Feasibility of Brachial Occlusion Technique for Beat-to-Beat Pulse Wave Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Devices
2.3. Study Design
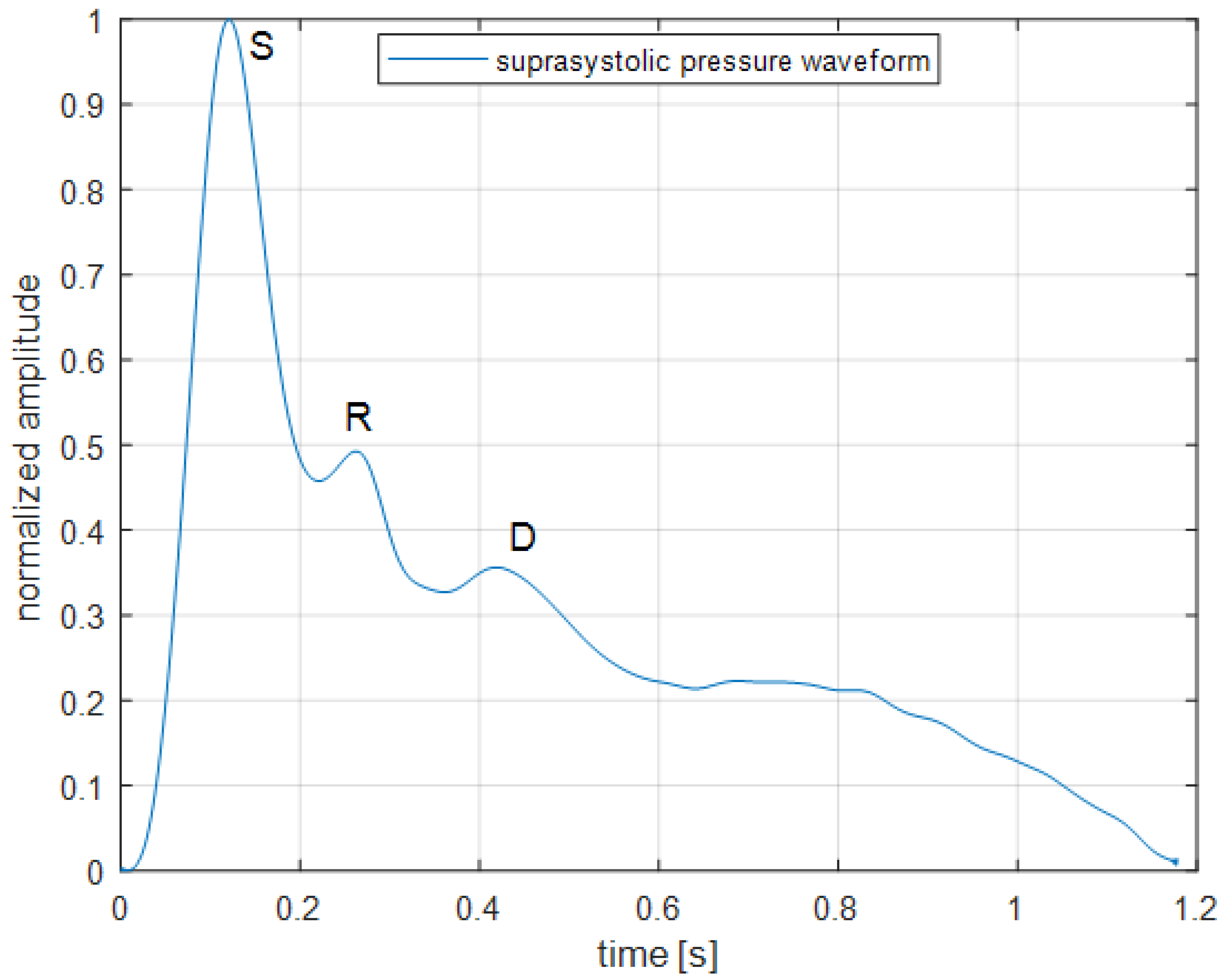
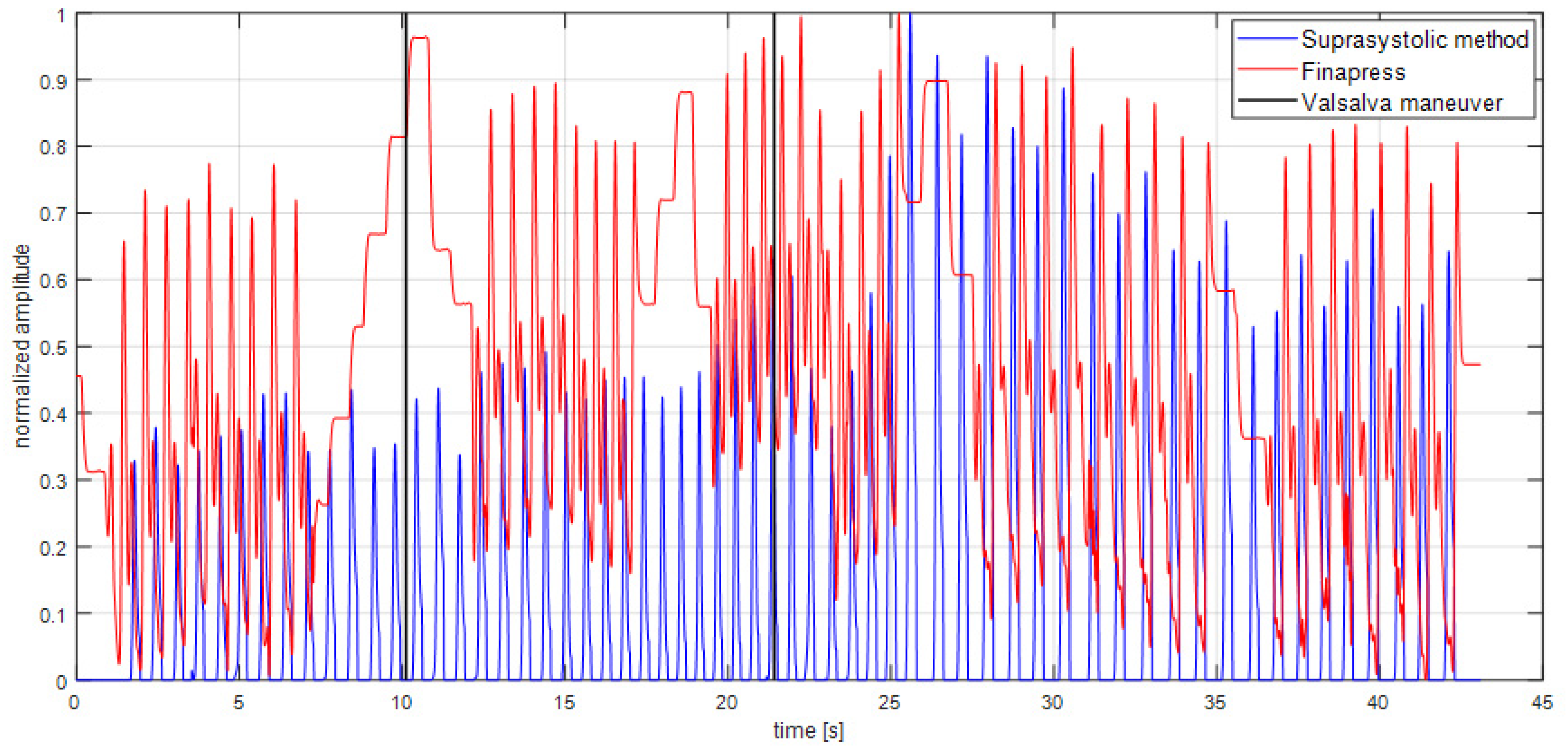
2.4. Signal Processing
2.5. Statistical Analysis
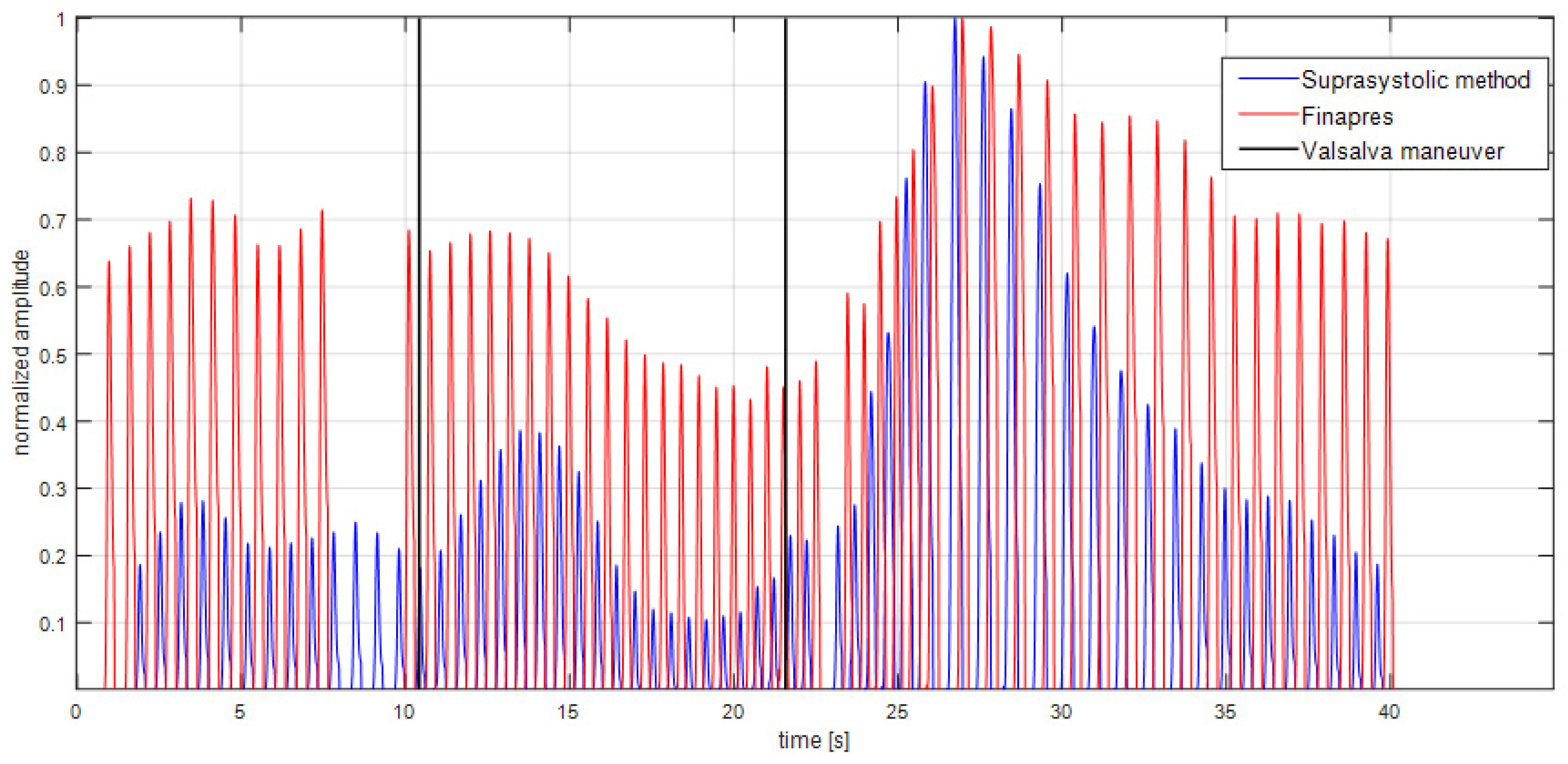
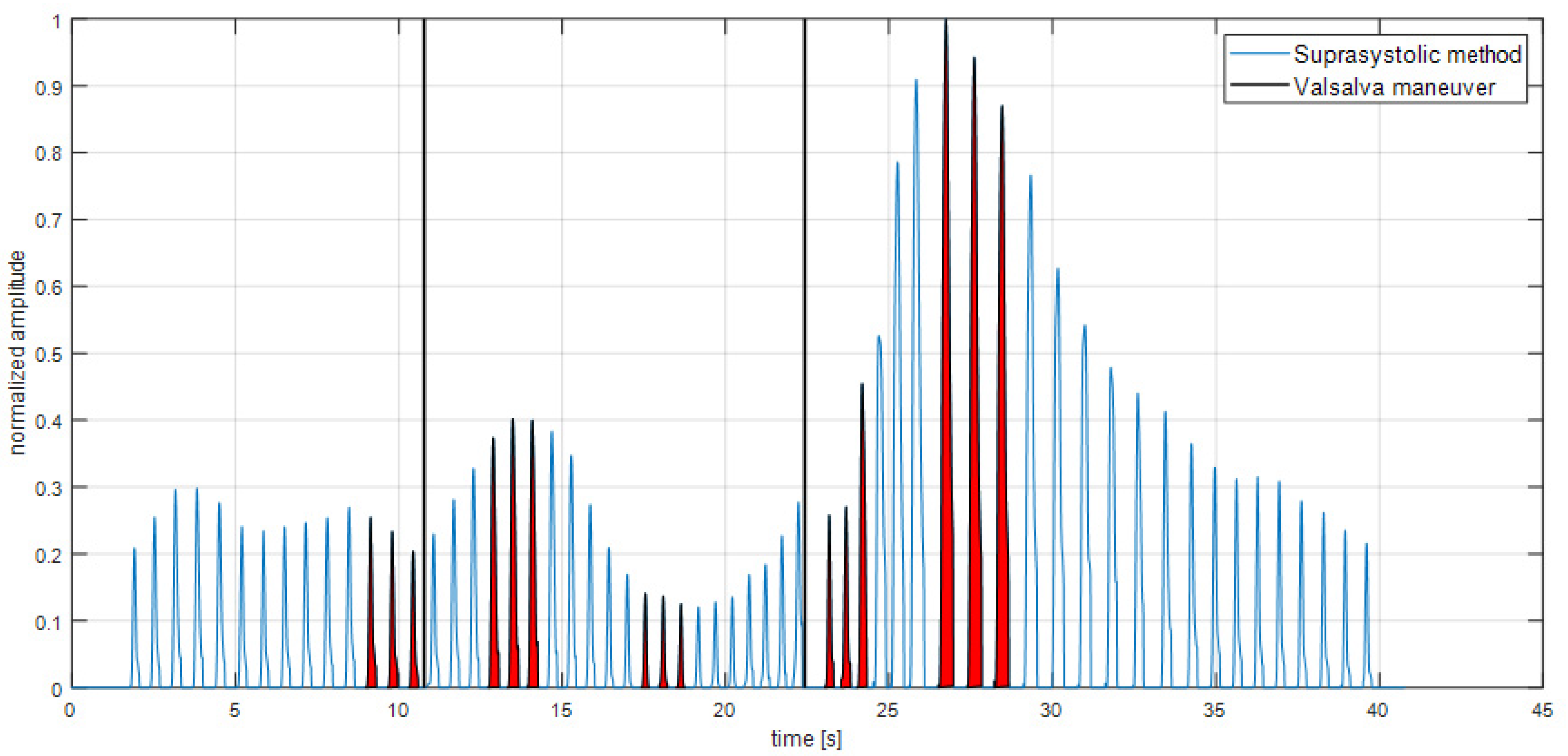
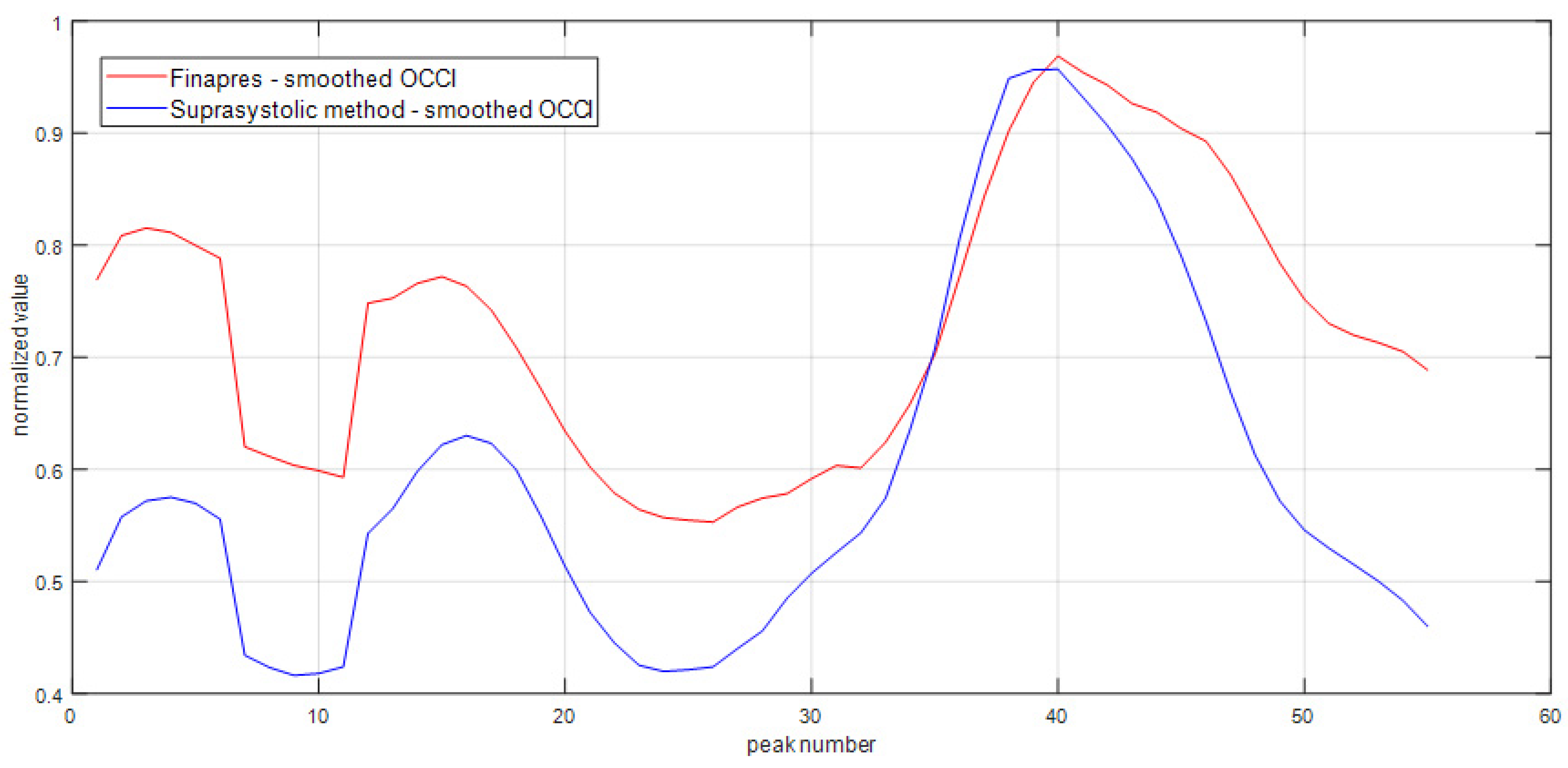
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdouw, P.D.; Beaune, J.; Roelandt, J.; Hugenholtz, P.G. Stroke volume from central aortic pressure? A critical assessment of the various formulae as to their clinical value. Basic Res. Cardiol. 1975, 70, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Reisner, A.T.; Saeed, M.; Heldt, T.; Mark, R.G. The cardiac output from blood pressure algorithms trial. Crit. Care Med. 2009, 37, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.K.; Senn, A.; Weibel, L.; Zollinger, A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit. Care 2012, 67, 377–383. [Google Scholar] [CrossRef]
- Broch, O.; Renner, J.; Gruenewald, M.; Meybohm, P.; Schöttler, J.; Caliebe, A.; Steinfath, M.; Malbrain, M.; Bein, B. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 2012, 67, 377–383. [Google Scholar] [CrossRef]
- Wesseling, K.H. Finapres, continuous noninvasive finger arterial pressure based on the method of Penaz. In Blood Pressure Measurements; Springer: Berlin/Heidelberg, Germany, 1990; pp. 161–172. [Google Scholar]
- Truijen, J.; Van Lieshout, J.J.; Wesselink, W.A.; Westerhof, B.E. Noninvasive continuous hemodynamic monitoring. J. Clin. Monit. Comput. 2012, 26, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Alhashemi, J.A.; Cecconi, M.; Hofer, C.K. Cardiac output monitoring: An integrative perspective. Annu. Update Intensive Care Emerg. Med. 2011, 2011, 443–456. [Google Scholar]
- Stouffer, G.A. (Ed.) Cardiovascular Hemodynamics for the Clinician; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lal, S.K.L.; Henderson, R.J.; Cejnar, M.; Hart, M.G.; Hunyor, S.N. Physiological influences on continuous finger and simultaneous intra-arterial blood pressure. Hypertension 1995, 26, 307–314. [Google Scholar] [CrossRef]
- Imholz, B.P.; Langewouters, G.J.; van Montfrans, G.A.; Parati, G.; van Goudoever, J.; Wesseling, K.H.; Wieling, W.; Mancia, G. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertension 1993, 21, 65–73. [Google Scholar] [CrossRef]
- Goldblatt, A.; Harrison, D.C.; Glick, G.; Braunwald, E. Studies on cardiac dimensions in intact, unanaesthetised man. Circ. Res. 1963, 13, 448–467. [Google Scholar]
- Hoffman, J.E.; Guz, A.; Charlier, A.A.; Wilcker, D.E.L. Stroke volume in conscious dogs; effect of respiration, posture and vascular occlusion. J. Appl. Physiol. 1965, 20, 865–877. [Google Scholar] [CrossRef]
- Karam, M.; Wise, R.A.; Natarajan, T.K.; Permutt, S.; Wagner, H.N. Mechanism of decreased left ventricular stroke volume during inspiration in man. Circulation 1984, 69, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Guz, B.Y.A.; Innes, J.A.; Murphy, K. Respiratory modulation of left ventricular stroke volume in man measured using pulsed doppler ultrasound. J. Physiol. 1987, 393, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Zema, M.J.; Masters, A.P.; Margouleff, D. Dyspnea: The heart or the lungs? Differentiation at bedside by use of the simple Valsalva maneuver. Chest 1984, 85, 59–64. [Google Scholar] [CrossRef]
- Zema, M.J.; Restivo, B.; Sos, T.; Sniderman, K.W.; Kline, S. Left ventricular dysfunction–bedside Valsalva manoeuvre. Br. Heart J. 1980, 44, 560–569. [Google Scholar] [CrossRef]
- Gorlin, R.; Knowles, J.H.; Storey, C.F. The valsalva maneuver as a test of cardiac function. Am. J. Med. 1957, 22, 197–212. [Google Scholar] [CrossRef]
- Horváth, I.G.; Németh, A.; Lenkey, Z.; Alessandri, N.; Tufano, F.; Kis, P.; Gaszner, B.; Cziráki, A. Invasive validation of a new oscillometric device (arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J. Hypertens. 2010, 28, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Sajgalik, P.; Kremen, V.; Carlson, A.R.; Vratislav, F.; Kim, C.-H.; Wheatley, C.; Gerla, V.; Schirger, J.A.; Olson, T.P.; Johnson, B.D. Non-invasive assessment of cardiac output by brachial cuff technique: Comparison to the open circuit acetylene washin method. J. Appl. Physiol. 2016, 121, 1319–1325. [Google Scholar] [CrossRef]
- Sajgalik, P.; Kremen, V.; Fabian, V.; Maltais, S.; Stulak, J.M.; Kushwaha, S.S.; Joyce, L.D.; Schirger, J.A.; Johnson, B.D. Non-invasive Blood pressure monitor designed for heart failure patients supported with continuous-flow left ventricular assist devices. ASAIO J. 2018, 65, 127–133. [Google Scholar] [CrossRef]
- Fabian, V.; Matera, L.; Bayerova, K.; Havlik, J.; Kremen, V.; Pudil, J.; Sajgalik, P.; Zemanek, D. Noninvasive assessment of aortic pulse wave velocity by the brachial occlusion-cuff technique: Comparative study. Sensors 2019, 19, 3467. [Google Scholar] [CrossRef]
- Cross, T.J.; Sajgalik, P.; Fabian, V.; Matera, L.; Kushwaha, S.S.; Maltais, S.; Johnson, B.D. Non-invasive assessment of arterial pulsatility in patients with continuous-flow left ventricular assist devices. Int. J. Artif. Organs 2020, 43, 99–108. [Google Scholar] [CrossRef]
- Fabian, V.; Kremen, V.; Dobias, M. Method for an Accurate Automated Non-Invasive Measurement of Blood Pressure Waveform and Apparatus to Carry Out the Same. U.S. Patent US10251567B2, 9 January 2017. [Google Scholar]
- Alexander, R.A. A note on averaging correlations. Bull. Psychon. Soc. 1990, 28, 335–336. [Google Scholar] [CrossRef]
- Stewart, J.M.; Medow, M.A.; Bassett, B.; Montgomery, L.D. Effects of thoracic blood volume on Valsalva maneuver. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H798–H804. [Google Scholar] [CrossRef] [PubMed]
- Kurki, T.S.; Smith, N.T.; Sanford, T.J., Jr.; Head, N. Pulse oximetry and finger blood pressure measurement during open-heart surgery. J. Clin. Monit. 1989, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- de Simone, G.; Roman, M.J.; Koren, M.J.; Mensah, G.A.; Ganau, A.; Devereux, R.B. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 1999, 33, 800–805. [Google Scholar] [CrossRef]
- Lind, L.; Andrén, B.; Sundström, J. The stroke volume/pulse pressure ratio predicts coronary heart disease mortality in a population of elderly men. J. Hypertens. 2004, 22, 899–905. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Tanaka, H. Distal shift of arterial pressure wave reflection sites with aging. Hypertension 2010, 56, 920–925. [Google Scholar] [CrossRef]
- Monge García, M.I.; Gil Cano, A.; Díaz Monrové, J.C. Arterial pressure changes during the Valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009, 35, 77–84. [Google Scholar] [CrossRef]
- Tagawa, K.; Takahashi, A.; Yokota, A.; Sato, T.; Maeda, S. Aortic diastolic pressure decay modulates relation between worsened aortic stiffness and myocardial oxygen supply/demand balance after resistance exercise. surgery patient? J. Appl. Physiol. 2019, 127, 737–744. [Google Scholar] [CrossRef]
| Subject | Number of Peaks | z | |
|---|---|---|---|
| 1 | 104 | 0.753 | 0.979 |
| 2 | 163 | 0.450 | 0.485 |
| 3 | 166 | 0.549 | 0.617 |
| 4 | 195 | 0.522 | 0.579 |
| 5 | 162 | 0.714 | 0.895 |
| 6 | 154 | 0.330 | 0.343 |
| 7 | 166 | 0.640 | 0.760 |
| 8 | 174 | 0.660 | 0.794 |
| 9 | 120 | 0.708 | 0.884 |
| 10 | 105 | 0.643 | 0.764 |
| Totally | 1499 | 0.597 | 0.689 |
| Subject | OOCIBefore | OCCIMax During | OCCIMin During | Before/Max During | Before/Min During | Max During/Min During |
|---|---|---|---|---|---|---|
| 1 | 0.163 | 0.211 | 0.124 | 0.296 | −0.234 | 0.705 |
| 2 | 0.159 | 0.168 | 0.134 | 0.056 | −0.157 | 0.257 |
| 3 | 0.154 | 0.157 | 0.121 | 0.020 | −0.212 | 0.298 |
| 4 | 0.150 | 0.163 | 0.118 | 0.090 | −0.206 | 0.381 |
| 5 | 0.155 | 0.150 | 0.120 | −0.029 | −0.221 | 0.248 |
| 6 | 0.156 | 0.153 | 0.127 | −0.011 | −0.177 | 0.207 |
| 7 | 0.136 | 0.147 | 0.123 | 0.083 | −0.096 | 0.198 |
| 8 | 0.167 | 0.181 | 0.134 | 0.081 | −0.190 | 0.357 |
| 9 | 0.212 | 0.223 | 0.137 | 0.050 | −0.354 | 0.624 |
| 10 | 0.125 | 0.142 | 0.104 | 0.133 | −0.163 | 0.368 |
| 11 | 0.134 | 0.147 | 0.123 | 0.101 | −0.082 | 0.204 |
| Mean ± SD | 0.156 ± 0.022 | 0.167 ± 0.026 | 0.124 ± 0.009 | 0.079 ± 0.083 | −0.190 ± 0.069 | 0.350 ± 0.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matera, L.; Sajgalik, P.; Fabian, V.; Mikhailov, Y.; Zemanek, D.; Johnson, B.D. Feasibility of Brachial Occlusion Technique for Beat-to-Beat Pulse Wave Analysis. Sensors 2022, 22, 7285. https://doi.org/10.3390/s22197285
Matera L, Sajgalik P, Fabian V, Mikhailov Y, Zemanek D, Johnson BD. Feasibility of Brachial Occlusion Technique for Beat-to-Beat Pulse Wave Analysis. Sensors. 2022; 22(19):7285. https://doi.org/10.3390/s22197285
Chicago/Turabian StyleMatera, Lukas, Pavol Sajgalik, Vratislav Fabian, Yegor Mikhailov, David Zemanek, and Bruce D. Johnson. 2022. "Feasibility of Brachial Occlusion Technique for Beat-to-Beat Pulse Wave Analysis" Sensors 22, no. 19: 7285. https://doi.org/10.3390/s22197285
APA StyleMatera, L., Sajgalik, P., Fabian, V., Mikhailov, Y., Zemanek, D., & Johnson, B. D. (2022). Feasibility of Brachial Occlusion Technique for Beat-to-Beat Pulse Wave Analysis. Sensors, 22(19), 7285. https://doi.org/10.3390/s22197285








