Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review
Abstract
1. Introduction
1.1. Spectrophotometric Methods for the Analysis of Total Phenol Content
1.2. High-Performance Liquid Chromatography with UV-VIS and MS Detectors
1.3. Electrochemical Methods
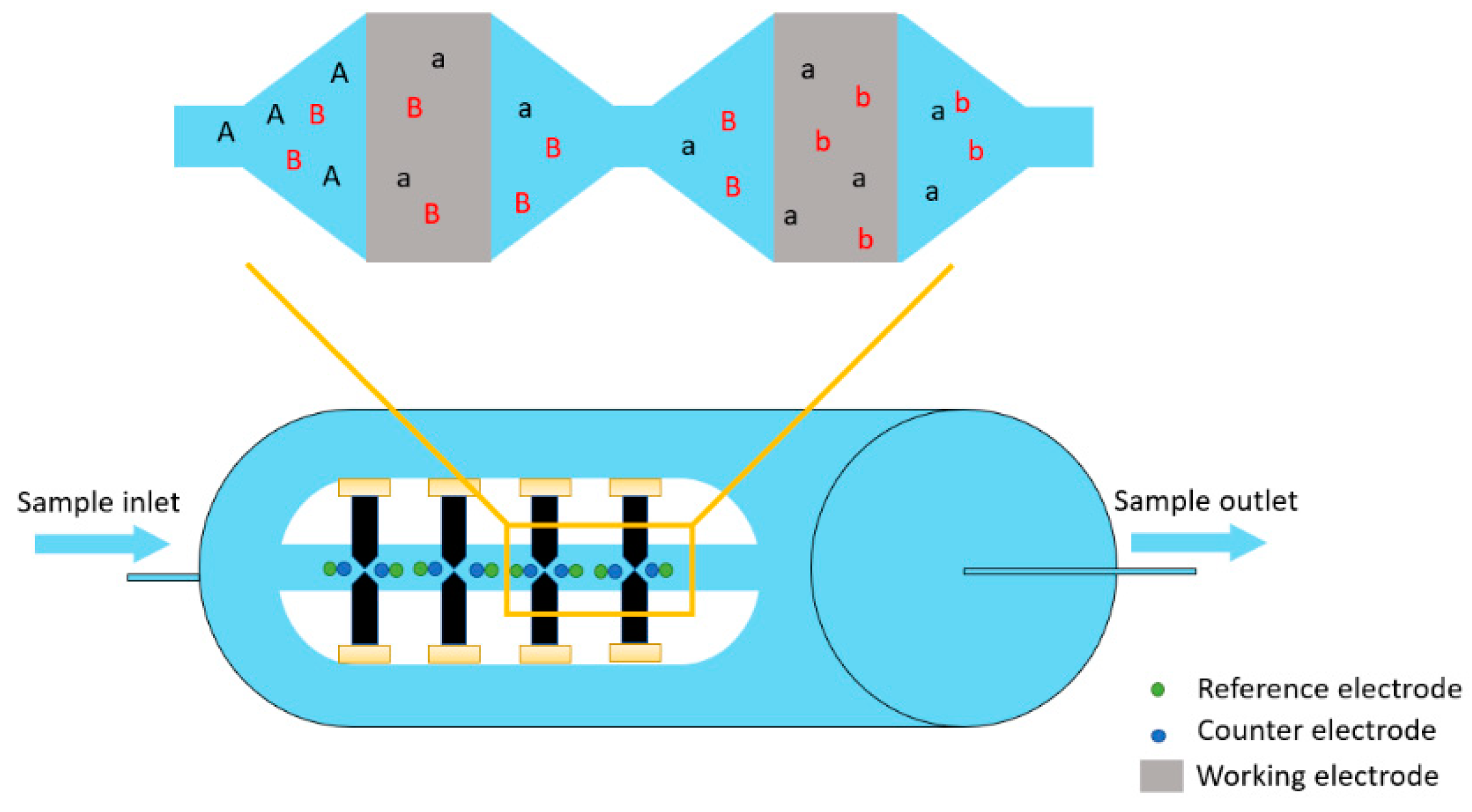
2. Principles of the Coulometric Array Detector
2.1. Description of the Coulometric Array Detector
2.2. Coulometric Array Detector Apparatus
2.3. The Application of Hydrodynamic Voltammograms (HDVs) and Faraday’s Law
3. Phenolic Compounds in Food
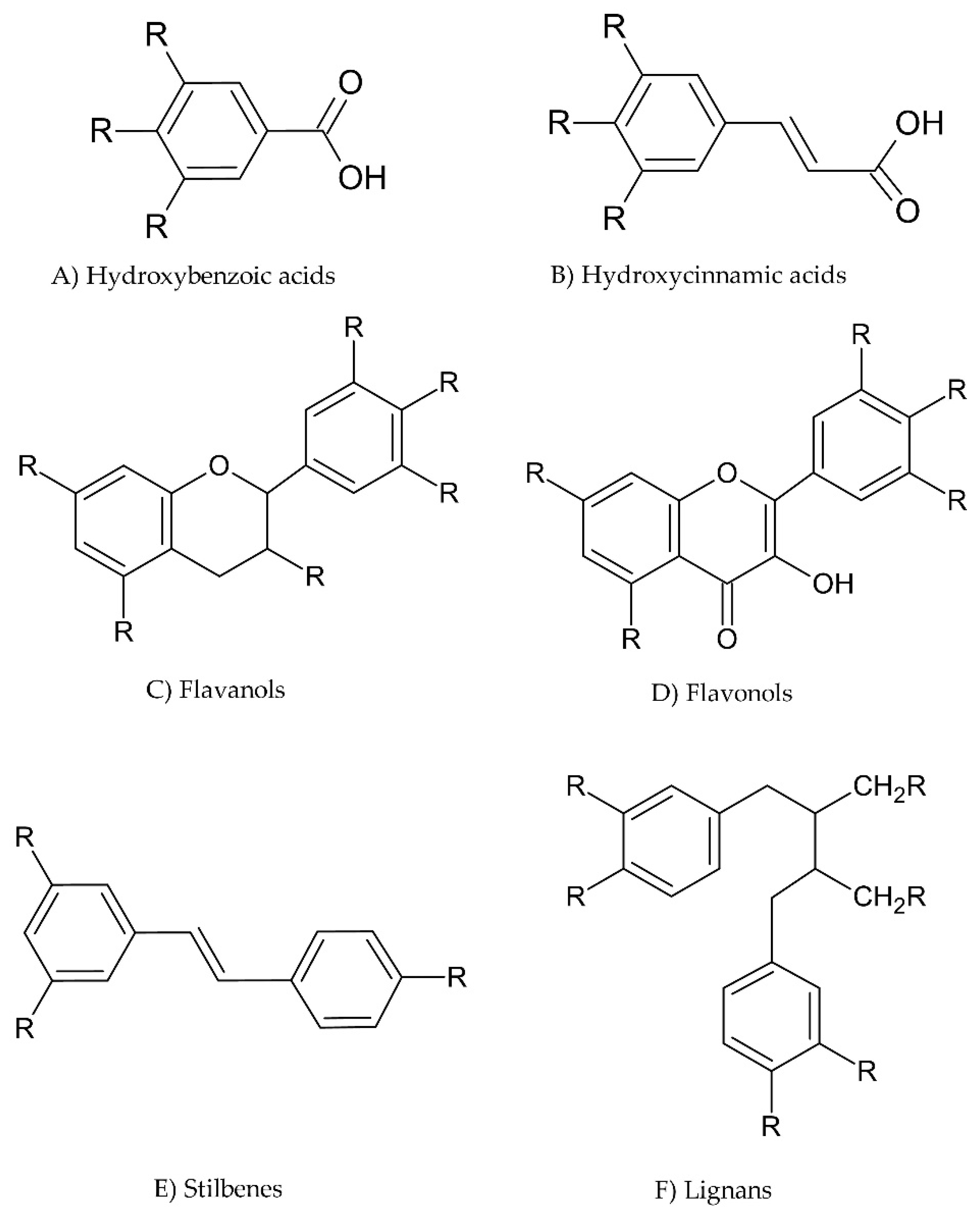
3.1. What Are Phenols?
3.2. Food Sources of Phenolic Compounds
3.3. The Extraction of Phenolic Compounds in Food
4. The Application of the CoulArray for Analysis of Phenolic Compounds in Food
4.1. Fruits
4.2. Herbs
4.3. Beverages
4.4. Cereals
4.5. Others
| Food Product Type | Food Matrix | Method | Working Electrode and Potential Range | Recovery % | Reproducibility (Coefficient of Variation (CV)) | Limit of Detection (LOD) | Compounds | Reference |
|---|---|---|---|---|---|---|---|---|
| Fruit | Currant, gooseberry | FIA-ECD | 4 PGEs, +200 to +800 mV | N/A | N/A | N/A | N/A | [71] |
| Fruit | Bilberry, lingonberry, cloudberry, seabuckthorn berry | RP-HPLC, gradient elution | 8 PGEs, 0 to +840 mV | 76.4% to 153.8% | N/A | N/A | Gallic acid | [72] |
| vanillic acid | ||||||||
| caffeic acid | ||||||||
| p-coumaric acid | ||||||||
| Ferulic acid, myricetin, quercetin, isorhamnetin | ||||||||
| Fruit | Swiss chard | RP-HPLC, gradient elution | 2 PGEs, −50 to +825 mV | N/A | 0.06%–1.05% CV | 1 ng mL−1 | Gallic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, myricetin, quercetin, p-OH-benzoic acid, proto-catechuic acid, chlorogenic acid, syringic acid, catechin, kaempferol | [74] |
| Fruit | Blue honeysuckle | RP-HPLC, gradient elution | 12 PGEs, −80 to +800 mV | N/A | N/A | N/A | N/A | [101] |
| Saskatoon berry | ||||||||
| Chinese hawthorn | ||||||||
| Fruit | Honeysuckle berries | RP-HPLC, N/A | 12 PGEs, N/A | N/A | N/A | N/A | Gallic acid, catalposide, rutin, resveratrol, quercitrin, chlorogenic acid | [75] |
| Fruit | Strawberries | RP-HPLC, gradient elution | 8 PGEs, +100 to +800 mV | N/A | N/A | N/A | Catechin, cinnamic acid derivatives, anthocyanin derivatives | [78] |
| Herbs | Moringa oleifera, Melissa officinalis, Fraxinus excelsior, and other officinal plants | RP-HPLC, gradient elution | 16 PGEs, −50 to +700 mV | N/A | 1.5% to 2% | 1.3 ± 0.1 µM | Chlorogenic acid, isoquercetin, phloretic acid, oleuropein, osivitexin, gallic acid, catechin, protocatechuic acid, and others | [42] |
| Beverages | Red and white wines, meads | RP-HPLC, gradient elution | 8 PGEs, +200 to +900 mV | N/A | N/A | 2.8 to 15.0 µg L−1 | Cinnamic acid derivatives, benzoic acid derivatives, and others | [87] |
| Beverages | Meads | RP-HPLC, gradient elution | 8 PGEs, +200 to +900 mV | N/A | N/A | 4 to 29 µg L−1 | Gallic acid, protocatechuic acid, gentisic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, and others | [88] |
| Beverages | Beer, tea | RP-HPLC, gradient elution | 8 PGEs, +250 to +900 mV | N/A | N/A | 1 to 5 µg L−1 | 4-Hydroxycoumarin, gallic acid, vanillic acid, rutin, caffeic acid, naringenin, and others | [84] |
| Cereals | Barley and malt extracts | RP-HPLC, gradient elution | 8 PGEs, +250 to +900 mV | N/A | N/A | N/A | (+)-Catechin, (−)-epicatechin, esculin, umbeliferone, scopoletin, rutin, quercetin, and others | [93] |
| Cereals | Wholegrain wheat flour, wheat semolina. barley, rye bran, spelt, oats | RP-HPLC, gradient elution | 8 PGEs, 0 to +850 mV | 98.4% to 107.5% | 0.8% to >10% CV, | 1 ng g−1 | Alkylresorcinols | [94] |
| depending on the different homologues | ||||||||
| Spices | Parsley | RP-HPLC, gradient elution | 4 PGEs, +300 to +900 mV | N/A | N/A | 4.75 µg mL−1 | Chlorogenic acid | [95] |
| celery | ||||||||
| onion | ||||||||
| dill leaves | ||||||||
| Fruit | Tomatoes | RP-HPLC, gradient elution | 16 PGEs, +50 to +750 mV | 81.1% to 89.8 ±2.8% | N/A | 3 to 13 µg mL−1 | Naringenin, rutin, ferulic acid, p-hydroxybenzaldehyde, p-coumaric acid, and others | [96] |
| Fruit | Almonds | RP-HPLC, gradient elution | 13 PGEs, +60 to +720 mV | N/A | 1.24% to 5.17% | N/A | Catechin, procatechuic acid, epicatechin, quercetin, and others | [97] |
| Oils | Olive oil | RP-HPLC, gradient elution | 4 PGEs, +250 to +750 mV | N/A | N/A | 0.03 to 1.7 ng mL−1 | Tyrosol, hydroxytyrosol, oleuropein, pinoresinol, caffeic acid, ferulic acid, vanillic acid, p-coumaric acid | [99] |
5. Relation with Other Antioxidant Assays
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of Main and Healthy Phenolic Compounds in Foods. J. AOAC Int. 2019, 102, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Compendium of International Methods of Analysis-OIV. Compendium of International Methods of Analysis–OIV, Folin-Ciocalteu Index. Paris. 2009. Available online: https://www.oiv.int/public/medias/2477/oiv-ma-as2-10.pdf (accessed on 4 August 2022).
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2017, 6, 107–115. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Medina-Remón, A.; Tresserra-Rimbau, A.; Estruch, R. Fruit and vegetable polyphenol consumption decreases blood pressure. ACS Symp. Ser. 2012, 1093, 443–461. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue Assays to Estimate The Total Phenolic Content of Juices and Teas Using 96-Well Microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G. Phenolic compounds. Chemistry and occurences in fruit and vegetables. Fruit Veg. Phytochem. Chem. Nutr. Value Stab. 2010, 1, 53–88. [Google Scholar]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- López-fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, X.; Xu, Y.; Liu, X.; Zhang, J.; He, Z. Simultaneous determination of 49 amino acids, B vitamins, flavonoids, and phenolic acids in commonly consumed vegetables by ultra-performance liquid chromatography–tandem mass spectrometry. Food Chem. 2021, 344, 128712. [Google Scholar] [CrossRef]
- Ayhan, N.K.; Rosenberg, E. Development of comprehensive liquid chromatography with diode array and mass spectrometric detection for the characterization of (poly-)phenolic and flavonoid compounds and application to asparagus. Food Chem. 2021, 354, 129518. [Google Scholar] [CrossRef] [PubMed]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Optimized Identification of Triacylglycerols in Milk by HPLC-HRMS. Food Anal. Methods 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Imperiale, S.; Morozova, K.; Ferrentino, G.; Alam, M.R.; Scampicchio, M. Fast Detection of 5-Hydroxymethylfurfural in Dulce de Leche by SPE-LC–MS. Food Anal. Methods 2022, 15, 1–9. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Bazar, G.; Gillay, Z.; Kovacs, Z. Emerging trends of advanced sensor based instruments for meat, poultry and fish quality– a review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3443–3460. [Google Scholar] [CrossRef]
- Merkyte, V.; Morozova, K.; Boselli, E.; Scampicchio, M. Fast and Simultaneous Determination of Antioxidant Activity, Total Phenols and Bitterness of Red Wines by a Multichannel Amperometric Electronic Tongue. Electroanalysis 2018, 30, 314–319. [Google Scholar] [CrossRef]
- Dejmkova, H.; Morozova, K.; Scampicchio, M. Estimation of Scoville index of hot chili peppers using flow injection analysis with electrochemical detection. J. Electroanal. Chem. 2018, 821, 82–86. [Google Scholar] [CrossRef]
- Classification and Definitions of Chemical Sensor Array Devices. Available online: https://www.degruyter.com/database/IUPAC/entry/iupac.77.0083/html (accessed on 23 June 2022).
- Nazari, M.; Kashanian, S.; Rafipour, R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 130–138. [Google Scholar] [CrossRef]
- Montereali, M.R.; della Seta, L.; Vastarella, W.; Pilloton, R. A disposable Laccase-Tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine. J. Mol. Catal. B Enzym. 2010, 64, 189–194. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, Y.; Zhang, Y.; Zhang, M.; Zhang, Y.; Qiao, L.; Yin, N.; Song, K.; Liu, M.; Wang, D. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem. J. 2021, 171, 106776. [Google Scholar] [CrossRef]
- Hauser, P.C. Coulometry. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 234–240. [Google Scholar] [CrossRef]
- Hicks, M.B.; Salituro, L.; Mangion, I.; Schafer, W.; Xiang, R.; Gong, X.; Welch, C.J. Assessment of coulometric array electrochemical detection coupled with HPLC-UV for the absolute quantitation of pharmaceuticals. Analyst 2017, 142, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Sontag, G.; Pinto, M.I.; Noronha, J.P.; Burrows, H.D. Analysis of Food by High Performance Liquid Chromatography Coupled with Coulometric Detection and Related Techniques: A Review. J. Agric. Food Chem. 2019, 67, 4113–4144. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Budnikov, H. Analytical capabilities of coulometric sensor systems in the antioxidants analysis. Chemosensors 2021, 9, 91. [Google Scholar] [CrossRef]
- Coularray, T.; Management, P.; Nt, W.; Panel, C.; Ram, F. Note for Coularray Users. no. 978. Available online: https://files.mtstatic.com/site_13984/25523/0?Expires=1664576311&Signature=ZGeX5eJ0oc8sgNDcME8gmrFqZQpXSp3NTDcFX6ulG7cMUGiuGYIYEFmkeYx-IwRrpd1NrPN4AyYhQe7-BplenHkbFz0s4ZN8hE5Hz86zBh1FRIk0jwMhjZCWqrOFYBVS59HuRF3aX9rWIL1VP5fORf4KI6lAojC2-6X4TtXXWq8_&Key-Pair-Id=APKAJ5Y6AV4GI7A555NA (accessed on 20 June 2022).
- Morozova, K.; Rodríguez-Buenfil, I.; López-Domínguez, C.; Ramírez-Sucre, M.; Ballabio, D.; Scampicchio, M. Capsaicinoids in Chili Habanero by Flow Injection with Coulometric Array Detection. Electroanalysis 2019, 31, 844–850. [Google Scholar] [CrossRef]
- Kongwong, P.; Morozova, K.; Ferrentino, G.; Poonlarp, P.; Scampicchio, M. Rapid Determination of the Antioxidant Capacity of Lettuce by an E-Tongue Based on Flow Injection Coulometry. Electroanalysis 2018, 30, 230–237. [Google Scholar] [CrossRef]
- Mikelova, R.; Prokop, Z.; Stejskal, K.; Adam, V.; Beklova, M.; Trnkova, L.; Kulichova, B.; Horna, A.; Chaloupkova, R.; Damborsky, J.; et al. Enzymatic reaction coupled with flow-injection analysis with charged aerosol, coulometric, or amperometric detection for estimation of contamination of the environment by pesticides. Chromatographia 2008, 67, 47–53. [Google Scholar] [CrossRef]
- Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors 2008, 8, 7097–7112. [Google Scholar] [CrossRef]
- Longo, E.; Morozova, K.; Scampicchio, M. Effect of light irradiation on the antioxidant stability of oleuropein. Food Chem. 2017, 237, 91–97. [Google Scholar] [CrossRef]
- Błazewicz, A.; Fijałek, Z.; Samsel, K. Determination of pipecuronium bromide and its impurities in pharmaceutical preparation by high-performance liquid chromatography with coulometric electrode array detection. J. Chromatogr. A 2008, 1201, 191–195. [Google Scholar] [CrossRef]
- de la Huebra, M.J.G.; Vincent, U.; von Holst, C. Sample preparation strategy for the simultaneous determination of macrolide antibiotics in animal feedingstuffs by liquid chromatography with electrochemical detection (HPLC-ECD). J. Pharm. Biomed. Anal. 2007, 43, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Lebiedzińska, A.; Marszałł, M.L.; Kuta, J.; Szefer, P. Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B1 (thiamine), B6 (pyridoxamine, pyridoxal and pyridoxine) and B12 in animal and plant foods. J. Chromatogr. A 2007, 1173, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hormann, A.M.; Vom Saal, F.S.; Nagel, S.C.; Stahlhut, R.W.; Moyer, C.L.; Ellersieck, M.R.; Welshons, W.V.; Toutain, P.-L.; Taylor, J.A. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS ONE 2014, 9, e110509. [Google Scholar] [CrossRef]
- Saravanabhavan, G.; Blais, E.; Vincent, R.; Kumarathasan, P. A high performance liquid chromatography-electrochemical array method for the measurement of oxidative/nitrative changes in human urine. J. Chromatogr. A 2010, 1217, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L. Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2- furfural in human subjects following consumption of dried plums or dried plum juice. J. Agric. Food Chem. 2006, 54, 3744–3749. [Google Scholar] [CrossRef]
- Webster, G.K.; Craig, R.A.; Pommerening, C.A.; Acworth, I.N. Selection of Pharmaceutical Antioxidants by Hydrodynamic Voltammetry. Electroanalysis 2012, 24, 1394–1400. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Morozova, K.; Ramírez-Sucre, M.O.; Scampicchio, M.; Rodríguez-Buenfil, I.M. Determination of Peak Purity in HPLC by Coupling Coulometric Array Detection and Two-Dimensional Correlation Analysis. Sensors 2022, 22, 1794. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Imperiale, S.; Angeli, L.; Asma, U.; Ferrentino, G.; Scampicchio, M. HPLC-Triple detector (Coulometric array, diode array and mass spectrometer) for the analysis of antioxidants in officinal plants. LWT 2022, 162, 113456. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Prenzler, P.; Antolovich, M. Applications of mass spectrometry to plant phenols. TrAC Trends Anal. Chem. 1999, 18, 362–372. [Google Scholar] [CrossRef]
- Borochov-Neori, H.; Judeinstein, S.; Tripler, E.; Harari, M.; Greenberg, A.; Shomer, I.; Holland, D. Seasonal and cultivar variations in antioxidant and sensory quality of pomegranate (Punica granatum L.) fruit. J. Food Compos. Anal. 2009, 22, 189–195. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Cardinali, A.; Linsalata, V. Plant Phenolics: A Biochemical and Physiological Perspective. Recent Adv. Polyphen. Res. 2012, 3, 1–39. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Rui, A.; Liu, H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols in plant foods: Nature, isolation, and analysis. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Academic Press: New York, NY, USA, 2014; pp. 203–218. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Bouhtoury, F.C. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Grasel, F.d.S.; Ferrão, M.F.; Wolf, C.R. Ultraviolet spectroscopy and chemometrics for the identification of vegetable tannins. Ind. Crop. Prod. 2016, 91, 279–285. [Google Scholar] [CrossRef]
- Martínez-Busi, M.; Arredondo, F.; González, D.; Echeverry, C.; Vega-Teijido, M.A.; Carvalho, D.; Rodríguez-Haralambides, A.; Rivera, F.; Dajas, F.; Abin-Carriquiry, J.A. Purification, structural elucidation, antioxidant capacity and neuroprotective potential of the main polyphenolic compounds contained in Achyrocline satureioides (Lam) D.C. (Compositae). Bioorg. Med. Chem. 2019, 27, 2579–2591. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Science 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M.A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20, S1201–S1221. [Google Scholar] [CrossRef]
- Sikora, E.; Cieślik, E.; Topolska, K. The Sources of Natural Antioxidants. ACTA Acta Sci. Pol. 2008, 7, 5–17. [Google Scholar]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Chavan, U.D.; Shahidi, F.; Naczk, M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chem. 2001, 75, 509–512. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, Esterified, and Insoluble-Bound Phenolic Acids. Composition of Phenolic. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic acid profiles and antioxidant activity of major cereal crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Hensley, K.; Williamson, K.S.; Maidt, M.L.; Gabbita, S.P.; Grammas, P.; Floyd, R.A. Determination of Biological Oxidative Stress Using High Performance Liquid Chromatography with Electrochemical Detection (HPLC-ECD). J. High Resolut. Chromatogr. 1999, 22, 429–437. [Google Scholar] [CrossRef]
- Vávra, R.; Voříšek, V.; Kabrhelová, J.; Eichlerová, E.; Machová, R.; Bílková, A.; Knapová, P.; Kaplan, J.; Novotná, I.; Danková, V.; et al. Evaluation of antioxidants in currant and gooseberry. Acta Hortic. 2021, 1329, 21–26. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT–Food Sci. Technol. 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012; Nordic Council of Ministers: Copenhagen, Denmark, 2008; Volume 5, pp. 1–3. [Google Scholar] [CrossRef]
- Pyo, Y.H.; Lee, T.C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Sochor, J.; Jurikova, T.; Pohanka, M.; Skutkova, H.; Baron, M.; Tomaskova, L.; Balla, S.; Klejdus, B.; Pokluda, R.; Mlcek, J.; et al. molecules Evaluation of Antioxidant Activity, Polyphenolic Compounds, Amino Acids and Mineral Elements of Representative Genotypes of Lonicera edulis. Molecules 2014, 19, 6504–6523. [Google Scholar] [CrossRef]
- Thompson, M.M.; Chaovanalikit, A. Preliminary observations on adaptation and nutraceutical values of blue honeysuckle (lonicera caerulea ) in Oregon, USA. Acta Hortic. 2003, 626, 65–72. [Google Scholar] [CrossRef]
- Juríková, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic Composition and Antioxidant Activities in Flesh and Achenes of Strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of Phenolic Compounds in Strawberry (Fragaria × ananassa) Fruits by Different HPLC Detectors and Contribution of Individual Compounds to Total Antioxidant Capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Cantalapiedra, A.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Sensitive and selective determination of phenolic compounds from aromatic plants using an electrochemical detection coupled with HPLC method. Phytochem. Anal. 2014, 25, 247–254. [Google Scholar] [CrossRef]
- Jandera, P.; Škeříková, V.; Řehová, L.; Hájek, T.; Baldriánová, L.; Škopová, G.; Kellner, V.; Horna, A. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a CoulArray detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Bonnely, S.; Berset, C. Determination of the antioxidant activity of phenolic compounds by coulometric detection. Talanta 2000, 51, 709–716. [Google Scholar] [CrossRef]
- Beňová, B.; Hájek, T. Utilization of coulometric array detection in analysis of beverages and plant extracts. Procedia Chem. 2010, 2, 92–100. [Google Scholar] [CrossRef][Green Version]
- Kahoun, D.; Řezková, S.; Veškrnová, K.; Královský, J.; Holčapek, M. Determination of phenolic compounds and hydroxymethylfurfural in meads using high performance liquid chromatography with coulometric-array and UV detection. J. Chromatogr. A 2008, 1202, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, C.; Careri, M.; Groppi, F.; Mangia, A.; Manini, E.; Moil, G. Comparative investigation of UV, electrochemical and particle beam mass spectrometric detection for the high-performance liquid chromatographic determination of benzoic and cinnamic acids and of their corresponding phenolic acids. J. Chromatogr. A 1996, 753, 157–170. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y.; Trabelsi, N. Functional Properties of Polyphenols in Grains and Effects of Physicochemical Processing on Polyphenols. J. Food Qual. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Carcea, M.; Narducci, V.; Turfani, V.; Giannini, V. Polyphenols in raw and cooked cereals/pseudocereals/legume pasta and couscous. Foods 2017, 6, 80. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Dvořáková, M.; Douanier, M.; Jurková, M.; Kellner, V.; Dostálek, P. Comparison of antioxidant activity of barley (Hordeum vulgare L.) and malt extracts with the content of free phenolic compounds measured by high performance liquid chromatography coupled with CoulArray detector. J. Inst. Brew. 2008, 114, 150–159. [Google Scholar] [CrossRef]
- Ross, A.B.; Kochhar, S. Rapid and Sensitive Analysis of Alkylresorcinols from Cereal Grains and Products Using HPLC-Coularray-Based Electrochemical Detection. J. Agric. Food Chem. 2009, 57, 5187–5193. [Google Scholar] [CrossRef] [PubMed]
- Stankevičius, M.; Akuåeca, I.; Jákobsone, I.; Maruška, A. Comparative analysis of radical scavenging and antioxidant activity of phenolic compounds present in everyday use spice plants by means of spectrophotometric and chromatographic methods. J. Sep. Sci. 2011, 34, 1261–1267. [Google Scholar] [CrossRef]
- Schindler, M.; Solar, S.; Sontag, G. Phenolic compounds in tomatoes. Natural variations and effect of gamma-irradiation. Eur. Food Res. Technol. 2005, 221, 439–445. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Bernwieser, I.; Sontag, G. Determination of phenolic acids in vegetable oil using HPLC coupled with multielectrode detection. Z. Lebensm. Unters. Forsch. 1992, 195, 559–561. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, M.; van Camp, J.; Pedra, M.; Renders, K.; Socaciu, C.; Verhé, R. Correlations of the phenolic compounds and the phenolic content in some Spanish and French olive oils. J. Agric. Food Chem. 2008, 56, 5181–5187. [Google Scholar] [CrossRef]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Pokorna, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical Determination of the Antioxidant Potential of Some Less Common Fruit Species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef]
- Guo, C.; Cao, G.; Sofic, E.; Prior, R.L. High-Performance Liquid Chromatography Coupled with Coulometric Array Detection of Electroactive Components in Fruits and Vegetables: Relationship to Oxygen Radical Absorbance Capacity. J. Agric. Food Chem. 1997, 45, 1787–1796. [Google Scholar] [CrossRef]
- Aaby, K.; Hvattum, E.; Skrede, G. Analysis of flavonoids and other phenolic compounds using high-performance liquid chromatography with coulometric array detection: Relationship to antioxidant activity. J. Agric. Food Chem. 2004, 52, 4595–4603. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razem, M.; Ding, Y.; Morozova, K.; Mazzetto, F.; Scampicchio, M. Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review. Sensors 2022, 22, 7498. https://doi.org/10.3390/s22197498
Razem M, Ding Y, Morozova K, Mazzetto F, Scampicchio M. Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review. Sensors. 2022; 22(19):7498. https://doi.org/10.3390/s22197498
Chicago/Turabian StyleRazem, Mutasem, Yubin Ding, Ksenia Morozova, Fabrizio Mazzetto, and Matteo Scampicchio. 2022. "Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review" Sensors 22, no. 19: 7498. https://doi.org/10.3390/s22197498
APA StyleRazem, M., Ding, Y., Morozova, K., Mazzetto, F., & Scampicchio, M. (2022). Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review. Sensors, 22(19), 7498. https://doi.org/10.3390/s22197498









