Visualization-Driven Time-Series Extraction from Wearable Systems Can Facilitate Differentiation of Passive ADL Characteristics among Stroke and Healthy Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Visualization Method for Time-Series Extraction
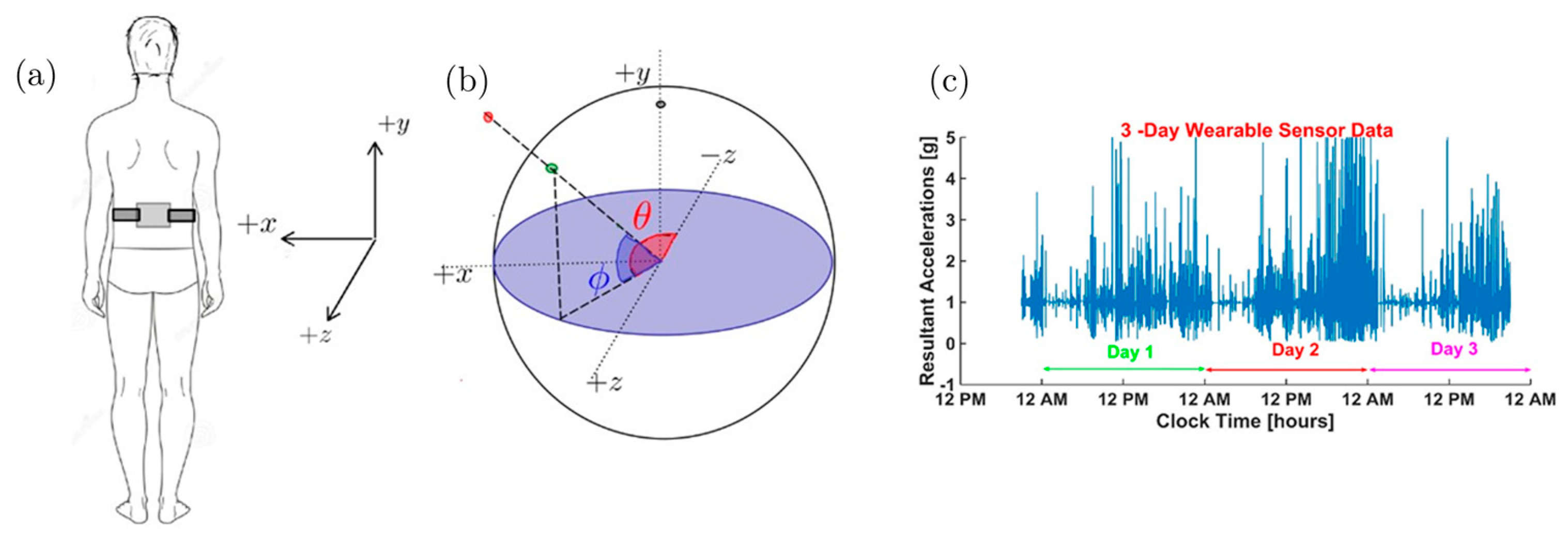
2.1.1. Unit Acceleration Sphere (UAS)
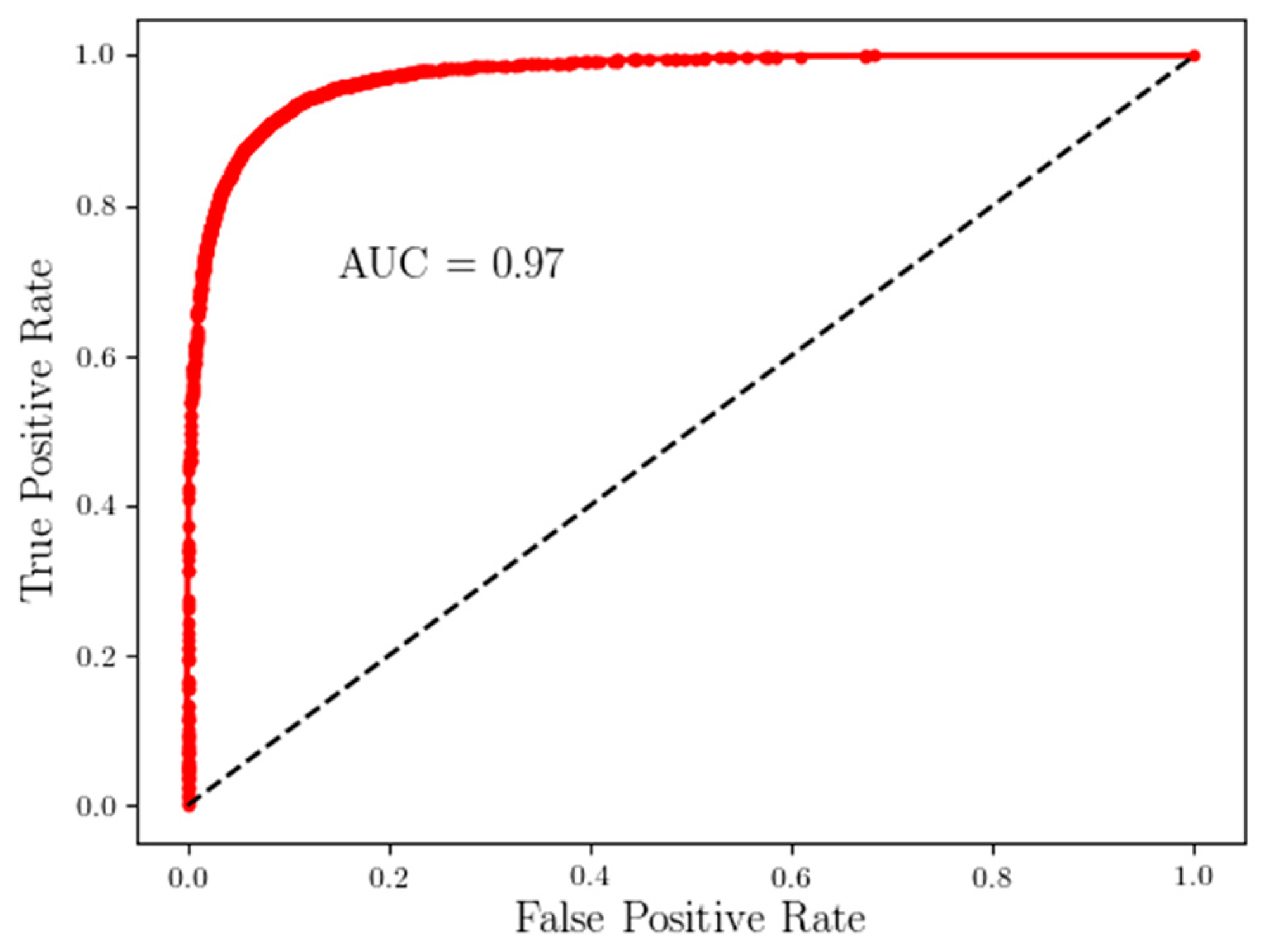
2.1.2. Task-Agnostic Time-Series Extraction for Recurrent Neural Networks (RNNs)
- (1)
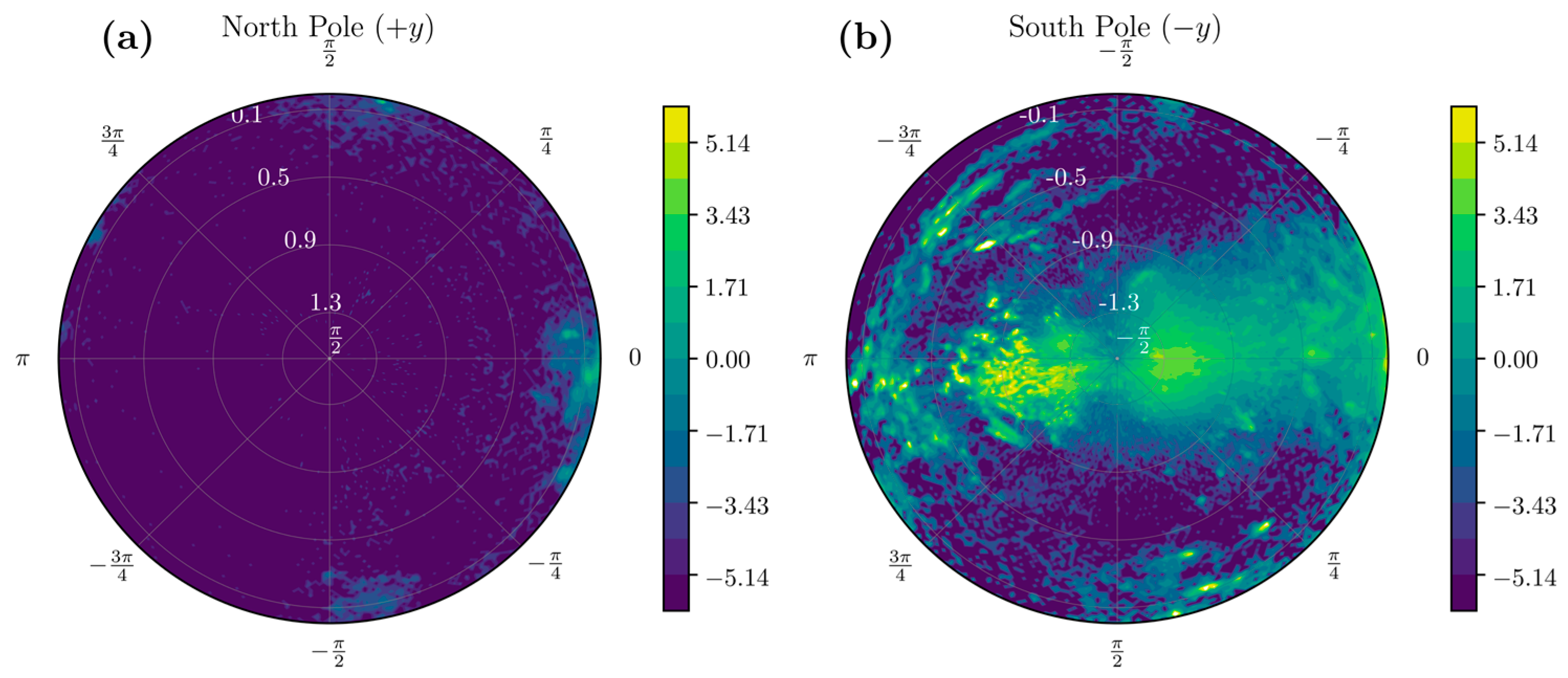
- Considering that the resultant acceleration vector a = [0, −1, 0] g’ in the stationary standing position corresponds to the south pole on the UAS, we look for data only in the southern hemisphere, or θ < 0.
- (2)
- Considering movements when in standing position, we look for data such that θ < −π/3, a neighborhood of the south pole, i.e. we consider samples with .
- (3)
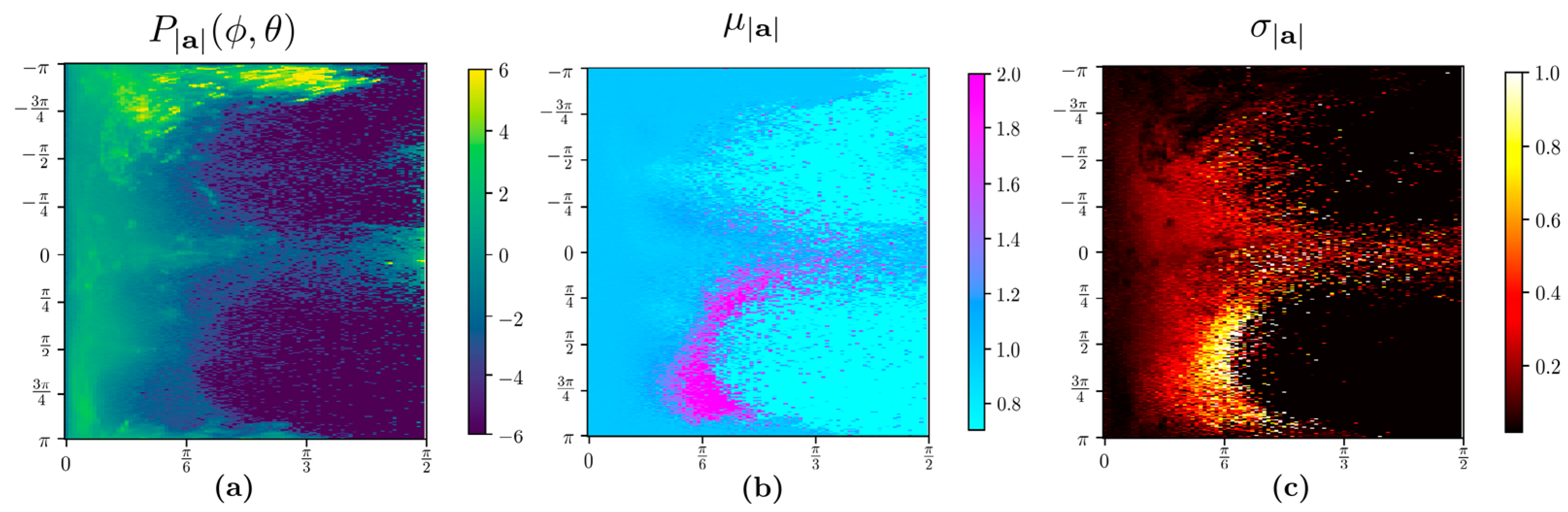
- The algorithm selected all bins with a standard deviation of the bin σ|a| ≥ 0.02 and the mean |µ|a| − | > 0.02 for input to recurrent neural networks. The rationale is that the bin corresponding to orientations where resultant acceleration signals show near-zero standard deviations and mean close to gravitational acceleration UAS (|a| = ) fits the rest of the scenarios and, therefore, there is not much movement-related information. For example, these scenarios can be regular breathing artifacts and posture re-orientations during sitting, standing, or other static postures. Furthermore, only daytime samples collected between 7 a.m. and 8 p.m. were considered since subjects were more active when awake during the day. The data samples that met the above time constraints were split into continuous 3 s time-series samples using the timestamp. Since postural transitions typically take up to 3 s, choosing this window size can capture such transitions. We used such 3 s long time-series samples with thirteen features to train a deep long short-term memory (LSTM) neural network using twelve features include: tri-axial acceleration, tri-axial angular velocity, resultant acceleration, acceleration components on the UAS, polar angle θ, and azimuthal angle . We obtained 37,383 and 19,067 data samples from healthy and stroke participants, respectively. Each sample has 3 s worth of data containing 11.21 × 106 samples for the healthy group and 5.72 × 106 samples for individuals with stroke. With such large numbers, the Z-test comparison yields extremely small p-values, and therefore, we report the effect size using Cohen’s d. The mean and standard deviations of these features and the effect size measured by Cohen’s d are listed in Table 1 below. We note that most of the features have only a small difference in the two populations, as evidenced by the small Cohen’s d values.
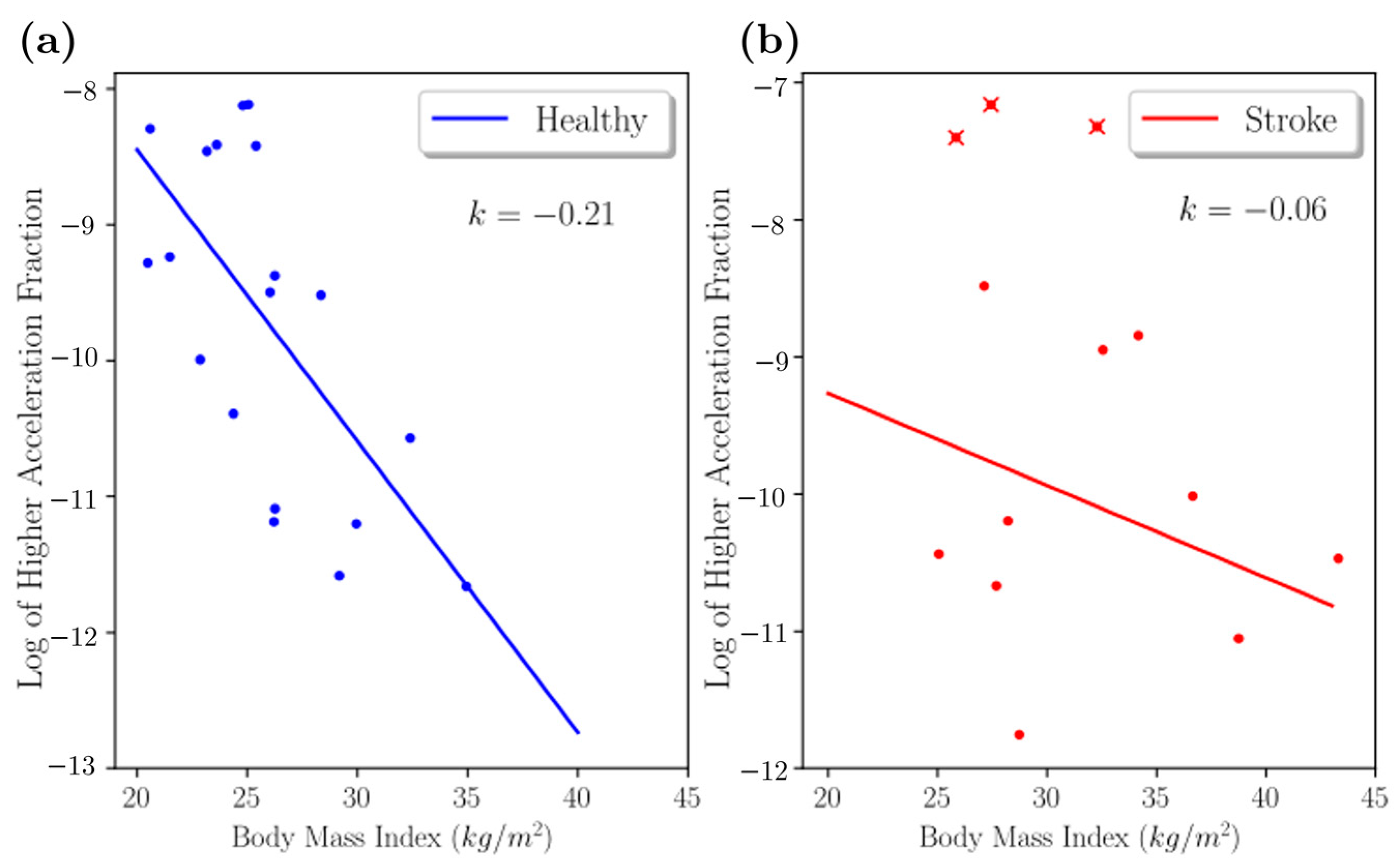
2.1.3. Higher Acceleration Fraction
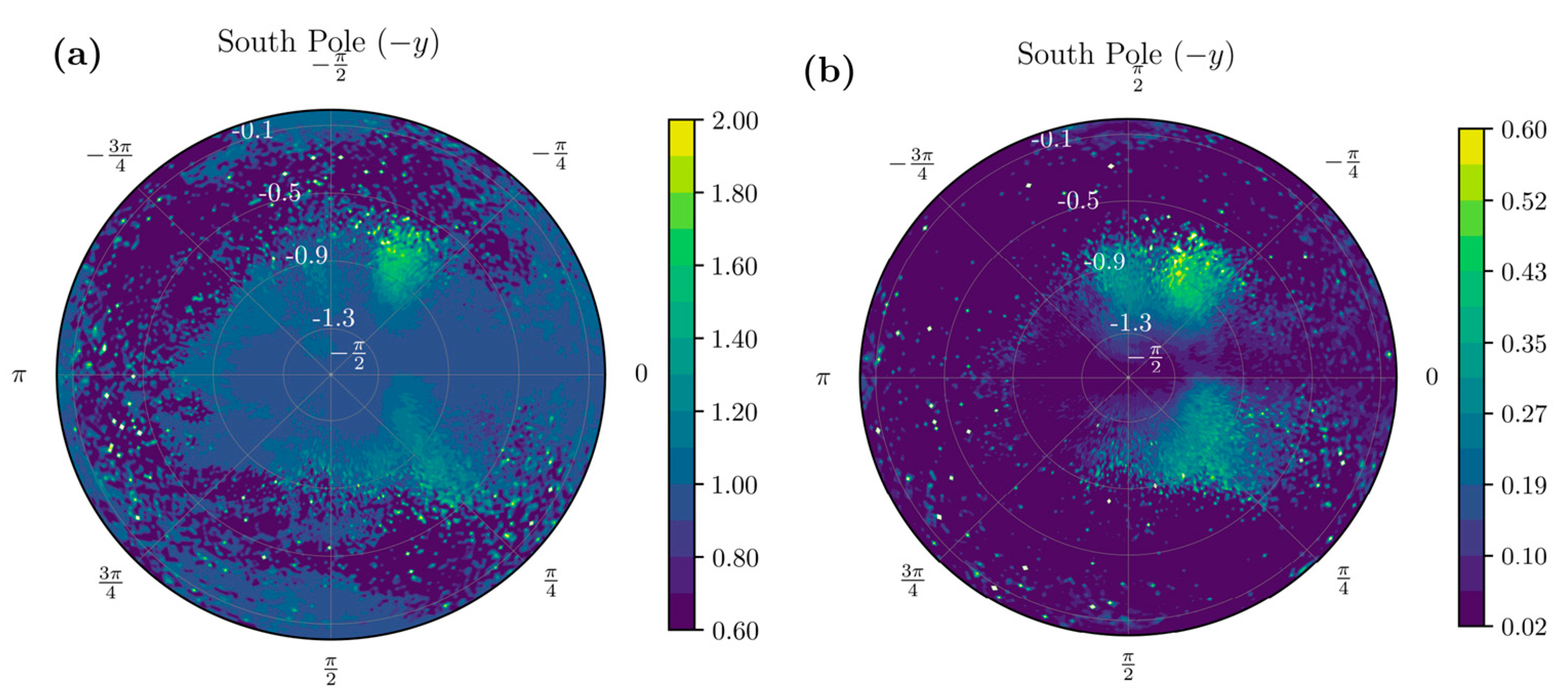
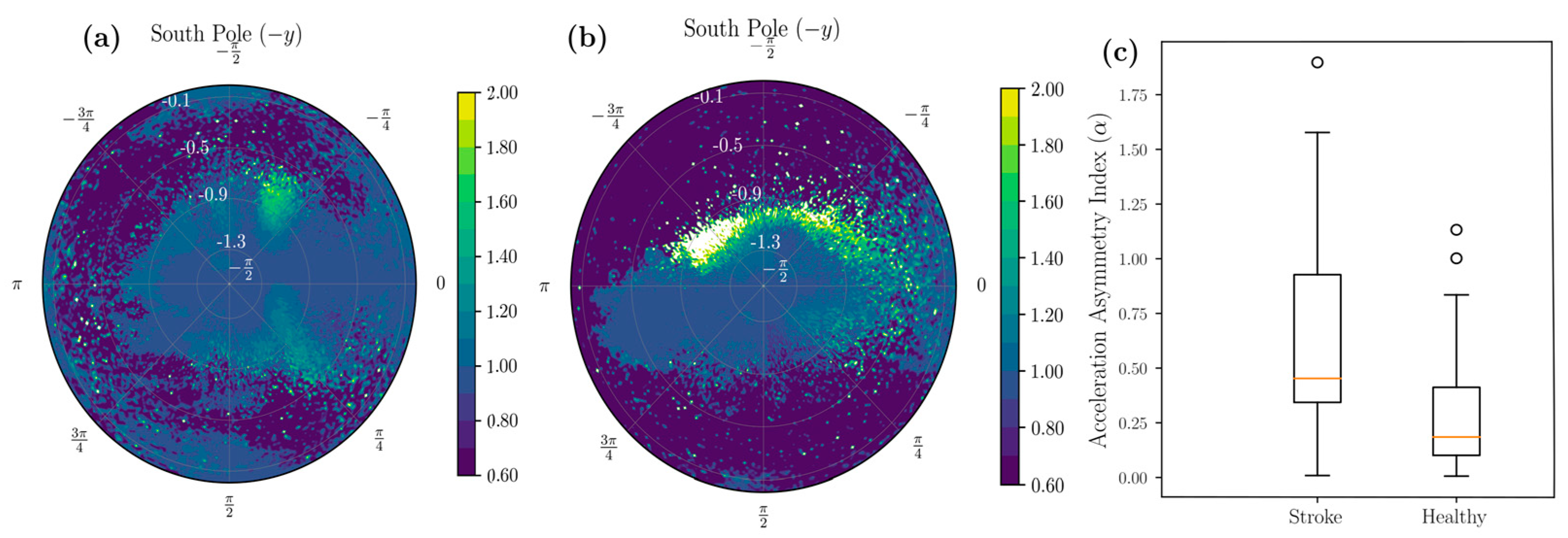
2.1.4. Stroke-Related Asymmetry Quantification
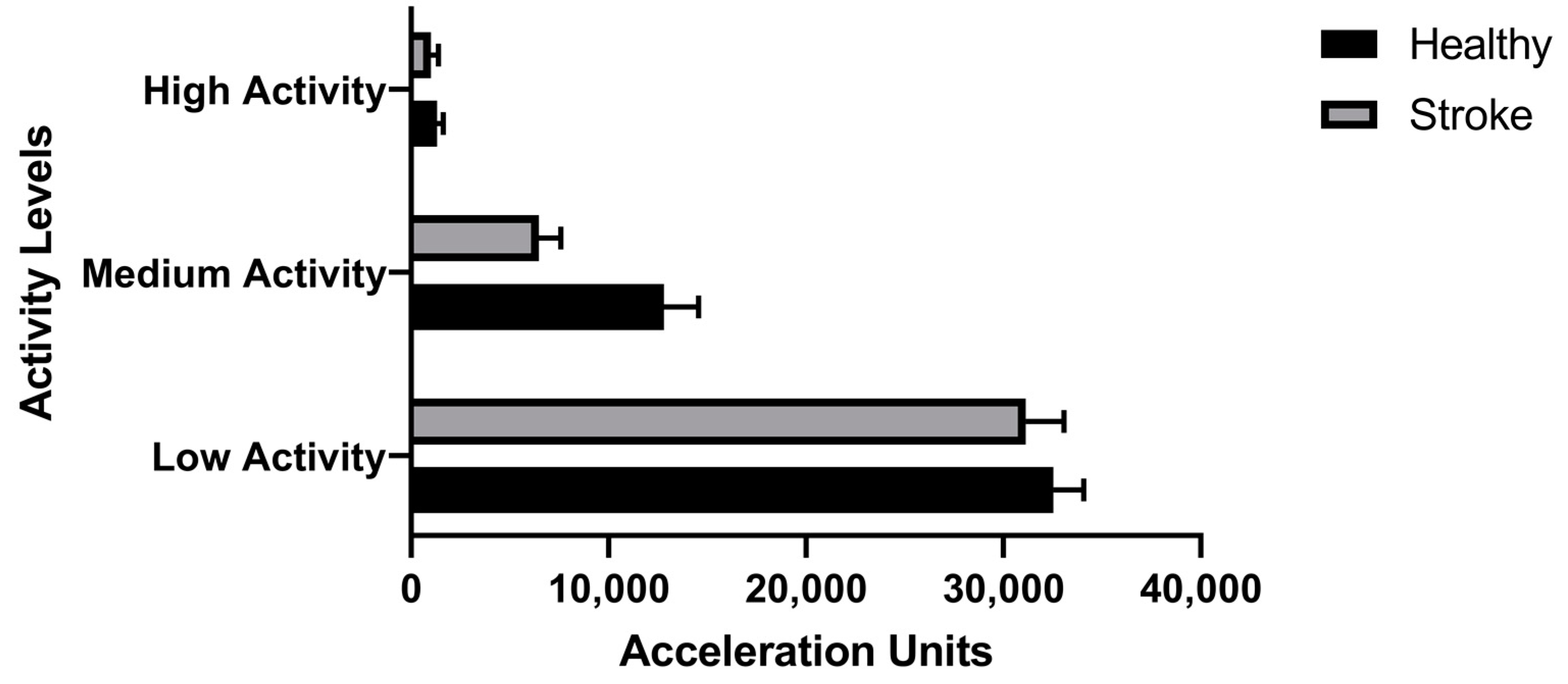
2.1.5. Movement Transitions and Activities in Frequency Bands
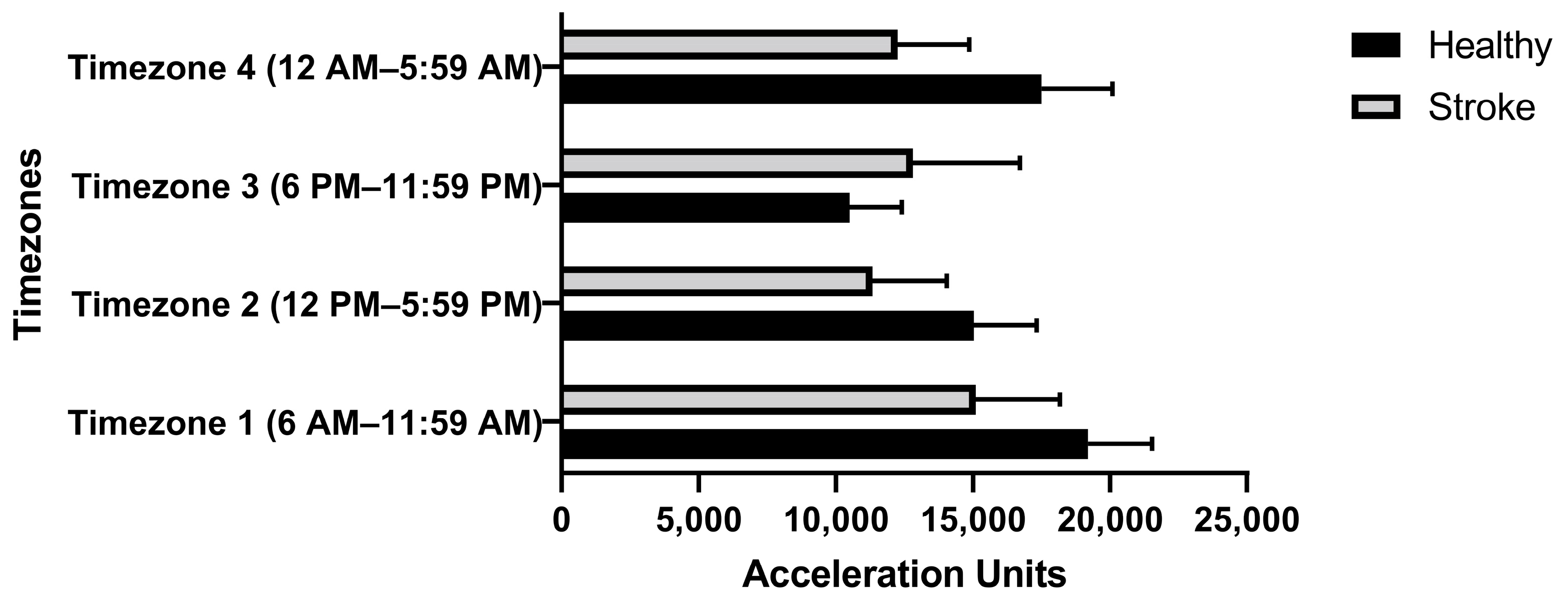
2.2. Experiment and Data Collection
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovbiagele, B.; Nguyen-Huynh, M.N. Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011, 8, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Wade, D.T.; Hewer, R.L. Functional abilities after stroke: Measurement, natural history and prognosis. J. Neurol. Neurosurg. Psychiatry 1987, 50, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Sivenius, J.; Pyorala, K.; Heinonen, O.P.; Salonen, J.T.; Riekkinen, P. The significance of intensity of rehabilitation of stroke—A controlled trial. Stroke 1985, 16, 928–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, K.; Brocklehurst, J.C.; Richards, B.; Laycock, P.J. The influence of age on the clinical presentation and outcome of stroke. Int. Rehabil. Med. 1984, 6, 49–53. [Google Scholar] [CrossRef]
- Feigenson, J.S.; McCarthy, M.L.; Meese, P.D.; Feigenson, W.D.; Greenberg, S.D.; Rubin, E.; McDowell, F.H. Stroke rehabilitation I. Factors predicting outcome and length of stay—An overview. N. Y. State J. Med. 1977, 77, 1426–1430. [Google Scholar] [PubMed]
- Stevens, R.S.; Ambler, N.R.; Warren, M.D. A randomized controlled trial of a stroke rehabilitation ward. Age Ageing 1984, 13, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Capistrant, B.D.; Wang, Q.; Liu, S.Y.; Glymour, M.M. Stroke-associated differences in rates of activity of daily living loss emerge years before stroke onset. J. Am. Geriatr. Soc. 2013, 61, 931–938. [Google Scholar] [CrossRef]
- Duncan, P.W.; Jorgensen, H.S.; Wade, D.T. Outcome measures in acute stroke trials: A systematic review and some recommendations to improve practice. Stroke 2000, 31, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, D.T.; Legh-Smith, J.; Langton Hewer, R. Social activities after stroke: Measurement and natural history using the Frenchay Activities Index. Int. Rehabil. Med. 1985, 7, 176–181. [Google Scholar] [CrossRef]
- Whiting, S.; Lincoln, N.; An, A.D.L. Assessment for Stroke Patients. Br. J. Occup. Ther. 2016, 43, 44–46. [Google Scholar] [CrossRef]
- Ebrahim, S.; Nouri, F.; Barer, D. Measuring disability after a stroke. J. Epidemiol. Community Health 1985, 39, 86–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Fagerberg, B.; Claesson, L.; Gosman-Hedstrom, G.; Blomstrand, C. Effect of acute stroke unit care integrated with care continuum versus conventional treatment: A randomized 1-year study of elderly patients: The Goteborg 70+ Stroke Study. Stroke 2000, 31, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Black, S.E.; Lawrence, J.; Szekely, C.; Szalai, J.P. The Sunnybrook Stroke Study: A prospective study of depressive symptoms and functional outcome. Stroke 1998, 29, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Kwok, T.; Lo, R.S.; Wong, E.; Tang, W.-K.; Mok, V.; Wong, K.-S. Quality of life of stroke survivors: A 1-year follow-up study. Arch. Phys. Med. Rehabil. 2006, 87, 1177–1182. [Google Scholar] [CrossRef]
- Park, S.; Jayaraman, S. Enhancing the quality of life through wearable technology. IEEE Eng. Med. Biol. Mag. 2003, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.E.; Storti, K.L.; Brach, J.S. Using activity monitors to measure physical activity in free-living conditions. Phys. Ther. 2006, 86, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortone, I.; Castellana, F.; Lampignano, L.; Zupo, R.; Moretti, B.; Giannelli, G.; Panza, F.; Sardone, R. Activity Energy Expenditure Predicts Clinical Average Levels of Physical Activity in Older Population: Results from Salus in Apulia Study. Sensors 2020, 20, 4585. [Google Scholar] [CrossRef]
- Redmond, S.J.; Lovell, N.H.; Yang, G.Z.; Horsch, A.; Lukowicz, P.; Murrugarra, L.; Marschollek, M. What Does Big Data Mean for Wearable Sensor Systems? Contribution of the IMIA Wearable Sensors in Healthcare WG. Yearb. Med. Inform. 2014, 9, 135–142. [Google Scholar]
- Chen, P.W.; Baune, N.A.; Zwir, I.; Wang, J.; Swamidass, V.; Wong, A.W.K. Measuring Activities of Daily Living in Stroke Patients with Motion Machine Learning Algorithms: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 1634. [Google Scholar] [CrossRef] [PubMed]
- Soangra, R.; Krishnan, V. Wavelet-Based Analysis of Physical Activity and Sleep Movement Data from Wearable Sensors among Obese Adults. Sensors 2019, 19, 3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soangra, R. Multi-day Longitudinal Assessment of Physical Activity and Sleep Behavior Among Healthy Young and Older Adults Using Wearable Sensors. IRBM 2020, 41, 80–87. [Google Scholar] [CrossRef]
- Little, V.L.; Perry, L.A.; Mercado, M.W.V.; Kautz, S.A.; Patten, C. Gait asymmetry pattern following stroke determines acute response to locomotor task. Gait Posture 2020, 77, 300–307. [Google Scholar] [CrossRef]
- Jette, A.M.; Keysor, J.; Coster, W.; Ni, P.; Haley, S. Beyond function: Predicting participation in a rehabilitation cohort. Arch. Phys. Med. Rehabil. 2005, 86, 2087–2094. [Google Scholar] [CrossRef]
- Mayo, N.E.; Anderson, S.; Barclay, R.; Cameron, J.I.; Desrosiers, J.; Eng, J.J.; Huijbregts, M.; Kagan, A.; MacKay-Lyons, M.; Moriello, C.; et al. Getting on with the rest of your life following stroke: A randomized trial of a complex intervention aimed at enhancing life participation post stroke. Clin. Rehabil. 2015, 29, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, J.; Noreau, L.; Rochette, A.; Bourbonnais, D.; Bravo, G.; Bourget, A. Predictors of long-term participation after stroke. Disabil. Rehabil. 2006, 28, 221–230. [Google Scholar] [CrossRef]
- Hoyle, M.; Gustafsson, L.; Meredith, P.; Ownsworth, T. Participation After Stroke: Do We Understand All the Components and Relationships As Categorised in the ICF? Brain Impair. 2012, 13, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Pang, M.Y.; Eng, J.J.; Miller, W.C. Determinants of satisfaction with community reintegration in older adults with chronic stroke: Role of balance self-efficacy. Phys. Ther. 2007, 87, 282–291. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Wondergem, R.; Pisters, M.F.; Wouters, E.J.; Olthof, N.; de Bie, R.A.; Visser-Meily, J.M.; Veenhof, C. The Course of Activities in Daily Living: Who Is at Risk for Decline after First Ever Stroke? Cerebrovasc. Dis. 2017, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Legg, L.; Drummond, A.; Leonardi-Bee, J.; Gladman, J.R.F.; Corr, S.; Donkervoort, M.; Edmans, J.; Gilbertson, L.; Jongbloed, L.; Logan, P.; et al. Occupational therapy for patients with problems in personal activities of daily living after stroke: Systematic review of randomised trials. BMJ 2007, 335, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babulal, G.M.; Huskey, T.N.; Roe, C.M.; Goette, S.A.; Connor, L.T. Cognitive impairments and mood disruptions negatively impact instrumental activities of daily living performance in the first three months after a first stroke. Top. Stroke Rehabil. 2015, 22, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Pohjasvaara, T.; Vataja, R.; Leppavuori, A.; Kaste, M.; Erkinjuntti, T. Cognitive functions and depression as predictors of poor outcome 15 months after stroke. Cerebrovasc. Dis. 2002, 14, 228–233. [Google Scholar] [CrossRef]
- Tatemichi, T.K.; Desmond, D.W.; Stern, Y.; Paik, M.; Sano, M.; Bagiella, E. Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. J. Neurol. Neurosurg. Psychiatry 1994, 57, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumming, T.B.; Marshall, R.S.; Lazar, R.M. Stroke, cognitive deficits, and rehabilitation: Still an incomplete picture. Int. J. Stroke 2013, 8, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L. Predictors of Functional Outcome Following Stroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 583–598. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Kwakkel, G.; van Wegen, E.E.; Ket, J.C.; Heymans, M.W. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke 2011, 42, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioncoloni, D.; Martini, G.; Piu, P.; Taddei, S.; Acampa, M.; Guideri, F.; Tassi, R.; Mazzocchio, R. Predictors of long-term recovery in complex activities of daily living before discharge from the stroke unit. NeuroRehabilitation 2013, 33, 217–223. [Google Scholar] [CrossRef]
- del Ser, T.; Barba, R.; Morin, M.M.; Domingo, J.; Cemillan, C.; Pondal, M.; Vivancos, J. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke 2005, 36, 2670–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazoura, O.; Papadaki, P.J.; Antoniadou, E.; Groumas, N.; Papadimitriou, A.; Thriskos, P.; Fezoulidis, I.V.; Vlychou, M. Skeletal and body composition changes in hemiplegic patients. J. Clin. Densitom. 2010, 13, 175–180. [Google Scholar] [CrossRef]
- Ryan, A.S.; Ivey, F.M.; Serra, M.C.; Hartstein, J.; Hafer-Macko, C.E. Sarcopenia and Physical Function in Middle-Aged and Older Stroke Survivors. Arch. Phys. Med. Rehabil. 2017, 98, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, L.; Jacobsen, B.K. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: A 1 year prospective study. Bone 2001, 28, 655–659. [Google Scholar] [CrossRef]
- Pietraszkiewicz, F.; Pluskiewicz, W.; Drozdzowska, B. Skeletal and functional status in patients with long-standing stroke. Endokrynol. Pol. 2011, 62, 2–7. [Google Scholar]
- Patterson, S.L.; Forrester, L.W.; Rodgers, M.M.; Ryan, A.S.; Ivey, F.M.; Sorkin, J.D.; Macko, R.F. Determinants of walking function after stroke: Differences by deficit severity. Arch. Phys. Med. Rehabil. 2007, 88, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shan, J.; Ding, W.; Wang, C.; Yuan, S.; Sun, F.; Li, H.; Fang, B. Cough Recognition Based on Mel-Spectrogram and Convolutional Neural Network. Front. Robot. AI 2021, 8, 580080. [Google Scholar] [CrossRef] [PubMed]
| Features | Healthy (μ, σ) | Stroke (μ, σ) | Effect Size (Cohen’s d) |
|---|---|---|---|
| −0.01, 0.14 | 0.01, 0.15 | 0.16 | |
| −0.93, 0.14 | −0.91, 0.14 | 0.16 | |
| 0.05, 0.33 | 0.14, 0.35 | 0.28 | |
| ωx | −0.06, 13.82 | −0.29, 15.15 | −0.02 |
| ωy | −0.60, 28.18 | 0.34, 19.49 | 0.04 |
| ωz | −0.20, 12.09 | −0.13, 9.98 | 0.01 |
| 1.00, 0.11 | 1.00, 0.09 | 0.01 | |
| −0.01, 0.13 | 0.01, 0.15 | 0.17 | |
| −0.93, 0.09 | −0.91, 0.11 | 0.24 | |
| 0.05, 0.33 | 0.14, 0.35 | 0.28 | |
| ϕ | −0.30, 2.03 | 0.09, 2.19 | 0.19 |
| θ | −1.24, 0.20 | −1.19, 0.22 | 0.26 |
| Layer | Output Shape | Parameters |
|---|---|---|
| LSTM | (None,300,200) | 170,400 |
| Batch Normalization | (None,300,200) | 800 |
| LSTM | (None,50) | 50,200 |
| Dropout | (None,50) | 0 |
| Dense (ReLU) | (None,50) | 2550 |
| Dropout | (None,50) | 0 |
| Dense (ReLU) | (None,15) | 765 |
| Dense (Sigmoid) | (None,1) | 16 |
| Stroke | Control | |
|---|---|---|
| Age (years) | 69 ± 8.4 | 74 ± 8.7 |
| BMI (kg/m2) | 30.8 ± 5.6 | 26.1 ± 3.0 |
| Gender | Six females and eight males | Eight females and six males |
| Fugl–Meyer Scores | Stroke | Healthy |
|---|---|---|
| Lower Extremity Score | 18.5 ± 3.3 | 28 ± 0 |
| Coordination Speed | 4.1 ± 1.0 | 6 ± 0 |
| Motor Function | 22.7 ± 3.7 | 34 ± 0 |
| Sensation Score | 9.2 ± 3.4 | 12 ± 0 |
| Passive Joint Motion | 15.7 ± 2 | 20 ± 0 |
| Joint Pain | 19.7 ± 0.5 | 20 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, J.; Soangra, R. Visualization-Driven Time-Series Extraction from Wearable Systems Can Facilitate Differentiation of Passive ADL Characteristics among Stroke and Healthy Older Adults. Sensors 2022, 22, 598. https://doi.org/10.3390/s22020598
John J, Soangra R. Visualization-Driven Time-Series Extraction from Wearable Systems Can Facilitate Differentiation of Passive ADL Characteristics among Stroke and Healthy Older Adults. Sensors. 2022; 22(2):598. https://doi.org/10.3390/s22020598
Chicago/Turabian StyleJohn, Joby, and Rahul Soangra. 2022. "Visualization-Driven Time-Series Extraction from Wearable Systems Can Facilitate Differentiation of Passive ADL Characteristics among Stroke and Healthy Older Adults" Sensors 22, no. 2: 598. https://doi.org/10.3390/s22020598
APA StyleJohn, J., & Soangra, R. (2022). Visualization-Driven Time-Series Extraction from Wearable Systems Can Facilitate Differentiation of Passive ADL Characteristics among Stroke and Healthy Older Adults. Sensors, 22(2), 598. https://doi.org/10.3390/s22020598






