Abstract
Background: This systematic review aimed to assess the effectiveness and safety of aPDT for the treatment of halitosis. Methods: Search strategies were conducted in October 2021 without language or data restrictions, on the following databases: MEDLINE, EMBASE, CENTRAL, LILACS and BBO, as well as a manual search. Randomized clinical trials (RCTs) with parallel design were considered for inclusion, assessing individuals (adolescents and adults) with a clinical diagnosis of halitosis treated with photodynamic therapy (aPDT). Primary outcomes assessed were halitosis measurements, adverse events and quality of life. The risk of bias for each included study was evaluated with the Cochrane Risk of Bias tool and the certainty of the body of the evidence was assessed with the GRADe approach. Results: Six RCTs (total of 225 participants) were included and due to clinical diversities it was not possible to group the outcome data in meta-analyses. Based on very low-certainty evidence (GRADE) the results showed that, when compared to tongue scraper, aPDT seems to promote a little to no difference in reducing halitosis and in the microbiological analysis. No adverse events were reported. Considering aPDT combined with tongue scraper, better outcome results were observed when compared to tongue scraper alone. Conclusions: Based on very low-certainty evidence, the findings of this review are uncertain about the effects of aPDT for halitosis control. Further RCTs with higher number of participants and long term assessments need to be conducted to support the use of this intervention. The protocol was registered in the PROSPERO database (number: CRD42020215319) on 19 November 2020—retrospectively registered.
1. Introduction
Halitosis is a term that consists of any unpleasant odor emanating from the oral cavity, the source of which may be local or systemic [1]. This alteration in mouth odor is the third major cause of the search for oral treatment [2]. Anaerobic bacteria are identified as the main cause of halitosis. These microorganisms produce sulfur-rich gases, which cause the presence of smell. Three volatile sulfur compounds (VSCs) are related to halitosis: hydrogen sulfide (H2S), methylmercaptan (CH3SH), and dimethyl sulfide (CH3SCH3) [3,4,5,6]. The high concentration of mucin in the saliva aids the adhesion of anaerobic microorganisms and epithelial cells in the posterior third of the lingual dorsum [7]. This biofilm is called tongue coating [8], and it is the most common cause of halitosis. Hydrogen sulfide (H2S) is the main gas liberated by bacteria from the tongue surface [9].
The different diagnostic methods for halitosis include a clinical assessment, known as the organoleptic test, which is a subjective method that consists of smelling the air exhaled from the mouth and quantifying the odor with the use of a scale. Halitosis can also be measured with a sulfide monitor, as described by Guedes et al. [10]; this method is an alternative to the organoleptic test and has high sensitivity and specificity [10]. Gas chromatography is the most appropriate method for the diagnosis of halitosis of any origin, as this method measures the three main sulfur gases [11,12,13]. The prevalence of this condition is high, with percentages above 50% found in articles [14].
Many interventions to improve halitosis have already been tested, but there is no evidence of superiority between them [15]. Existing treatments have disadvantages, such as staining of mucous membranes and teeth [16,17]. Conventional treatments for the control of halitosis basically consist of the use of toothpastes and mouthwashes with bactericidal substances, the correct use of tongue scrapers, the removal of dental caries lesions, the treatment of periodontal diseases, and the control of possible cases of xerostomia [12,13]. The tongue scraper has the disadvantage of causing excessive excoriation of the surface of the tongue, which is also a discomfort when eating acidic or bitter foods after excoriation of the tongue [18]. Oral hygiene behavior (OHB) is a very efficient complementary method [19].
Antimicrobial photodynamic therapy (aPDT) [18,20,21] has been tested in an attempt to treat halitosis [14,22]. It involves a photosensitizing agent, which produces free oxygen radicals (type I reaction) and singlet oxygen (type II reaction) in the presence of light, thereby destroying the cell wall of bacteria and causing cell death. This approach avoids both the occurrence of resistant bacteria and harm to the adjacent tissues, as the antimicrobial effect is confined to the areas covered by the photosensitizer and irradiated with light, acting quickly on the target microorganisms [18,20]. For halitosis, the main etiological factor of which is anaerobic bacteria, this therapy has achieved positive results regarding the reduction in hydrogen sulfide as well as a reduction in the bacterial load on the dorsum of the tongue, using a laser at a red wavelength and methylene blue. The advantages of this alternative approach are the reduction in damage to the tissues, the avoidance of bacterial resistance, and the development of a treatment protocol for halitosis that may be effective and lasting by eliminating the anaerobic bacteria related to this condition [18,20,23]. However, it is important to consider that even with immediate positive results, it was demonstrated that after 7 days, the participants returned to the initial halitosis values, which reinforces that the treatment for halitosis could be accompanied by oral hygiene behavior [18]. This probably occurs because bacteria residing in other niches of the oral cavity could recolonize the back of the newly treated tongue [24].
With the emergence of alternative treatments, such as aPDT, it is essential to analyze the level of evidence and the results of these novel protocols to assist dentists in the management of halitosis. Thus, the aim of the present study is to perform a systematic review of randomized controlled clinical trials that used aPDT protocols for the treatment of halitosis.
A large number of studies are led in the health field and need to be summarized [25] in systematic reviews. Such studies assist in the development of novel protocols for daily clinical practice [26], as decisions based on a single study could lead to errors. Moreover, this type of study enables an appraisal of the level of evidence found in articles and the effectiveness of the proposed treatments [27].
Halitosis is considered an important social drawback that affects interpersonal relations. In addition to raising concerns regarding the physical health of the individual, this condition can generate psychological problems and constitute a social barrier [28]. A systematic review enables the reproducibility of treatments proposed in clinical trials and therefore aggregates scientific evidence to these protocols. This type of study also assists in the development of new questions for future studies [29]. In the case of aPDT for the treatment of halitosis, it is necessary to investigate whether this therapy is more effective when used alone or whether it should be combined with another treatment modality. Therefore, the present systematic review aimed to assess the effectiveness and safety of aPDT for the treatment of halitosis.
2. Materials and Methods
This systematic review follows the methodological recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [30] and the report guidance of PRISMA [31]. The protocol registration was made in International prospective register of systematic reviews (PROSPERO) (retrospective register, under the number CRD42020215319).
2.1. Eligibility Criteria
2.1.1. Types of Studies
We considered for inclusion only randomized clinical trials (RCTs) with parallel design.
2.1.2. Types of Participants
Individuals (adolescents and adults) with a clinical diagnosis of halitosis.
2.1.3. Types of Interventions and Comparators
We included RCT that assessed the use of photodynamic therapy at any therapeutic parameter, dose and duration, compared with placebo, no intervention or another active treatment such as tongue scraper. If a co-intervention was administered in combination with aPDT, the study was included only if this co-intervention was given to both groups.
2.2. Outcomes Assessed
2.2.1. Primary
Halitosis measurements: hydrogen sulfide (H2S) gas, measured in parts per billion (ppb), for example by a gas chromatography test.
Adverse events (such as discomfort, gagging sensation, among others).
Quality of life (measured for example OHIP 14).
2.2.2. Secondary
Microbiological analysis, measured in colony forming unit (CFU/mL).
Patient-reported halitosis perception (measured as reported by the included studies).
2.2.3. Search Strategies
Broad and sensitive search strategies were performed on each of the following electronic databases: Medical Literature Analysis and Retrieval System Online (MEDLINE, via Pubmed), Cochrane Central Register of Controlled Trials (CENTRAL, via Wiley), Excerpta Medica database (EMBASE, via Elsevier), BBO (Bibliografia Brasileira de Odontologia) (both via BVS—Biblioteca Virtual em Saúde) and LILACS (Literatura Latino-Americana e do Caribe em Ciências da Saúde). An additional manual search was conducted in the reference lists of the relevant studies. The searches were run on 12 January 2021 and updated on 28 October 2021, without date and language restrictions. The detailed search strategies for each database were presented in the Supplementary Materials Table S1.
2.3. Study Selection Process and Data Extraction
The references screened from the search strategies were selected by two independent authors through the Rayyan platform [32]. After the removal of duplicates, the references were analyzed based on the eligibility criteria by titles and abstracts. Those studies that fulfil the eligibility criteria were assessed in a second stage by full texts in order to confirm inclusion or exclusion. A third author solved the disagreements.
The data extraction procedure was also performed by two independent authors, using a standard form through Microsoft Excel®. Data that were extracted were: year of publication, number of patients, wavelength, photosensitizer, pre-irradiation time, energy, power, number of points irradiated, application time per point, number of sessions, and follow-up time. When necessary, the RCT authors were contacted for additional information.
2.4. Methodological Quality of the Included Studies
The risk of bias of each included study was assessed using the Cochrane Risk of Bias tool [30], which is composed of seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. As recommended, we performed an outcome-level assessment for the domains: blinding of participants and researchers, blinding of outcome evaluators, incomplete outcome data. Each domain was judged according to the risk of bias as high, low, or unclear.
2.5. Data Synthesis
We planned to conduct meta-analyses using the software Review Manager 5.4.1, when the data from the included studies were available and homogenous. However, due to the lack of numerical data and the clinical diversity between included studies, the outcome data were described narratively and the estimated effects were calculated when possible (data availability). For continuous data, mean differences (MDs) were calculated between treatment groups. For dichotomous data, we planned to use the number of events in the intervention and control groups of each study to calculate risk ratios (RRs). A 95% confidence interval (CI) was considered. We planned to identify the methodological and clinical diversity of the studies if meta-analyses were conducted, as well. The presence of statistical heterogeneity among studies would be assessed by Chi² test, and its extension by the I2 test (I2 > 50% indicates substantial heterogeneity).
2.6. Assessing the Certainty of the Evidence
The GRADE approach (Grading of Recommendations, Assessment, Development and Evaluations) was used to evaluate the certainty of the overall body of evidence [33]. The GRADE encompasses five domains to downgrade the certainty of the evidence from RCTs (methodological limitations, inconsistency, imprecision, indirectness and publication bias). A summary of the findings table using the GRADEpro GDT software was generated, and the reasons to downgrade the certainty of the evidence were detailed.
3. Results
Database search identified 364 references. After removing 26 duplicates, 338 were screened by title and abstract and 324 were excluded for not fulfilling the inclusion criteria. Fourteen studies were analysed in full text and one was excluded due to wrong study design [34]. Thus, six randomized clinical trials (RCTs) (reported in 13 references) [18,20,24,34,35,36,37,38,39,40,41,42,43,44] were included in this systematic review (Figure 1).
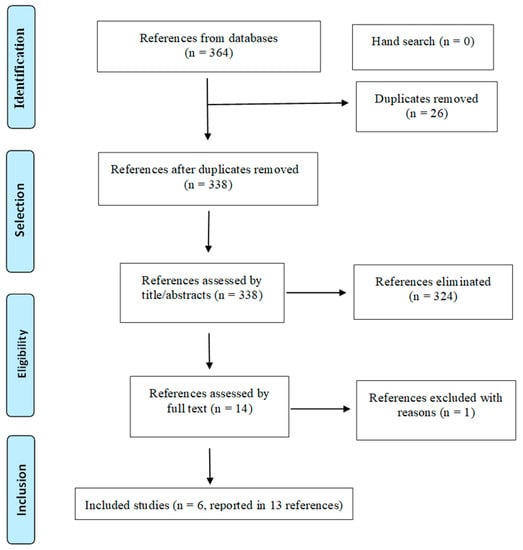
Figure 1.
PRISMA flowchart of the study selection process.
3.1. Characteristics of the Included Studies
The six RCTs involved a total of 255 participants and assessed halitosis reduction after the application of aPDT. They were published between 2016 and 2021 and were led in two countries: Brazil and Saudi Arabia. Table 1 detailed their main characteristics.

Table 1.
Main characteristics of included studies.
3.2. Methodological Quality Assessment
Figure 2 presented a summary of the risk of bias for each domain for each included study. Only one study [36] presented an inadequate random sequence generation and allocation concealment and was rated as having a high risk of selection bias. Another study [41] presented a high risk of bias for allocation concealment because of the use of a non sealed envelopes to maintain the concealment of the random sequence. One study [44] did not provide information on both selection domains and was considered as having an unclear risk of selection bias. Regarding performance bias, two studies [42,43] assessed outcomes that could be compromised by the lack of participant blinding, occurring given the nature of the interventions including different procedures. These studies were judged as high risk of bias for the subjective outcomes. All included studies presented a low risk of detection bias because the outcomes assessed could not be influenced by the lack of outcome assessor blinding. Three studies [24,41,42] did not provide any information on losses of participants and were, therefore, classified as unclear risk of attrition bias. One study [42] had the clinical trial register not found and was considered with an unclear risk of reporting bias. Ultimately, one study [44] did not present the baseline characteristics of the participants and was classified as high risk of bias for the other sources of bias domain.
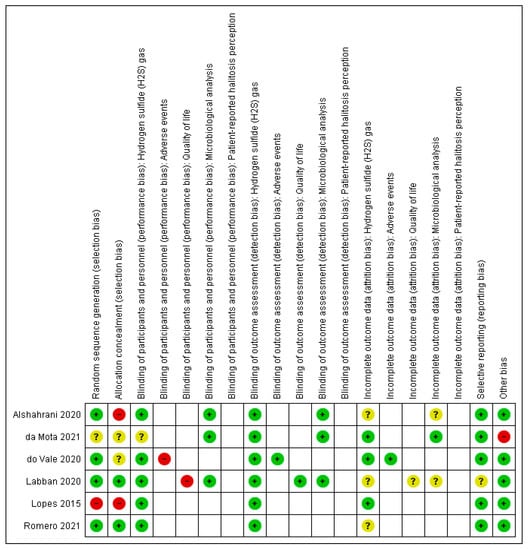
Figure 2.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.3. Effects of Intervention
3.3.1. Comparison 1. Antimicrobial Photodynamic Therapy (aPDT) versus Tongue Scraper
Hydrogen Sulfide (H2S) Gas Measurement
Five RCTs assessed the reduction in halitosis, which was determined by the measurement of H2S using the OralChromaTM device [24,36,41,43,44]. Considering the clinical differences and the lack of some numerical data it was not possible to group the results in meta-analysis. Thus, the estimated effects were reported individually on Table 2.

Table 2.
Main results of the included studies on the reduction in Hydrogen Sulfide (H2S) gas concentration measured in ppb (parts per billion).
Adverse Events
Only one RCT [43] (40 participants) assessed the presence of adverse events during the study and showed that most of the participants in the tongue scraper group reported discomfort or a gagging sensation during the procedure. No adverse events were observed in the aPDT group (very low-certainty evidence).
Microbiological Analysis
Three RCTs conducted microbiological analysis but it was not possible to conduct a meta-analysis due to the lack of numerical data; thus, the findings of each study were described individually. One RCT [44] reported no statistical difference between aPDT and tongue scrape for the following bacteria investigated: Porphyromonas gingivalis and Tannerella forsythia (p > 0.05, 30 participants). However, the analysis of Treponema denticola identified a statistical difference in favour of aPDT after 7 days (p = 0.004) and 14 days (p = 0.006) post-treatment. Another RCT [36] reported no statistical difference between groups (p = 0.05, 30 participants) regarding microbiological examination. Lastly, one RCT reported no difference between groups [41].
3.3.2. Comparison 2. Antimicrobial Photodynamic Therapy (aPDT) plus Tongue Scraper versus Tongue Scraper
Hydrogen Sulfide (H2S) Gas Measurement
Three studies evaluated this outcome but the lack of numerical data precluded performing a meta-analysis and data were described individually:
Labban et al. (2020) [42]: the authors reported significant improvement in H2S concentration in the aPDT plus tongue scraper group when compared with the tongue scraper group (median 148 versus 0 ppb; p = 0.001, 40 participants).
Alshahrani et al. (2020) [41]: the authors reported a reduced H2S concentration in favour of aPDT plus tongue scraper (median [IQT] 0 [0] versus 65 ppb, p = 0.0001, 29 participants).
Lopes et al. (2015) [36]: the authors reported a reduced H2S concentration in favour of aPDT plus tongue scraper (median [IQT] 0 [0] versus 53 [7] ppb, p = 0.0003, 29 participants).
Quality of Life
Estimated effect from one RCT showed an imprecise result on the improvement in the quality of life measured by the OHIP-14 summary scores. There was a wide confidence interval compatible with both a decrease and an increase in the score, and a small sample size (MD 20.05, 95% CI−53.22 to 93.23, 40 participants) [43].
Microbiological Analysis
One RCT reported a statistically significant reduction in Porphyromonas gingivalis with aPDT only after 5 days of treatment (p < 0.05, 40 participants) (no numerical data was provided) [42]. Another RCT reported a statistical bacterial reduction in the aPDT plus tongue scrape group when compared to tongue scrape (p = 0.0003, 29 participants) [36]. Lastly, one RCT reported no difference between groups [41].
Certainty of the Evidence
The certainty of the body of evidence was evaluated with the GRADE approach for primary outcomes assessed in the main comparison: antimicrobial photodynamic therapy (aPDT) versus tongue scraper. The evidence was rated as very low due to methodological limitations and imprecision (wide confidence interval, small sample size and few events). This indicates very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The summary of findings in Table 3 presented the assessment and judgements.

Table 3.
Summary of findings—GRADE approach.
4. Discussion
Halitosis is the third major cause of the search for oral treatment. Thus, it is relevant to address this problem, which can exert a considerable impact on quality of life [2]. The main cause of halitosis is gas (volatile sulfur compounds) produced by bacteria found in coated tongue. Therefore, treatment consists of the control of these bacteria through mechanical removal, chemical removal, or cell death due to phototoxicity [44]. Tongue scraping could be easily carried out by patients themselves and is widely recommended, but it is little practiced due to discomfort, as it can cause nausea, or lack of awareness regarding its use. In addition, studies have shown that self-cleaning of the tongue alone is not completely efficient for reducing halitosis and it should be associated with in-office treatments, such as periodontal ones. Consequently, alternative forms of treatment, such as aPDT, which can be performed in an office, and is less aggressive to the papillae (that can be hurt during the scraping process), are being researched.
The objective of the present study was to evaluate the efficacy of aPDT at reducing halitosis in comparison to other therapies. The authors of this review believe that alternative therapies, such as the one studied herein, have an advantage over tongue scrapers, as the mechanical removal of coated tongue can cause damage to the lingual papillae. In all articles analyzed, aPDT seems to be effective at achieving an immediate reduction in halitosis. Do Vale et al. [43] and Romero et al. [24] found that aPDT was more effective for the treatment of halitosis when compared to the use of a tongue scraper, whereas Mota et al. [44], Lopes et al. [36] and Alshahrani [41] found that this therapy was more effective when combined with the mechanical removal of coated tongue.
The majority of studies used a red laser (λ = 660 nm) as the energy source and the photosensitizer was methylene blue at a concentration of 0.005%. The predominant pre-irradiation time was five minutes, but Romero et al. [24] used a pre-irradiation time of one minute and found that the treatment was effective with this shorter time. The most used energy was 9 J with a power of 100 mW at six points distributed on the dorsum of the tongue. Treatment was performed in a single session in all articles. The researchers who performed follow-up after seven days reported the return to initial H2S concentrations [24,43] whose halitosis remained low until the seventh day. These two authors evaluated edentulous patients, and prosthesis were also cleaned. Interestingly, patients were undergoing fixed orthodontic treatment. Alshahrani 2020 [41] also maintains low concentrations of H2S.
The primary outcome in the majority of articles included in the present review was a reduction in halitosis, which was determined based on the measurement of H2S using the OralChroma device. The studies that employed this device reported a reduction in the concentration of H2S immediately after the application of aPDT. This device employs gas chromatography for the determination of concentrations of volatile sulfur compounds produced by anaerobic bacteria, which is the main cause of halitosis [36]. The authors used a H2S concentration ≥112 ppb for the determination of halitosis. OralChroma is currently considered the gold standard. The diagnosis was previously performed using the organoleptic method, which has fallen into disuse because of the subjectivity of the evaluation.
All researchers who used the OralChroma device followed the manufacturer’s instructions and obeyed the following sequence: the participants were instructed to avoid spicy foods, alcohol, coffee, chewing gum, and mouthwash. On the day of the reading, the participants needed to fast for at least two hours and rinsed their mouths with cysteine (10 mM).
It was not possible to perform a meta-analysis (or sensitivity analysis) for the outcome of the research question of this systematic review. Despite being well designed, the studies showed clinical heterogeneity, especially in relation to the characteristics of the population such as different age range. Literature shows the occurrence of halitosis at all ages, but the causes and habits can change the characteristics in different age groups. In view of the heterogeneity of the studies, it was decided to carry out only the qualitative analysis of the studies. The results of the present systematic review show uncertain evidence about the effects of antimicrobial photodynamic therapy compared to the tongue scraper. The results seem to present better effects with the combination of both methods (aPDT and tongue scraper) when compared to the tongue scraper. However, since the available evidence was classified as very low certainty by the GRADE approach, further studies should be conducted to support these findings and to establish the number and periodicity of sessions needed to achieve the complete resolution of this problem.
The predominant use of methylene blue is related to the wavelengths employed by these researchers, which ranged from 655 to 660 nm (red laser). Mota et al. [44], Lopes et al. [36], and Vale et al. [43] applied the photosensitizer and waited for five minutes of pre-irradiation time, and Romero et al. [24] waited only one minute.
The parameters used for aPDT were quite similar among the studies, as the majority were performed by the same research group. All studies used red laser. Mota et al. [44], Lopes et al. [36], and Vale et al. [43] and Romero et al. [24] and used an energy of 9 J and power of 100 mW at six points on the dorsum of the tongue for 90 s per point. All papers in the present review performed a single session of aPDT.
Mota et al. [44], Lopes et al. [36] and Alshahrani 2020 [41] conducted similar studies, in which three groups were compared: (1) treatment with aPDT; (2) treatment with a tongue scraper; and (3) combination of aPDT and tongue scraper. In the study by Lopes et al. [36], the reduction in the concentration of H2S was 97% in Group 1, 88.6% in Group 2, and 100% in Group 3. Additionally, in the Alshahrani 2020 [41] study, Group 1 (aPDT) decreased 95%, Group 2 (TS) 89,4% and Group 3 (aPDT + TS) 100% in 15 days. Mota et al. [44] did not provide numerical data.
Do Vale et al. [43] and Romero et al. [24] allocated the participants into two groups: experimental (treatment with aPDT) and control (treatment with tongue scraper). In the study by do Vale et al. [43], the authors found a reduction in halitosis after both treatments. However, significant differences between groups were found immediately after treatment and at the seven-day follow-up, with greater reductions in hydrogen sulfide concentrations in the group treated with aPDT.
Lopes et al. [36] found that aPDT was effective at achieving an immediate reduction in halitosis and therefore constitutes a treatment option for this condition that does not harm the papillae, as occurs in conventional treatment with a tongue scraper. However, the authors found that the application of 90 s per point at six points on the dorsum of the tongue caused certain discomfort among the patients and suggested further studies to test different energies. Do Vale et al. [43] conducted a study with patients who wore complete dentures. The authors concluded that aPDT seems to be effective at reducing H2S immediately after treatment and that this effect was maintained at the seventh day follow-up. Laban et al. [42] concluded that antimicrobial PDT seems to help in reducing H2S concentration and improving quality of life in elderly patients wearing dentures. There also a reduction in P. gingivalis that occurred only in the short-term follow-up. Da Mota et al. [44] concluded that aPDT using a red LED and 0.005% methylene blue caused an immediate reduction in halitosis, but the effect was not maintained after 7, 14, or 30 days. Additionally, they found no reduction in the number of bacteria investigated or the quantification of universal 16S rRNA. Romero et al. [24] reinforces the oral hygiene behavior associated with aPDT or tongue scraper was not able to reduce halitosis after 90-day follow-up. Despite halitosis remaining higher than 112 ppb in all follow-up periods, the mean values remain two- or three-fold smaller than baseline values. Future studies should include other oral hygiene behavior to achieve better results in the treatment of halitosis. Alshahrani [41] concludes that PDT along with tongue scraping showed immediate reduction in H2S and reduction in oral pathogens in adolescent patients undergoing fixed orthodontic treatment for 15 days.
5. Conclusions
The results of the present systematic review show that antimicrobial photodynamic therapy administered alone seems to be an effective treatment for the control of halitosis, achieving better results than the sole use of a tongue scraper. Considering the small number of participants in the included studies and some methodological limitations identified, future randomized clinical trials are still necessary, with higher sample size and long-term outcome assessments to provide a confident guidance for decision-making.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s22020469/s1, Table S1: Search strategies.
Author Contributions
P.d.B.M., L.J.M. and S.K.B. had the idea for the article. P.d.B.M., L.J.M., T.M.C., A.L.C.M. and A.C.R.T.H. performed the literature search and data analysis. P.d.B.M. and M.L.L.G. drafted the manuscript and E.M.S., D.J.C.d.A., R.A.M.-F., K.P.S.F. and S.K.B. critically revised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This article was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2019/14229-6, by Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES), grant number 88887.646066/2021-00, and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 306577/2020-8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data extracted from included studies are available from the corresponding author at a reasonable request.
Acknowledgments
The authors would like to thank Universidade Nove de Julho for the support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Armstrong, B.L.; Sensat, M.L.; Stoltenberg, J.L. Halitosis: A review of current literature. J. Dent. Hyg. Assoc. 2010, 84, 65–74. [Google Scholar]
- Du, M.; Li, L.; Jiang, H.; Zheng, Y.; Zhang, J. Prevalence and relevant factors of halitosis in Chinese subjects: A clinical research. BMC Oral Health 2019, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Calil, C.M.; Marcondes, F.K. Influence of anxiety on the production of oral volatile sulfur compounds. Life Sci. 2006, 79, 660–664. [Google Scholar] [CrossRef]
- Springfield, J.; Suarez, F.; Majerus, G.; Lenton, P.; Furne, J.; Levitt, M. Spontaneous Fluctuations in the Concentrations of Oral Sulfur-containing Gases. J. Dent. Res. 2001, 80, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A.; Winkel, E.G. The portable gas chromatograph OralChroma™: A method of choice to detect oral and extra-oral halitosis. J. Breath Res. 2008, 2, 017010. [Google Scholar] [CrossRef]
- Tolentino, E.D.S.; Chinellato, L.E.M.; Tarzia, O. Saliva and tongue coating pH before and after use of mouthwashes and relationship with parameters of halitosis. J. Appl. Oral Sci. 2011, 19, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Hine, K.H. Halitosis. JADA 1957, 55, 37–46. [Google Scholar] [CrossRef]
- Marocchio, L.; Da Conceição, M.; Tárzia, O. Remoção da saburra lingual: Comparação da efciência de três técnicas. RGO 2009, 57, 443–448. [Google Scholar]
- Quirynen, M.; Dadamio, J.; Van Den Velde, S.; De Smit, M.; Dekeyser, C.; Van Tornout, M.; Vandekerckhove, B. Characteristics of 2000 patients who visited a halitosis clinic. J. Clin. Periodontol. 2009, 36, 970–975. [Google Scholar] [CrossRef]
- Guedes, C.C.; Bussadori, S.K.; Garcia, A.C.M.; Motta, L.J.; Gomes, A.O.; Weber, R.; Amancio, O.M.S. Accuracy of a portable breath meter test for the detection of halitosis in children and adolescents. Clinics 2020, 75, e1764. [Google Scholar] [CrossRef]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef]
- Kara, C.; Demir, T.; Orbak, R.; Tezel, A. Effect of Nd: YAG laser irradiation on the treatment of oral malodour associated with chronic periodontitis. Int. Dent. J. 2008, 58, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.; Tezel, A.; Orbak, R. Effect of oral hygiene instruction and scaling on oral malodour in a population of Turkish children with gingival inflammation. Int. J. Paediatr. Dent. 2006, 16, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bicak, D.A. A Current Approach to Halitosis and Oral Malodor- A Mini Review. Open Dent. J. 2018, 12, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kumbargere Nagraj, S.; Eachempati, P.; Uma, E.; Singh, V.P.; Ismail, N.M.; Varghese, E. Interventions for managing halitosis. Cochrane Database Syst. Rev. 2019, 12, CD012213. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 2042098619854881. [Google Scholar] [CrossRef]
- Marrelli, M.; Amantea, M.; Tatullo, M. A comparative, randomized, controlled study on clinical efficacy and dental staining reduction of a mouthwash containing Chlorhexidine 0.20% and Anti Discoloration System (ADS). Ann. Stomatol. 2015, 6, 35–42. [Google Scholar]
- da Mota, A.C.C.; França, C.M.; Prates, R.; Deana, A.M.; Costa Santos, L.; Lopes Garcia, R.; Gonçalves, M.L.L.; Ferrari, R.A.M.; Fernandes, K.P.S.; Bussadori, S.K. Effect of photodynamic therapy for the treatment of halitosis in adolescents—A controlled, microbiological, clinical trial. J. Biophotonics 2016, 9, 1337–1343. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chou, H.-H.; Wu, T.-L.; Yang, Y.-H.; Ho, K.-Y.; Wu, Y.-M.; Ho, Y.-P. The levels of volatile sulfur compounds in mouth air from patients with chronic periodontitis. J. Periodontal Res. 2007, 43, 186–193. [Google Scholar] [CrossRef]
- Ciarcia, A.C.C.D.M.; Gonçalves, M.L.L.; Horliana, A.C.R.T.; Suguimoto, E.S.A.; Araujo, L.; Laselva, A.; Mayer, M.P.A.; Motta, L.J.; Deana, A.M.; Mesquita-Ferrari, R.A.; et al. Action of antimicrobial photodynamic therapy with red leds in microorganisms related to halitosis: Controlled and randomized clinical trial. Medicine 2019, 98, e13939. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Al-Kheraif, A.A.; Al-Askar, M.; Yunker, M.; Javed, F. Effect of antimicrobial photodynamic therapy and laser alone as adjunct to mechanical debridement in the management of halitosis: A systematic review. Quintessence Int. 2017, 48, 575–583. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoneda, M.; Takeshita, T.; Hirofuji, T.; Hanioka, T. Induction and inhibition of oral malodor. Mol. Oral Microbiol. 2019, 34, 85–96. [Google Scholar] [CrossRef]
- Hope, C.K.; Wilson, M. Induction of lethal photosensitization in biofilms using a confocal scanning laser as the excitation source. J. Antimicrob. Chemother. 2006, 57, 1227–1230. [Google Scholar] [CrossRef]
- Romero, S.S.; Vale, K.L.D.; Remolina, V.G.; Silva, T.G.; Schalch, T.O.; Ramalho, K.M.; Negreiros, R.M.; Ando, E.S.; Mayer, M.P.A.; Ferrari, R.A.M.; et al. Oral hygiene associated with antimicrobial photodynamic therapy or lingual scraper in the reduction of halitosis after 90 days follow up: A randomized, controlled, single-blinded trial. Photodiagnosis Photodyn. Ther. 2021, 33, 102057. [Google Scholar] [CrossRef]
- Cordeiro, A.; Oliveira, G.D.; Renteria, J.; Guimarães, C. Revisão sistemática: Uma revisão narrativa. Grupo de Estudo de Revisão Sistemática do Rio de Janeiro (GERS-Rio). Rev. Col. Bras. Cir. 2007, 34, 428–431. [Google Scholar] [CrossRef]
- Ciliska, D.; Cullum, N.; Marks, S. Evaluation of systematic reviews of treatment or prevention interventions. Évid. Based Nurs. 2001, 4, 100–104. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Reprint—Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Sá Elias, M.; das Graças Carvalho Ferriani, M. Aspectos históricos e sociais da halitose. Rev. Lat.-Am. Enferm. 2006, 14, 821–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sampaio, R.F.; Mancini, M.C. Estudos de revisão sistemática: Um guia para síntese criteriosa da evidência científica. Braz. J. Phys. Ther. 2007, 11, 83–89. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Available online: www.training.cochrane.org/handbook (accessed on 29 October 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Javed, F. Halitosis and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 32, 102006. [Google Scholar] [CrossRef]
- Lopes, R.G.; de Godoy, C.H.L.; Deana, A.M.; de Santi, M.E.S.O.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Bussadori, S.K. Photodynamic therapy as a novel treatment for halitosis in adolescents: Study protocol for a randomized controlled trial. Trials 2014, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.G.; Da Mota, A.C.C.; Soares, C.; Tarzia, O.; Deana, A.M.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Ferrari, R.A.M.; Bussadori, S.K. Immediate results of photodynamic therapy for the treatment of halitosis in adolescents: A randomized, controlled, clinical trial. Lasers Med. Sci. 2015, 31, 41–47. [Google Scholar] [CrossRef]
- Mota-Ciarcia, A.C.; Prates, R.A.; Gonçalves, M.L.L.; Costa, L.; Deana, A.; Mesquita-Ferrari, R.; Fernandes, K.; Horliana, A.C.; França, C.; Bussaddori, S. Effect of Photodynamic Therapy in the Treatment of Halitosis in Adolescents—Clinical Trial and Microbiological Analysis [Abstract]. American Society for Laser Medicine and Surgery Abstracts 2016; Wiley Online Library: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Vale, K.L.; Horliana, A.C.R.T.; Romero, S.S.; Deana, A.M.; La Selva, A.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Fernandes, K.P.S. Treatment of halitosis with photodynamic therapy in older patients with complete denture: A randomized, controlled trial. Abstracts Laser Florence 2019. Lasers Med. Sci. 2020, 35, 233–293. [Google Scholar] [CrossRef]
- Vale, K.L.D.; Horliana, A.C.R.T.; Romero, S.D.S.; Deana, A.D.M.; Gonçalves, M.L.L.; Ferrari, R.A.M.; Bussadori, S.K.; Fernandes, K.P.S. Evaluation of the treatment of halitosis with photodynamic therapy in older patients with complete denture: Protocol for a randomized, controlled trial. Medicine 2019, 98, e16275. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.D.S.; Schalch, T.O.; Vale, K.L.D.; Ando, E.S.; Mayer, M.P.A.; Feniar, J.P.G.; Fernandes, K.; Bussadori, S.K.; Motta, L.; Negreiros, R.M.; et al. Evaluation of halitosis in adult patients after treatment with photodynamic therapy associated with periodontal treatment: Protocol for a randomized, controlled, single-blinded trial with 3-month follow up. Medicine 2019, 98, e16976. [Google Scholar] [CrossRef] [PubMed]
- Abdul, A.A.; Ali, A.; Muhammad, A.K.; Ibrahim, A. Efficacy of antimicrobial photodynamic therapy against halitosis in adolescent patients undergoing orthodontic treatment. Photodiagnosis Photodyn. Ther. 2020, 32, 102019. [Google Scholar] [CrossRef]
- Labban, N.; Assery, M.K.; Al-Kattan, R.; Al-Shibani, N.; Alfouzan, A.F.; Al Taweel, S.M. Antimicrobial capacity of photodynamic therapy on oral health-related quality of life and halitosis among elderly patients wearing removal dentures. Photodiagnosis Photodyn. Ther. 2020, 32, 102059. [Google Scholar] [CrossRef]
- do Vale, K.L.; Horliana, A.C.R.T.; Dos Santos, S.R.; Schalch, T.O.; de Ana, A.M.; Ferrari, R.A.M.; Bussadori, S.K.; Fernandes, K.P.S. Treatment of halitosis with photodynamic therapy in older adults with complete dentures: A randomized, controlled, clinical trial. Photodiagnosis Photodyn. Ther. 2021, 33, 102128. [Google Scholar] [CrossRef] [PubMed]
- da Mota, A.C.C.; Gonçalves, M.L.L.; Horliana, A.C.R.T.; Deana, A.M.; Cavalcante, L.A.D.S.; Gomes, A.O.; Mayer, M.P.A.; Suguimoto, E.S.A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; et al. Effect of antimicrobial photodynamic therapy with red led and methylene blue on the reduction of halitosis: Controlled microbiological clinical trial. Lasers Med. Sci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
