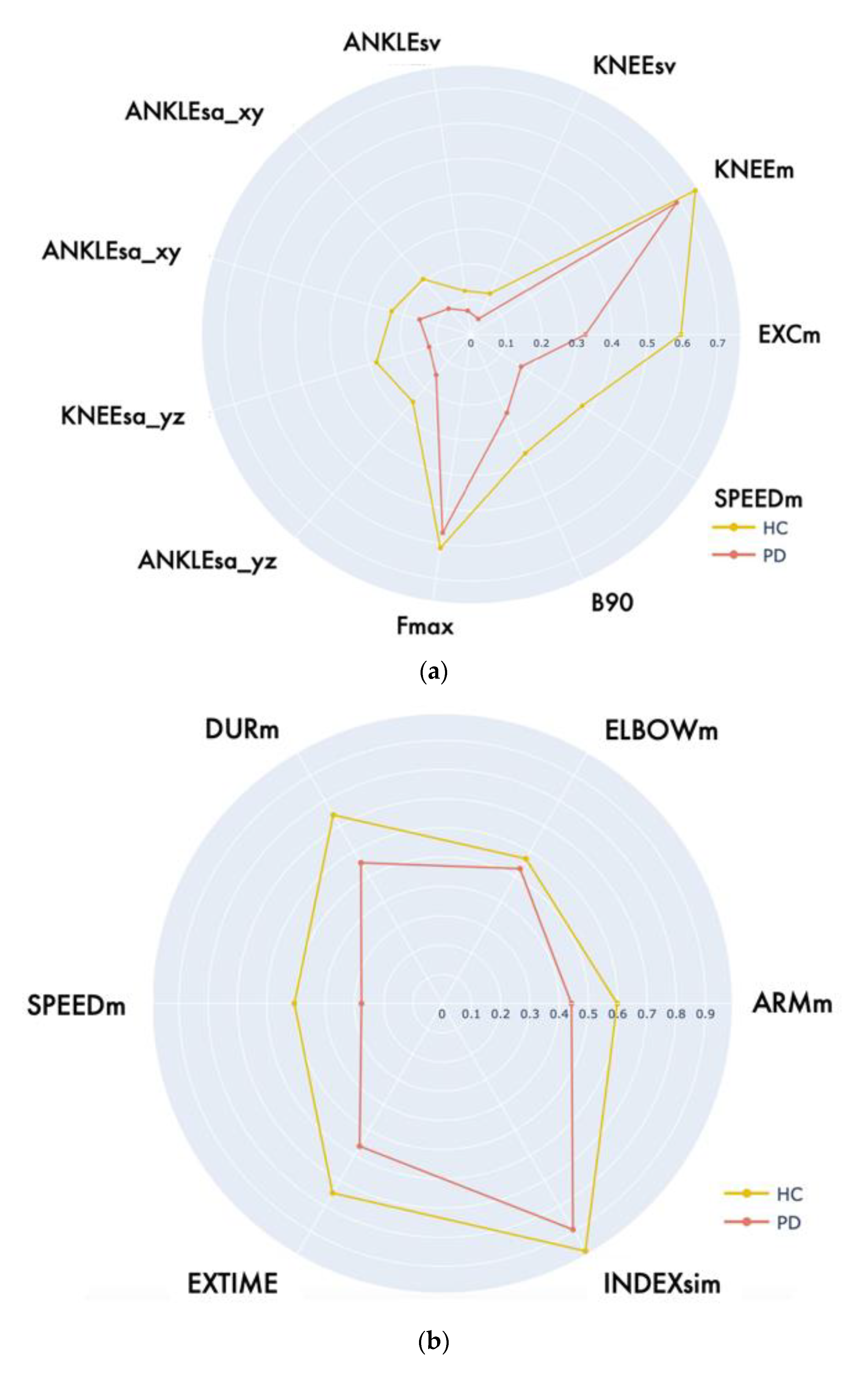
| LA | EXCM [deg] | 28.24 (22.64, 34.30) | 36.01 (22.23, 41.90) | 643.00 | 0.062 |
| KNEEM [deg] | 99.34 (88.92, 109.78) | 101.94 (99.40,106.63) | 735.00 | 0.277 | |
| ANKLESA_YZ [deg] | 120.05 (88.80, 165.76) | 168.52 (165.75, 279.11) | 567.00 | 0.010 * | |
| KNEESA_YZ [cm2] | 55.37 (38.17, 87.51) | 86.51 (57.83, 121.38) | 555.00 | 0.009 ** | |
| ANKLESA_XY [cm2] | 245.19 (130.52, 394.41) | 451.42 (249.79, 854.70) | 559.00 | <0.001 *** | |
| KNEESA_XY [cm2] | 60.13 (50.29, 79.23) | 113.40 (76.81, 147.22) | 412.00 | <0.001 *** | |
| ANKLESV [cm3] | 245.19 (130.52, 394.41) | 451.42 (249.78, 854.70) | 521.00 | 0.001 ** | |
| KNEESV [cm3] | 117.38 (73.38, 211.05) | 239.38 (131.69, 521.61) | 481.00 | 0.001 ** | |
| SPEEDM [deg/s] | 71.42 (46.41, 94.10) | 108.73 (86.18, 129.37) | 367.00 | <0.001 *** | |
| FMAX [Hz] | 0.98 (0.62, 1.40) | 1.32 (0.93, 2.19) | 572.00 | 0.014 * | |
| B90 [Hz] | 1.34 (0.98, 1.76) | 1.71 (1.16, 2.67) | 619.50 | 0.039 * | |
| PoS | APR [cm] | 2.47 (1.74, 3.93) | 1.75 (1.27, 2.16) | 269.00 | 0.001 ** |
| APT [cm] | 29.70 (24.60, 33.1) | 23.40 (20.90, 26.70) | 256.00 | <0.001 *** | |
| APS [cm/s] | 5.40 (4.59, 6.68) | 4.05 (3.44, 4.44) | 190.00 | <0.001 *** | |
| MLR [cm] | 1.55 (0.98, 2.14) | 0.87 (0.50, 1.46) | 277.00 | 0.002 ** | |
| MLT [cm] | 16.00 (13.90, 19.90) | 15.40 (12.00, 18.70) | 418.00 | 0.232 | |
| MLS [cm/s] | 3.75 (2.97, 4.54) | 3.26 (2.67, 3.65) | 368.00 | 0.061 | |
| AREA [cm2] | 1.97 (1.05, 3.93) | 0.85 (0.52, 1.99) | 265.00 | <0.001 *** | |
| G | CADENCE [step/min] | 100.67 (87.76, 117.31) | 108.11 (94.94, 115.38) | 972.00 | 0.144 |
| DSUP [s] | 0.34 (0.24, 0.50) | 0.17 (0.11, 0.31) | 612.00 | <0.001 *** | |
| STANCE [%] | 63.88 (61.84, 70.98) | 59.28 (56.44, 62.89) | 564.00 | <0.001 *** | |
| STEPL [m] | 0.55 (0.50, 0.58) | 0.66 (0.61, 0.71) | 324.00 | <0.001 *** | |
| SPEED [m/s] | 0.91 (0.71, 1.02) | 1.17 (1.01, 1.27) | 480.00 | <0.001 *** | |
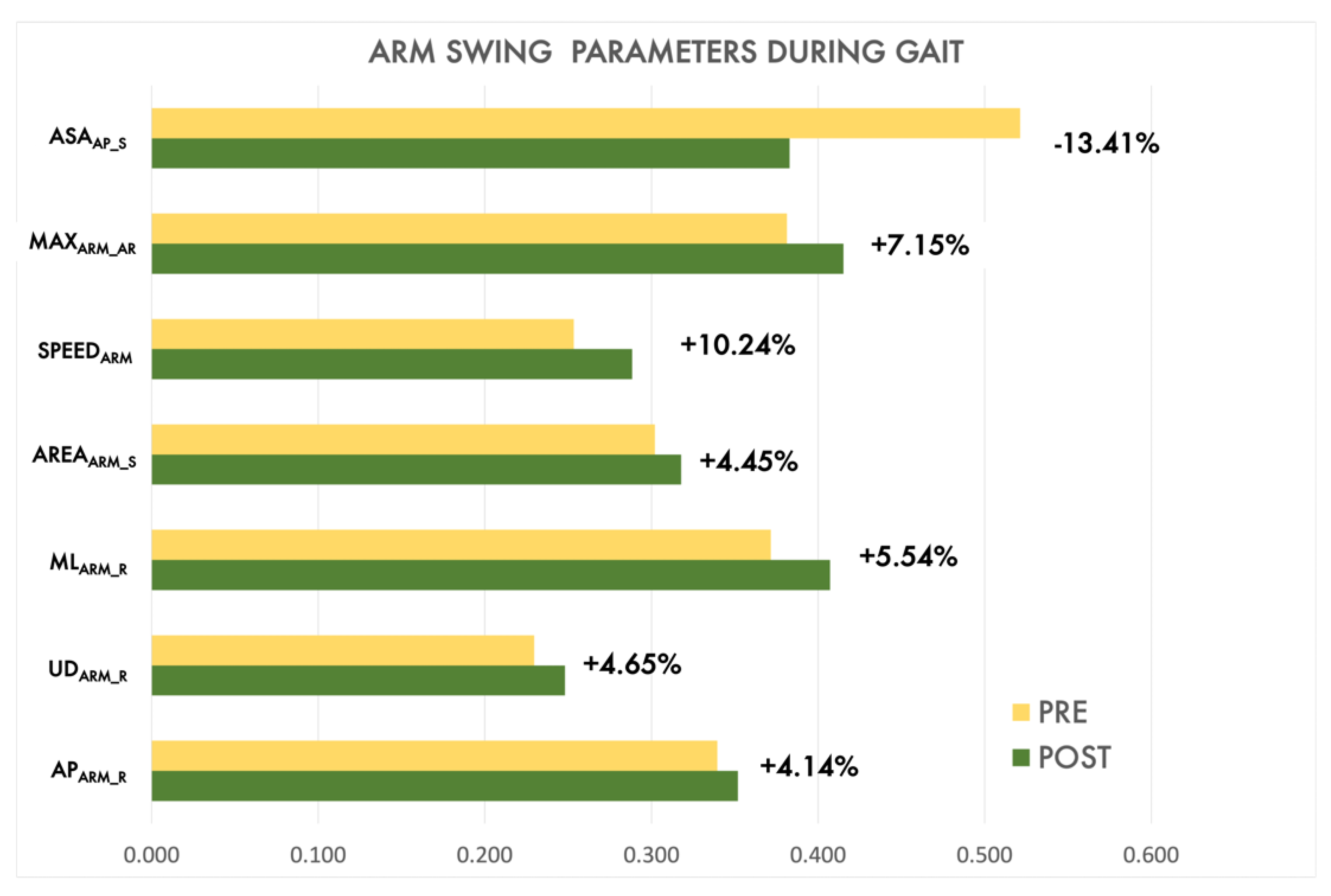
| SPEEDARM [cm/s] | 32.72 (21.53, 57.19) | 60.60 (29.67, 79.98) | 757.00 | 0.004 ** | |
| APARM_R [cm] | 15.11 (9.71, 21.74) | 20.73 (12.02, 28.17) | 813.00 | 0.012 * | |
| MLARM_R [cm] | 5.11 (4.00, 6.57) | 4.76 (4.11, 5.92) | 1080.00 | 0.566 | |
| UDARM_R [cm] | 4.66 (3.14, 6.06) | 5.41 (4.32, 7.10) | 816.00 | 0.013 * | |
| AREAARM_S [cm2] | 40.57 (23.18, 76.53) | 61.01 (40.71, 86.52) | 865.00 | 0.033 * | |
| MAXARM_AR [deg] | 14.96 (6.18, 21.94) | 21.82 (16.90, 26,88) | 756.00 | 0.004 ** | |
| ASAAP_S [%] | −14.98 (−18.3, −7.46) | −6.55 (−11.74, −2.72) | 704.00 | <0.001 *** | |
| Median (1° and 3° Percentiles) | Mann–Whitney | ||||
|---|---|---|---|---|---|
| EXERGAME | Parameter [Unit] | PD Group | HC Group | Statistic | p-Value |
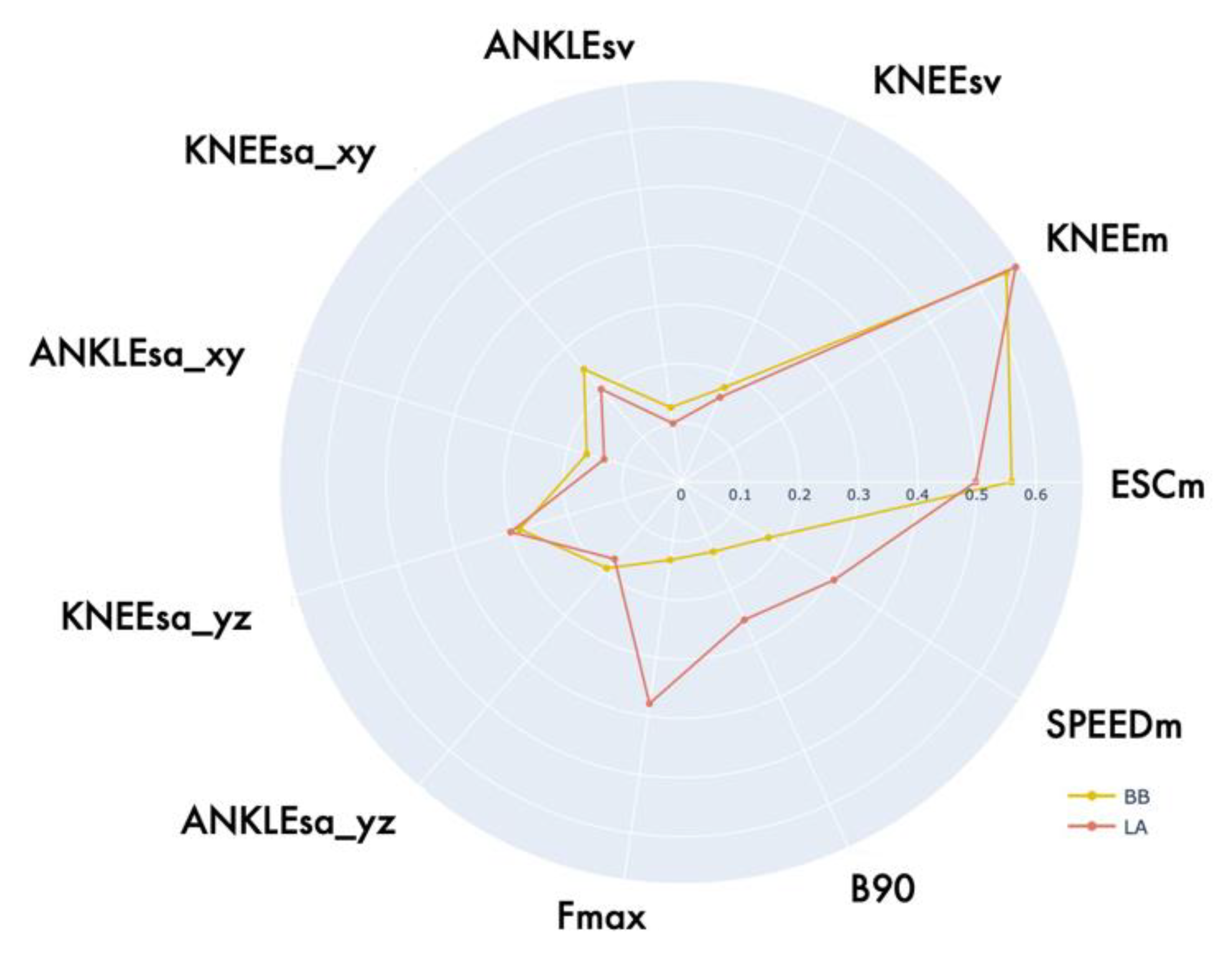
| BB | EXCM [deg] | 29.00 (24.01, 33.97) | 45.20 (29.76, 48.26) | 135.00 | <0.001 *** |
| KNEEM [deg] | 98.68 (88.87, 108.45) | 99.99 (97.17,104.83) | 283.00 | 0.671 | |
| ANKLESA_YZ [cm2] | 158.31 (59.74, 234.09) | 218.86 (132.33, 331.93) | 192.00 | 0.030 * | |
| KNEESA_YZ [cm2] | 54.29 (32.91, 89.11) | 93.77 (76.10, 160.04) | 149.00 | 0.002 ** | |
| ANKLESA_XY [cm2] | 59.32 (38.53, 88.61) | 98.21 (65.69, 124.67) | 170.00 | 0.009 ** | |
| KNEESA_XY [cm2] | 66.34 (50.07, 97.58) | 124.52 (77.09, 156.68) | 154.00 | 0.003 ** | |
| ANKLESV [cm3] | 323.26 (106.64, 516.91) | 553.44 (388.48, 775.76) | 189.00 | 0.025 ** | |
| KNEESV [cm3] | 117.93 (68.18, 259.63) | 284.29 (139.39, 498.37) | 170.00 | 0.009 ** | |
| SPEEDM [deg/s] | 38.69 (34.31, 52.28) | 81.79 (52.83, 92.77) | 83.00 | <0.001 *** | |
| FMAX [Hz] | 0.44 (0.34, 0.45) | 0.44 (0.39, 0.49) | 256.00 | 0.335 | |
| B90 [Hz] | 0.83 (0.67, 1.03) | 1.123 (0.98, 1.37) | 158.00 | 0.005 * | |
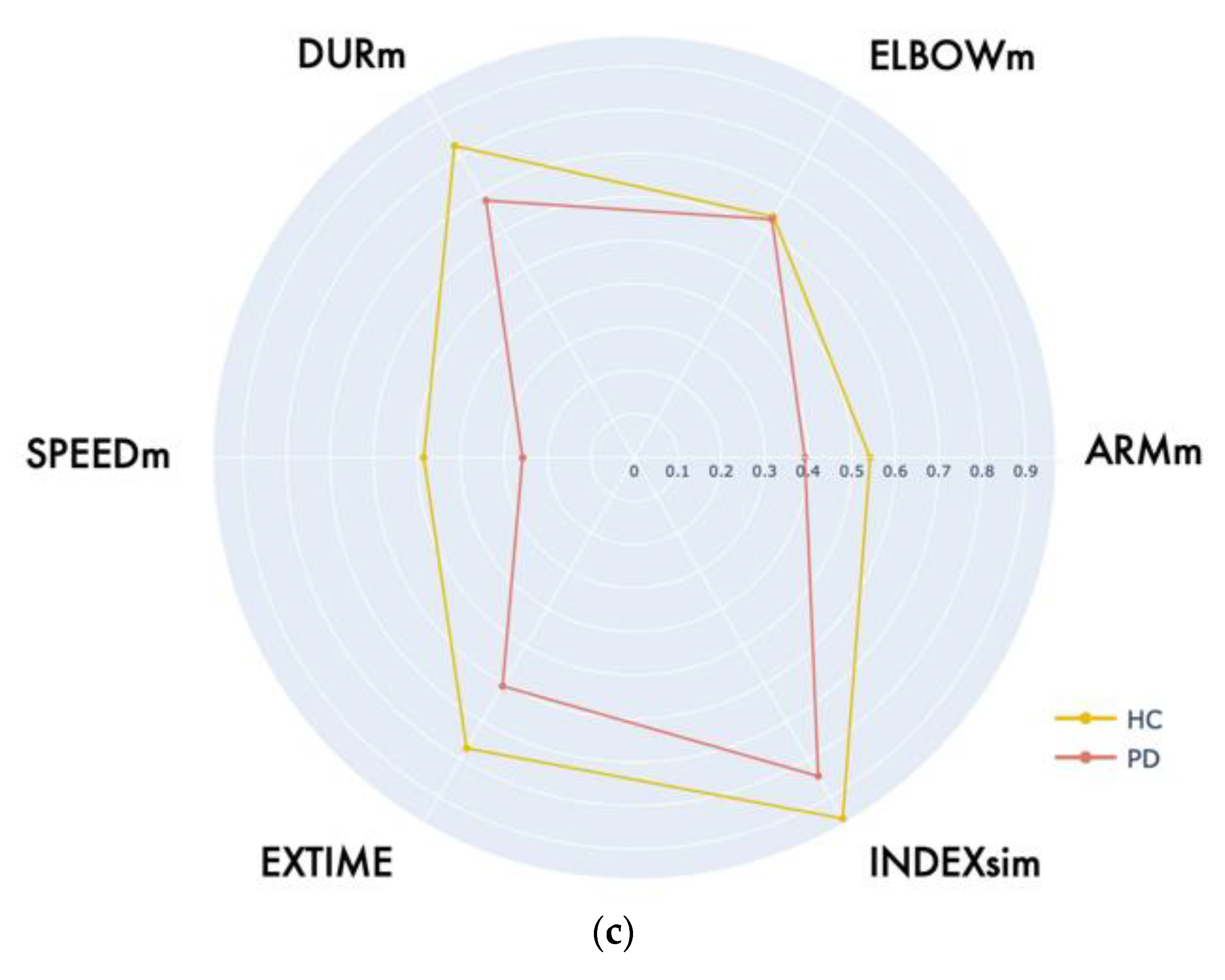
| LWL | ARMM [deg] | 97.00 (89.60, 105.00) | 108.00 (104.00, 114.00) | 764.00 | 0.001 ** |
| ELBOWM [deg] | 147.00 (141.00, 150.00) | 149.00 (144.00, 153.00) | 1519.00 | 0.244 | |
| DURM [s] | 2.32 (2.04, 2.82) | 1.95 (1.58, 2.13) | 708.00 | <0.001 *** | |
| SPEEDM [deg/s] | 74.00 (63.60, 87.70) | 106.00 (85.60, 148.00) | 567.00 | <0.001 *** | |
| INDEXSIM [-] | 0.030 (0.021, 0.042) | 0.022 (0.017, 0.023) | 39.5 | 0.004 ** | |
| EXTIME [s] | 12.2 (10.5, 15.2) | 10.5 (8.75, 11.6) | 839.00 | <0.001 *** | |
| FWL | ARMM [deg] | 110.07 (98.25, 119.92) | 120.28 (102.63, 142.74) | 1141.00 | 0.004 ** |
| ELBOWM [deg] | 144.75 (138.77, 149.53) | 145.58 (137.87, 149.59) | 1675.00 | 0.981 | |
| DURM [s] | 2.50 (2.17, 2.91) | 1.81 (1.50, 2.11) | 494 | <0.001 *** | |
| SPEEDM [deg/s] | 79.48 (62.53, 101.13) | 139.47 (91.86, 183.13) | 627.00 | <0.001 *** | |
| INDEXSIM [-] | 0.030 (0.020, 0.042) | 0.021 (0.016, 0.023) | 52.50 | 0.038 ** | |
| EXTIME [s] | 14.06 (11.83, 16.35) | 10.3 (10.37, 11.86) | 611.00 | <0.001 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amprimo, G.; Masi, G.; Priano, L.; Azzaro, C.; Galli, F.; Pettiti, G.; Mauro, A.; Ferraris, C. Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect. Sensors 2022, 22, 8173. https://doi.org/10.3390/s22218173
Amprimo G, Masi G, Priano L, Azzaro C, Galli F, Pettiti G, Mauro A, Ferraris C. Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect. Sensors. 2022; 22(21):8173. https://doi.org/10.3390/s22218173
Chicago/Turabian StyleAmprimo, Gianluca, Giulia Masi, Lorenzo Priano, Corrado Azzaro, Federica Galli, Giuseppe Pettiti, Alessandro Mauro, and Claudia Ferraris. 2022. "Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect" Sensors 22, no. 21: 8173. https://doi.org/10.3390/s22218173
APA StyleAmprimo, G., Masi, G., Priano, L., Azzaro, C., Galli, F., Pettiti, G., Mauro, A., & Ferraris, C. (2022). Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect. Sensors, 22(21), 8173. https://doi.org/10.3390/s22218173







