Non-Enzymatic Phenylboronic Acid-Based Optode Membrane for Glucose Monitoring in Serums of Diabetic Patients and in the Culture Medium of Human Embryos
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Solution Preparation
2.3. Instrumentations
2.4. Optode Preparation
2.5. Selectivity
2.6. Effect of Soaking Time and Regeneration
2.7. Response, Reversibility, and Lifetimes
2.8. Repeatability and Reproducibility
2.9. Analytical Application
3. Results and Discussion
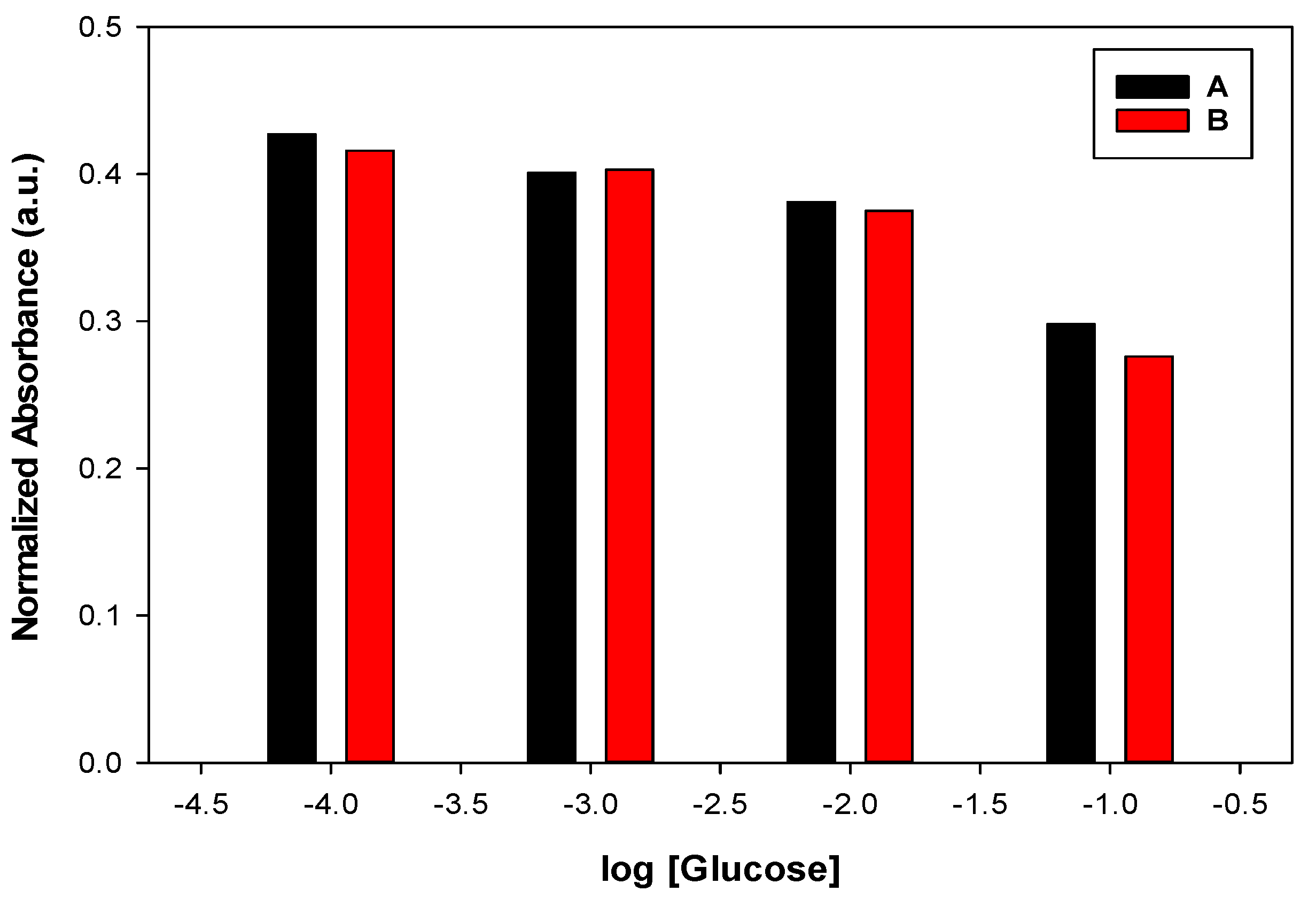
3.1. Effect of Membrane Composition
3.2. Recognition Mechanism
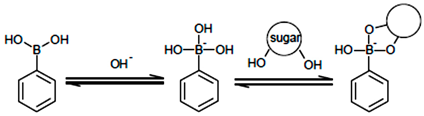
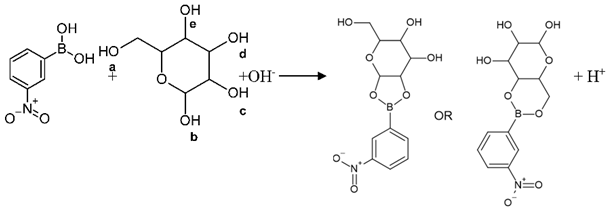
3.3. Effect of pH
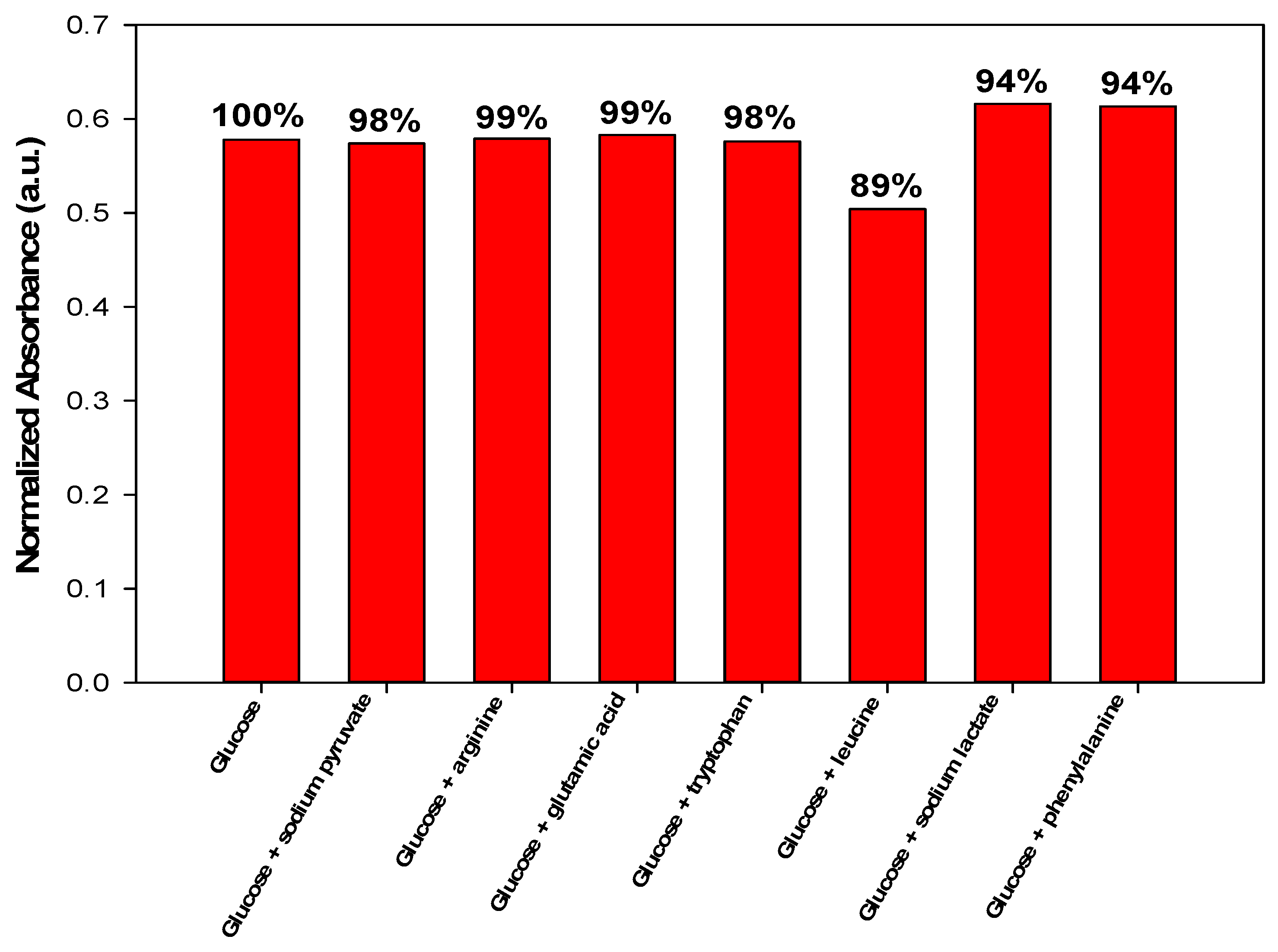
3.4. Interference Study
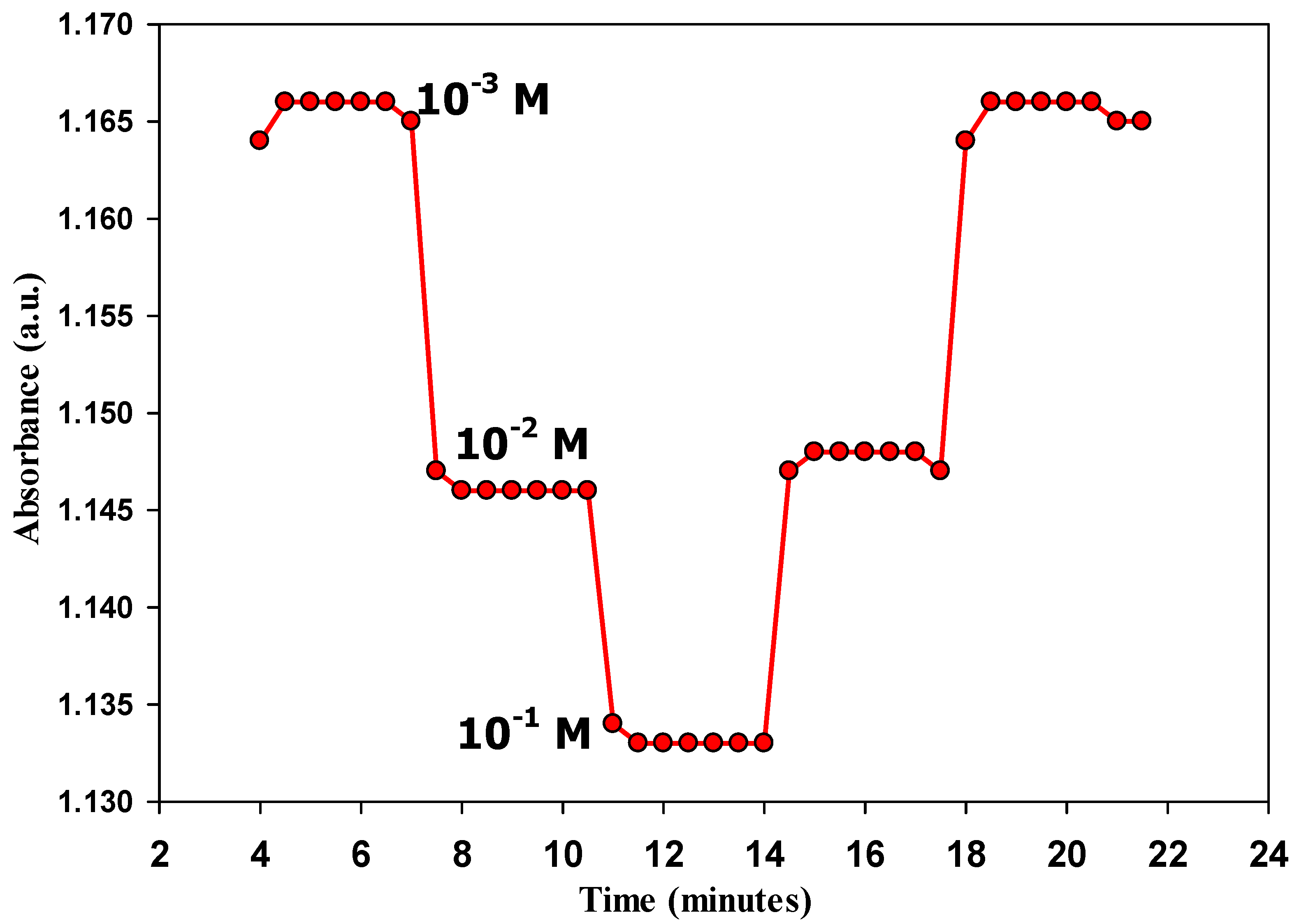
3.5. Response Time and Reversibility
3.6. Soaking, Regeneration, and Lifetime
3.7. Repeatability and Reproducibility
3.8. Analytical Detection of Glucose in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 4 April 2022).
- Washko, M.E.; Rice, E.W. Determination of glucose by an improved enzymatic procedure. Clin. Chem. 1961, 7, 542–545. Available online: https://pubmed.ncbi.nlm.nih.gov/14005104 (accessed on 6 August 2022). [CrossRef] [PubMed]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.; Baumann, N.A.; Matern, D.; Moyer, T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766.e9. [Google Scholar] [CrossRef] [PubMed]
- Gruber, I.; Klein, M. Embryo culture media for human IVF: Which possibilities exist? J. Turk. Gynecol. Assoc. 2011, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Uhde, K.; Van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Exposure to elevated glucose concentrations alters the metabolomic profile of bovine blastocysts. PLoS ONE 2018, 13, e0199310. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Embryo culture medium: Which is the best? Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 83–100. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schoolcraft, W.B. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil. Steril. 2001, 76, 1175–1180. [Google Scholar] [CrossRef]
- El Abd, M.M.; El Kaffash, D.N.-E.D.; El Samra, M.A.-E.M.; Sallam, N. Predictive Value Of Glucose And β-Hcg Concentration In The Embryo Culture Medium Of Patients Undergoing Intracytoplasmic Sperm Injection. Évid. Based Womens Health J. 2017, 7, 150–154. [Google Scholar] [CrossRef][Green Version]
- Gardner, D.K.; Wale, P.L.; Collins, R.; Lane, M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum. Reprod. 2011, 26, 1981–1986. [Google Scholar] [CrossRef]
- Renard, J.-P.; Philippon, A.; Menezo, Y. In-vitro uptake of glucose by bovine blastocysts. Reproduction 1980, 58, 161–164. [Google Scholar] [CrossRef]
- Gardner, D.K.; Leese, H.J. Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake. J. Exp. Zoöl. 1987, 242, 103–105. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Rizk, M.S. Highly selective thiocyanate optochemical sensor based on manganese(III)-salophen ionophore. Mater. Sci. Eng. C 2017, 75, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; El Nashar, R.M. Calixarene-doped PVC polymeric films as size-selective optical sensors: Monitoring of salicylate in real samples ETH 5294 ETH 7075. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F. Highly selective thiourea-based bulk optode for determination of salicylate in spiked urine samples, Aspirin® and Aspocid®. Sens. Actuators B Chem. 2016, 233, 257–262. [Google Scholar] [CrossRef]
- Sun, X.; James, T.D. Glucose Sensing in Supramolecular Chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials 2014, 7, 1201–1220. [Google Scholar] [CrossRef]
- Fujita, N.; Shinkai, S.; James, T.D. Boronic acids in molecular self-assembly. Chem.—Asian J. 2008, 3, 1076–1091. [Google Scholar] [CrossRef]
- Hall, D.G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications. In Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–99. [Google Scholar] [CrossRef]
- Peng, B.; Qin, Y. Lipophilic Polymer Membrane Optical Sensor with a Synthetic Receptor for Saccharide Detection. Anal. Chem. 2008, 80, 6137–6141. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Glucose-Mediated Assembly of Phenylboronic Acid Modified CdTe/ZnTe/ZnS Quantum Dots for Intracellular Glucose Probing. Angew. Chem. Int. Ed. 2010, 49, 6554–6558. [Google Scholar] [CrossRef]
- Hartley, J.H.; James, T.D.; Ward, C.J. Synthetic receptors. J. Chem. Soc. Perkin Trans. 1 2000, 4, 3155–3184. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Lopes, F.M.; Coltro, W.K.T. Paper-Based Colorimetric Biosensor for Tear Glucose Measurements. Micromachines 2017, 8, 104. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Saad, M.; Rizk, M.S. Development of new potentiometric sensors for the determination of proguanil hydrochloride in serum and urine. Chin. Chem. Lett. 2016, 27, 857–863. [Google Scholar] [CrossRef]
- Barhoum, A.; Forster, R.J. Label-Free Electrochemical Immunosensor for Picomolar Detection of the Cervical Cancer Biomarker MCM5. Anal. Chim. Acta 2022, 1225, 340226. [Google Scholar] [CrossRef]
- El-Beshlawy, M.M.; Abdel-Haleem, F.M.; Barhoum, A. Molecularly imprinted potentiometric sensor for nanomolar determination of pioglitazone hydrochloride in pharmaceutical formulations. Electroanalysis 2021, 33, 1244–1254. [Google Scholar] [CrossRef]
- Wang, E.; Meyerhoff, M.E. Anion selective optical sensing with metalloporphyrin-doped polymeric films. Anal. Chim. Acta 1993, 283, 673–682. [Google Scholar] [CrossRef]
- Roscales, S.; Csáky, A.G. How to make C–N bonds using boronic acids and their derivatives without transition metals. Chem. Soc. Rev. 2020, 49, 5159–5177. [Google Scholar] [CrossRef]
- Lerchi, M.; Bakker, E.; Rusterholz, B.; Simon, W. Lead-selective bulk optodes based on neutral ionophores with subnanomolar detection limits. Anal. Chem. 1992, 64, 1534–1540. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.; Mahmoud, S.; Abdel-Ghani, N.; El Nashar, R.; Bechelany, M.; Barhoum, A. polyvinyl chloride modified carbon paste electrodes for sensitive determination of levofloxacin drug in serum, urine, and pharmaceutical formulations. Sensors 2021, 21, 3150. [Google Scholar] [CrossRef]
- Gamsey, S.; Baxter, N.A.; Sharrett, Z.; Cordes, D.B.; Olmstead, M.M.; Wessling, R.A.; Singaram, B. The effect of boronic acid-positioning in an optical glucose-sensing ensemble. Tetrahedron 2006, 62, 6321–6331. [Google Scholar] [CrossRef]
- Monajemi, H.; Cheah, M.H.; Lee, V.S.; Zain, S.M.; Abdullah, W.A.T.W. On the kinetics and reaction mechanisms of boronic acid in interaction with diols for non-enzymatic glucose monitoring applications: A hybrid DFT study. RSC Adv. 2014, 4, 10505–10513. [Google Scholar] [CrossRef]
- Zhan, Z.; Li, Y.; Zhao, Y.; Zhang, H.; Wang, Z.; Fu, B.; Li, W.J. A Review of Electrochemical Sensors for the Detection of Glycated Hemoglobin. Biosensors 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Gozdalik, J.T.; Adamczyk-Woźniak, A.; Sporzyński, A. Influence of fluorine substituents on the properties of phenylboronic compounds. Pure Appl. Chem. 2017, 90, 677–702. [Google Scholar] [CrossRef]
- Zarzeczańska, D.; Adamczyk-Woźniak, A.; Kulpa, A.; Ossowski, T.; Sporzyński, A. Fluorinated Boronic Acids: Acidity and Hydrolytic Stability of Fluorinated Phenylboronic Acids. Eur. J. Inorg. Chem. 2017, 2017, 4493–4498. [Google Scholar] [CrossRef]
- Diamond, D.; McKervey, M.A. Calixarene-based sensing agents. Chem. Soc. Rev. 1996, 25, 15–24. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; Elhaes, H.; Jalbout, A.F.; De Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Onche, E.U.; Tukura, B.W.; Bako, S.S. Nuclear Magnetic Resonance (NMR) Analysis of D-(+)-Glucose: A Guide to Spectrometric Structural Elucidation of Sugars. IOSR J. Appl. Chem. (IOSR-JAC) 2013, 6, 45–51. Available online: www.iosrjournals.org (accessed on 6 January 2022).
- Dubach, J.M.; Harjes, A.D.I.; Clark, H.A. Ion-Selective Nano-optodes Incorporating Quantum Dots. J. Am. Chem. Soc. 2007, 129, 8418–8419. [Google Scholar] [CrossRef]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J.-I. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley-VCH Weinheim: Weinheim, Germany, 2011. [Google Scholar]
- Ptak, T.; Młynarz, P.; Dobosz, A.; Rydzewska, A.; Prokopowicz, M. Potentiometric and NMR complexation studies of phenylboronic acid PBA and its aminophosphonate analog with selected catecholamines. J. Mol. Struct. 2013, 1040, 59–64. [Google Scholar] [CrossRef]
- António, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Cheng, Y.; Cui, J.; Wang, B. Click Reactions and Boronic Acids: Applications, Issues, and Potential Solutions. Molecules 2010, 15, 5768–5781. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 648704. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Salah, A.; Rizk, M.S.; Moustafa, H.; Bechelany, M.; Barhoum, A. Carbon-based Nanosensors for Salicylate Determination in Pharmaceutical Preparations. Electroanalysis 2019, 31, 778–789. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Rizk, M.S.; El-Beshlawy, M.M. Molecularly-imprinted polymer-base bulk optode for the determination of ivabradine hydrochloride in Procoralan®. RSC Adv. 2022, 12, 17645–17654. [Google Scholar] [CrossRef]



| Optodes | Optode Composition, (wt%) | Optode Response, M | ||||
|---|---|---|---|---|---|---|
| PVC | Plasticizer | Ionophore | ETH7075 | Conc. Range | LOD | |
| Optode 1 | 49.0 | 49.0 (TCP) | 1.0 (Crown) | 1.0 | 10−3–10−4 | 1.0 × 10−4 |
| Optode 2 | 49.0 | 49.0 (TCP) | 1.0 (TFPBA) | 1.0 | 10−2–10−3 | 1.0 × 10−3 |
| Optode 3 | 49.0 | 49.0 (TCP) | 1.0 (NPBA) | 1.0 | 10−1–10−3 | 9.0 × 10−4 |
| Optode 4 | 49.0 | 49.0 (NOPE) | 1.0 (NPBA) | 1.0 | 10−1–10−2 | 1.0 × 10−2 |
| Glucose | NPBA | Mixture |
|---|---|---|
| 4.405 (CH-OHa) | ---- | 4.379 |
| 4.572, 4.588 (OHd) | ---- | 4.397, 4.409 |
| 4.772 (OHc) | ---- | 4.606 |
| 4.886 (OHe) | ---- | 4.837, 4.850 |
| 4.899 (CH-OHb) | ||
| 6.151, 6.166 (OHb) | ---- | 6.053, 6.065 |
| ---- | 8.615 (B-OH) | 8.610 |
| Samples | Theoretical Value | Optode 3 | Reference Method | Recovery |
|---|---|---|---|---|
| Synthetic culture media [3] | 1.00 × 10−3 M | 1.01 × 10−3 M | ---- | 101.5% |
| 1.00 × 10−4 M | 1.02 × 10−4 M | ---- | 102.4% | |
| Commercial culture Embryo media (GLOBAL® TOTAL® W/HEPES) [3] | ---- | 2.04 × 10−3 M | 2.01 × 10−3 M | 101.5% |
| Serum from normal and diabetic patients [4] | ---- | 155.8 mg/dL | 144.0 mg/dL | 108.2% |
| ---- | 382.2 mg/dL | 370.0 mg/dL | 103.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.M.; Rizk, M.S.; Zayed, M.A.; Abdel-Haleem, F.M.; Barhoum, A. Non-Enzymatic Phenylboronic Acid-Based Optode Membrane for Glucose Monitoring in Serums of Diabetic Patients and in the Culture Medium of Human Embryos. Sensors 2022, 22, 7135. https://doi.org/10.3390/s22197135
Taha MM, Rizk MS, Zayed MA, Abdel-Haleem FM, Barhoum A. Non-Enzymatic Phenylboronic Acid-Based Optode Membrane for Glucose Monitoring in Serums of Diabetic Patients and in the Culture Medium of Human Embryos. Sensors. 2022; 22(19):7135. https://doi.org/10.3390/s22197135
Chicago/Turabian StyleTaha, Mohamed M., Mahmoud S. Rizk, Mohamed A. Zayed, Fatehy M. Abdel-Haleem, and Ahmed Barhoum. 2022. "Non-Enzymatic Phenylboronic Acid-Based Optode Membrane for Glucose Monitoring in Serums of Diabetic Patients and in the Culture Medium of Human Embryos" Sensors 22, no. 19: 7135. https://doi.org/10.3390/s22197135
APA StyleTaha, M. M., Rizk, M. S., Zayed, M. A., Abdel-Haleem, F. M., & Barhoum, A. (2022). Non-Enzymatic Phenylboronic Acid-Based Optode Membrane for Glucose Monitoring in Serums of Diabetic Patients and in the Culture Medium of Human Embryos. Sensors, 22(19), 7135. https://doi.org/10.3390/s22197135










