Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration
Abstract
1. Introduction
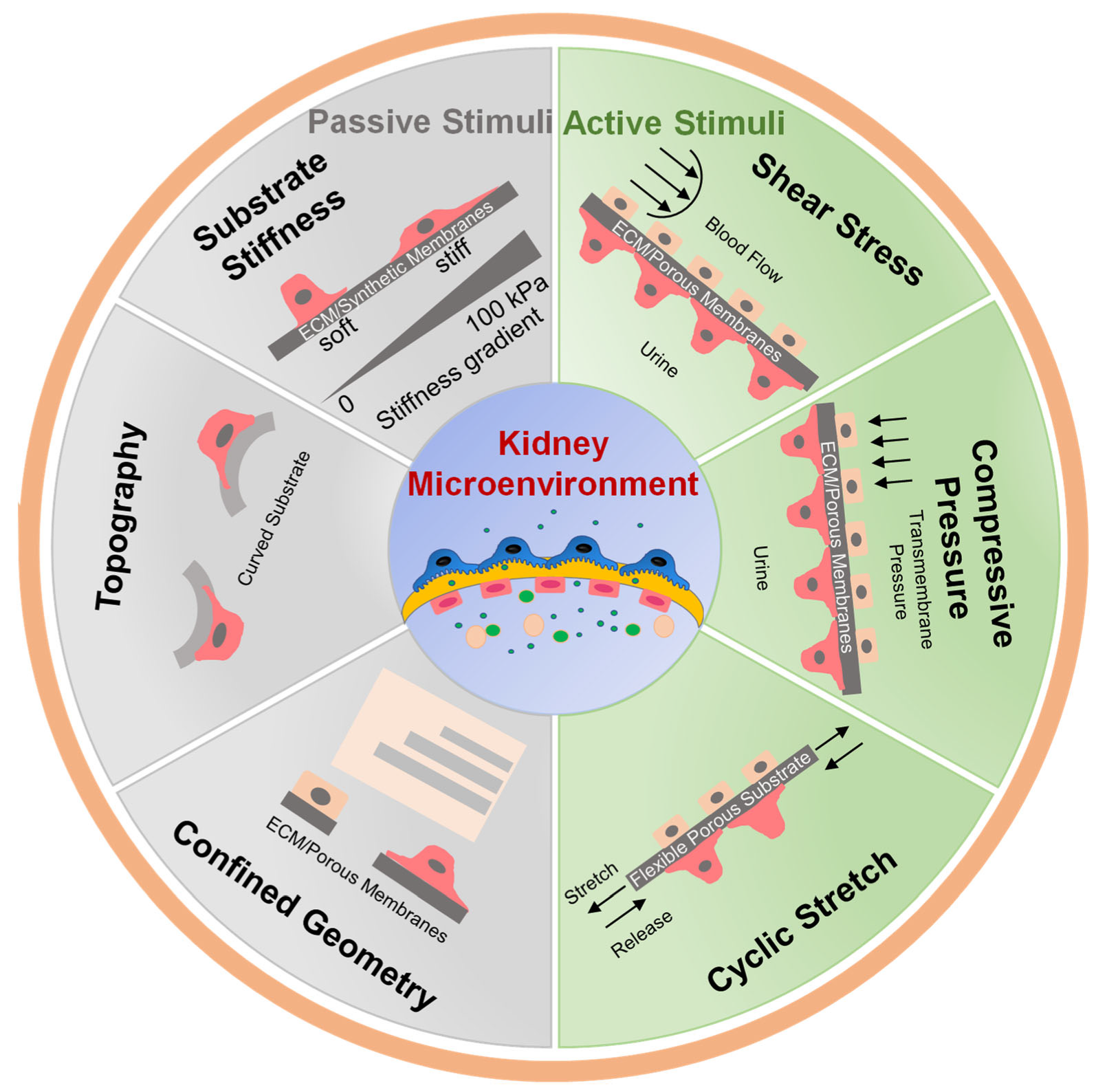
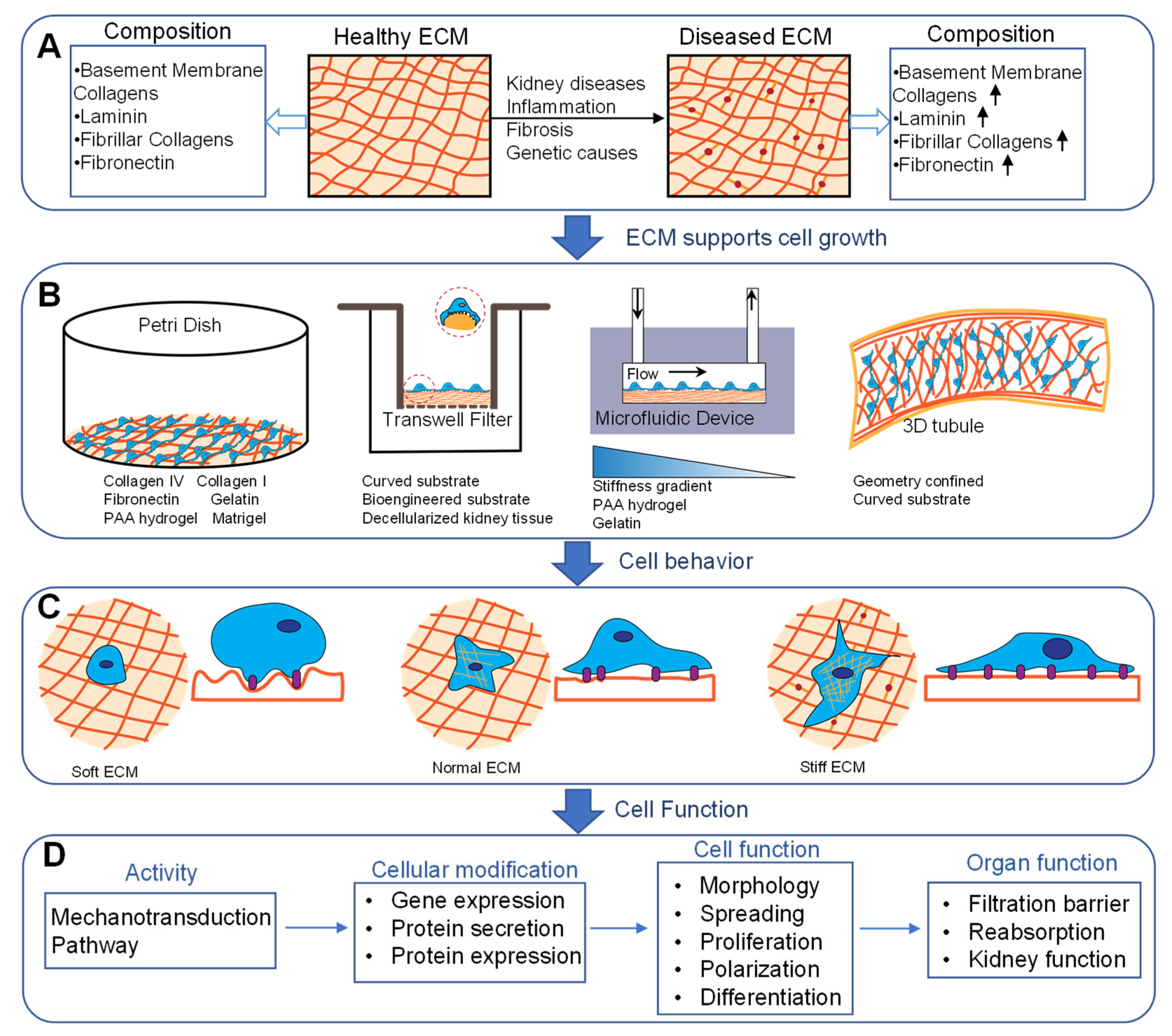
2. Importance of Mechanical Stimuli in Kidney
3. Passive Mechanical Simulation
3.1. Substrate Stiffness
3.2. Surface Topography and ECM Composition
3.3. Confined Geometry
4. Active Mechanical Simulation
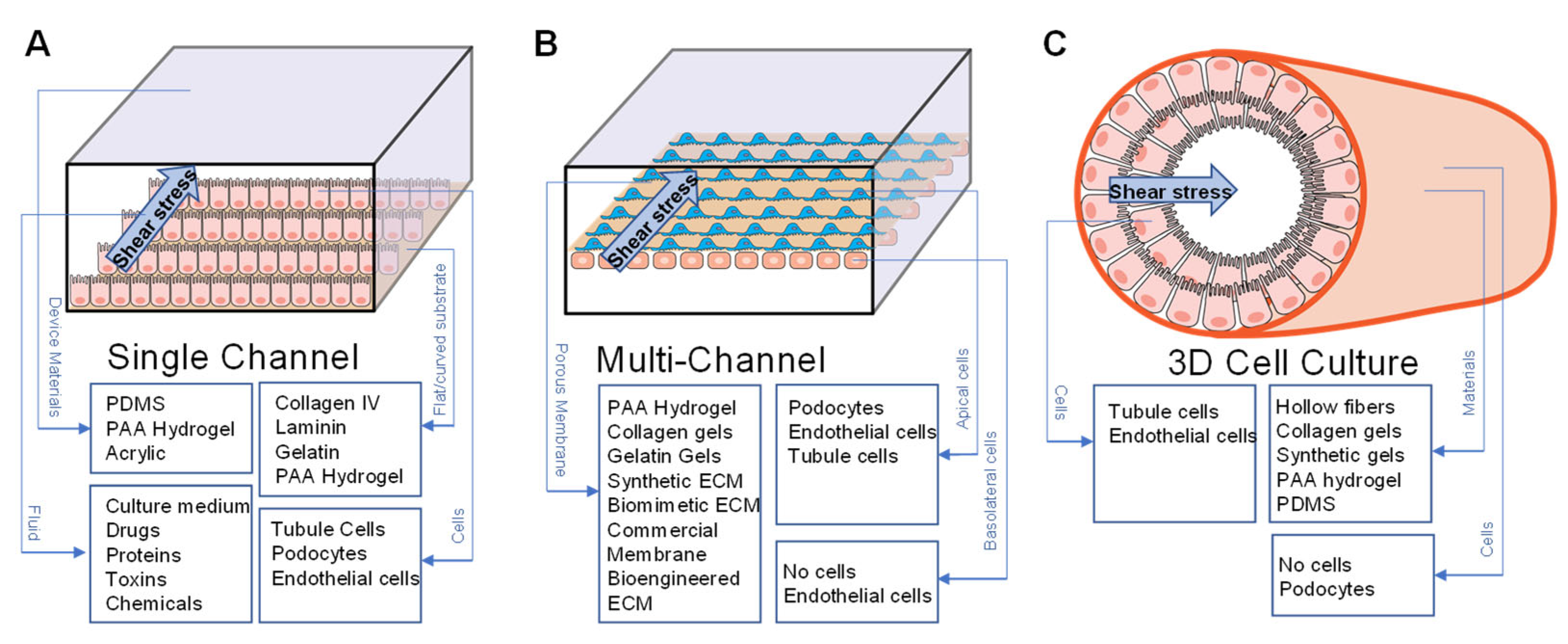
4.1. Fluid Shear Stress
4.1.1. FSS in Modeling Normal Physiology
4.1.2. FSS in Modeling Disease
4.1.3. FSS in Modeling Barrier Function
4.2. Compressive Pressure and Cyclic Stretch
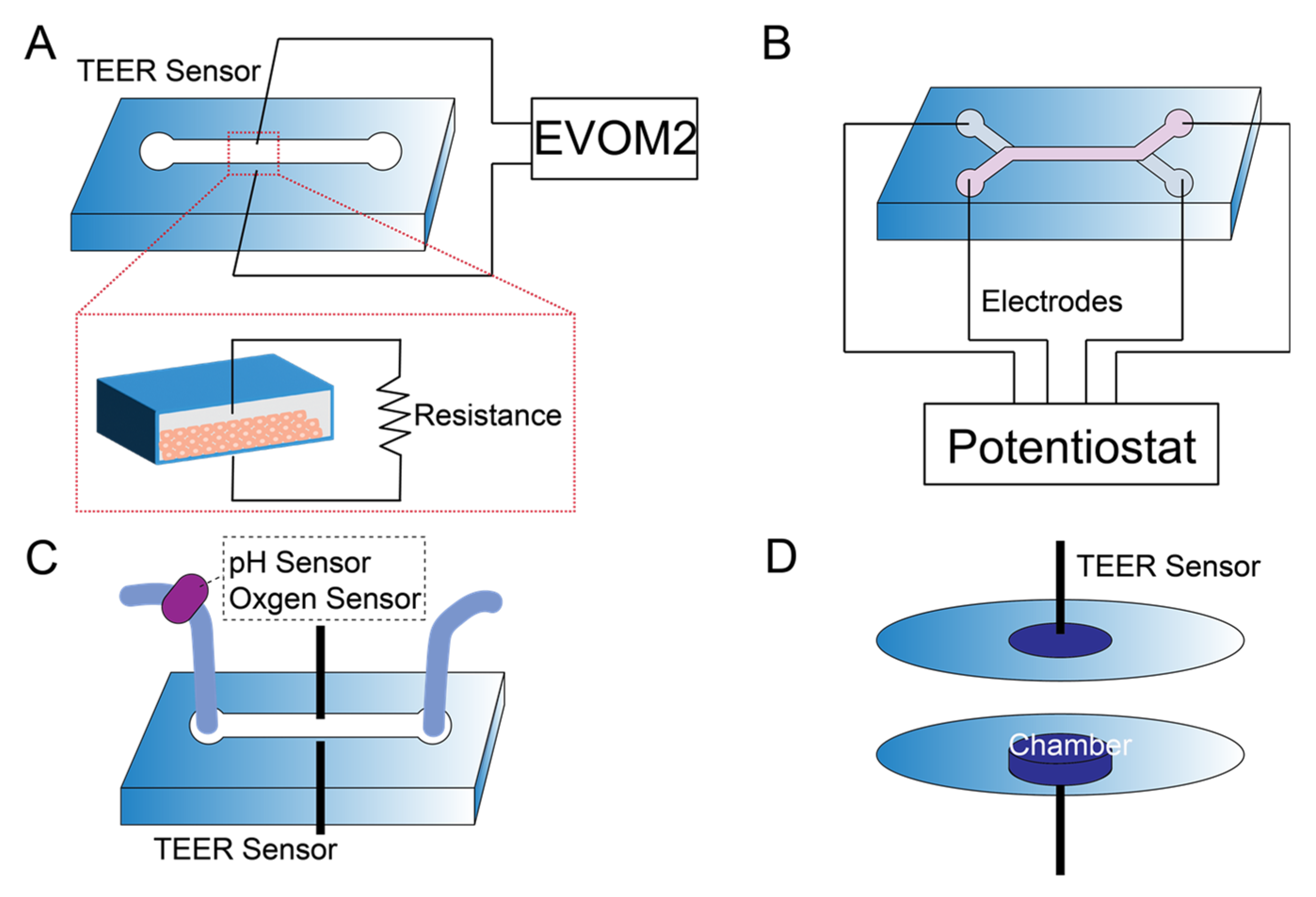
5. Sensor Integration in Kidney-on-a-Chip
6. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quaggin, S.E. Kindling the kidney. N. Engl. J. Med. 2016, 374, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S.; Rohacs, T.; Susztak, K. How many cell types are in the kidney and what do they do? Annu. Rev. Physiol. 2022, 84, 507–531. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.W.; Murphy, W.L.; Beebe, D.J. Screening the cellular microenvironment: A role for microfluidics. IEEE Rev. Biomed. Eng. 2008, 1, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Ewald, A.J. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Wesseling-Perry, K.; Hasan, A.; Elkhammas, E.; Zhang, Y.S. Kidney-on-a-chip: Untapped opportunities. Kidney Int. 2018, 94, 1073–1086. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Wang, L.; Tao, T.; Su, W.; Yu, H.; Yu, Y.; Qin, J. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip 2017, 17, 1749–1760. [Google Scholar] [CrossRef]
- Petrosyan, A.; Cravedi, P.; Villani, V.; Angeletti, A.; Manrique, J.; Renieri, A.; De Filippo, R.E.; Perin, L.; Da Sacco, S. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 2019, 10, 3656. [Google Scholar] [CrossRef]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 2017, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.G.; Mille, L.S.; Figler, K.P.; Cervantes, E.; Li, V.Y.; Bonventre, J.V.; Maseeeuw, R.; Zhang, Y.S. Biomimetic models of the glomerulus. Nat. Rev. Nephrol. 2022, 18, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ma, H.; Lin, H.; Qin, J. Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials 2014, 35, 1390–1401. [Google Scholar] [CrossRef]
- Baudoin, R.; Griscom, L.; Monge, M.; Legallais, C.; Leclerc, E. Development of a renal microchip for in vitro distal tubule models. Biotechnol. Prog. 2007, 23, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Jang, K.-J.; Suh, K.-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef]
- Rayner, S.G.; Phong, K.T.; Xue, J.; Lih, D.; Shankland, S.J.; Kelly, E.J.; Himmelfarb, J.; Zheng, Y. Reconstructing the human renal vascular-tubular unit in vitro. Adv. Healthc. Mater. 2018, 7, e1801120. [Google Scholar] [CrossRef]
- Rizki-Safitri, A.; Traitteur, T.; Morizane, R. Bioengineered kidney models: Methods and functional assessments. Function 2021, 2, zqab026. [Google Scholar] [CrossRef]
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and soft lithography for organ-on-chip applications. Methods Mol. Biol. 2022, 2373, 1–19. [Google Scholar]
- Sochol, R.D.; Gupta, N.R.; Bonventre, J.V. A role for 3D printing in kidney-on-a-chip platforms. Curr. Transplant. Rep. 2016, 3, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Rayner, S.G.; Howard, C.C.; Mandrycky, C.J.; Stamenkovic, S.; Himmelfarb, J.; Shih, A.Y.; Zheng, Y. Multiphoton-guided creation of complex organ-specific microvasculature. Adv. Healthc. Mater. 2021, 10, e2100031. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, N.; Desai, R.R.; Fleischman, A.J.; Roy, S.; Humes, H.D.; Fissell, W.H. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol. Bioeng. 2010, 107, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, M.J.; Ng, C.P.; Lanz, H.L.; Vulto, P.; Suter-Dick, L.; Masereeuw, R. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef]
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; MacDonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.M.; et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 2018, 3, e123673. [Google Scholar] [CrossRef]
- Ferrell, N.; Ricci, K.B.; Groszek, J.; Marmerstein, J.T.; Fissell, W.H. Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol. Bioeng. 2012, 109, 797–803. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Persson, M.; Kelly, E.J.; Sjögren, A.K. A multicompartment human kidney proximal tubule-on-a-chip replicates cell polarization-dependent cisplatin toxicity. Drug Metab. Dispos. 2020, 48, 1303–1311. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Wen, X.; Wu, T.; Wang, W.; Yang, M.; Wang, J.; Fang, M.; Lin, B.; Lin, H. Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci. Rep. 2016, 6, 31771. [Google Scholar] [CrossRef]
- Yu, P.; Duan, Z.; Liu, S.; Pachon, I.; Ma, J.; Hemstreet, G.P.; Zhang, Y. Drug-induced nephrotoxicity assessment in 3D cellular models. Micromachines 2022, 13, 3. [Google Scholar] [CrossRef]
- Fuchs, S.; Johansson, S.; Tjell, A.O.; Werr, G.; Mayr, T.; Tenje, M. In-line analysis of organ-on-chip systems with sensors: Integration, fabrication, challenges, and potential. ACS Biomater. Sci. Eng. 2021, 7, 2926–2948. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Asli, A.E.N.; Taghavimehr, M.; Hashemi, N.; Shirsavar, M.A.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J.; et al. Advancement of sensor integrated organ-on-chip devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; SHin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, G.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Block, F.E.; Cliffel, D.E.; Goodwin, C.R.; Marasco, C.C.; Markov, D.A.; McLean, D.L.; McLean, J.A.; McKenzie, J.R.; Reisserer, R.S.; et al. Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans. Biomed. Eng. 2013, 60, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Simmons, C.A. Macro-and microscale fluid flow systems for endothelial cell biology. Lab Chip 2010, 10, 143–160. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Heywood, H.K.; Knight, M.M.; Thorpe, S.D. Mechanical stimulation: A crucial element of organ-on-chip models. Front. Bioeng. Biotechnol. 2020, 8, 602646. [Google Scholar] [CrossRef]
- Charelli, L.E.; Ferreira, J.P.; Naveira-Cotta, C.P.; Balbino, T.A. Engineering mechanobiology through organoids-on-chip: A strategy to boost therapeutics. J. Tissue Eng. Regen. Med. 2021, 15, 883–899. [Google Scholar] [CrossRef]
- Vera, D.; Garcia-Diaz, M.; Torras, N.; Alvarez, M.; Villa, R.; Martinez, E. Engineering tissue barrier models on hydrogel microfluidic platforms. ACS Appl. Mater. Interfaces 2021, 13, 13920–13933. [Google Scholar] [CrossRef]
- Richfield, O.; Cortez, R.; Navar, L.G. Simulations of glomerular shear and hoop stresses in diabetes, hypertension, and reduced renal mass using a network model of a rat glomerulus. Physiol. Rep. 2020, 8, e14577. [Google Scholar] [CrossRef]
- Ferrell, N.; Sandoval, R.M.; Bian, A.; Campos-Bilderback, S.B.; Molitoris, B.A.; Fissell, W.H. Shear stress is normalized in glomerular capillaries following ⅚ nephrectomy. Am. J. Physiol. Ren. Physiol. 2015, 308, F588–F593. [Google Scholar] [CrossRef]
- Remuzzi, A.; Brenner, B.M.; Pata, V.; Tebaldi, G.; Mariano, R.; Belloro, A.; Remuzzi, G. Three-dimensional reconstructed glomerular capillary network: Blood flow distrituion and local filtration. Am. J. Physiol. Ren. Physiol. 1992, 263, F562–F572. [Google Scholar] [CrossRef]
- Kriz, W.; Lemley, K.V. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 2017, 91, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, N.; Sandoval, R.M.; Molitoris, B.A.; Brakeman, P.; Roy, S.; Fissell, W.H. Application of physiological shear stress to renal tubular epithelial cells. Methods Cell Biol. 2019, 153, 43–67. [Google Scholar] [PubMed]
- Friedrich, C.; Endlich, N.; Kriz, W.; Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol. Ren. Physiol. 2006, 291, F856–F865. [Google Scholar] [CrossRef]
- Duan, Y.; Weinstein, A.M.; Weinbaum, S.; Wang, T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21860–21865. [Google Scholar] [CrossRef] [PubMed]
- Maggiorani, D.; Dissard, R.; Belloy, M.; Saulnier-Blache, J.-S.; Casemayou, A.; Ducasse, L.; Grès, S.; Bellière, J.; Caubet, C.; Bascands, J.-L. Shear stress-induced alteration of epithelial organization in human renal tubular cells. PLoS ONE 2015, 10, e0131416. [Google Scholar] [CrossRef]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- Miner, J.H. The glomerular basement membrane. Exp. Cell Res. 2012, 318, 973–978. [Google Scholar] [CrossRef]
- Miner, J.H. Renal basement membrane components. Kidney Int. 1999, 56, 2016–2024. [Google Scholar] [CrossRef]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenes. Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Bülow, R.D.; Boor, P. Extracellular matrix in kidney fibrosis: More than just a scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Funk, S.D.; Lin, M.-H.; Miner, J.H. Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol. 2018, 71, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and response to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–4100. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Osório, L.A.; Silva, E.; Mackay, R.E. A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioengineering 2021, 8, 113. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 2019, 31, 1902042. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010, 47, 10.16.11–10.16.16. [Google Scholar] [CrossRef]
- Wyss, H.M.; Henderson, J.M.; Byfield, F.J.; Bruggeman, L.A.; Ding, Y.; Huang, C.; Suh, J.H.; Franke, T.; Mele, E.; Pollak, M.R.; et al. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011, 300, C397–C405. [Google Scholar] [CrossRef]
- Janmey, P.A.; Miller, R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011, 124, 9–18. [Google Scholar] [CrossRef]
- Embry, A.E.; Liu, Z.; Henderson, J.M.; Byfield, F.J.; Liu, L.; Yoon, J.; Wu, Z.; Cruz, K.; Moradi, S.; Gillombardo, C.B.; et al. Similar biophysical abnormalities in glomeruli and podocytes from two distinct models. J. Am. Soc. Nephrol. 2018, 29, 1501–1512. [Google Scholar] [CrossRef]
- Szeto, S.G.; Narimatsu, M.; Lu, M.; He, X.; Sidiqi, A.M.; Tolosa, M.F.; Chan, L.; Freitas, K.D.; Bialik, J.F.; Majumder, S.; et al. YAP/TAZ are mechanoregulators of TGF-Β-Smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 2016, 27, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Wang, D.; Agarwal, R.; Dillender, S.; Ferrell, N. Glycation alters the mechanical behavior of kidney extracellular matrix. Matrix Biol. Plus 2020, 8, 100035. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Wang, D.; Abidi, M.; Walker, G.; Ferrell, N. Mechanical characterization of native and sugar-modified decellularized kidneys. J. Mech. Behav. Biomed. Mater. 2021, 114, 104220. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Martin, M.; El Tahchi, M.R.; Balme, S.; Faour, W.H.; Varga, B.; Cloitre, T.; Pall, O.; Cuisinier, F.J.; Gergely, C. Influence of hydrolyzed polyacrylamide hydrogel stiffness on podocyte morphology, phenotype, and mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 32632. [Google Scholar] [CrossRef]
- Garcia, S.; Sunyer, R.; Olivares, A.; Noailly, J.; Atencia, J.; Trepat, X. Generation of stable orthogonal gradients of chemical concentration and substrate stiffness in a microfluidic device. Lab Chip 2015, 15, 2606–2614. [Google Scholar] [CrossRef]
- Hu, M.; Azeloglu, E.U.; Ron, A.; Tran-Ba, K.-H.; Calizo, R.C.; Tavassoly, I.; Bhattacharya, S.; Jayaraman, G.; Chen, Y.; Rabinovich, V. A biomimetic gelatin-based platform elicits a pro-differentiation effect on podocytes through mechanotransduction. Sci. Rep. 2017, 7, 43934. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, H.H.; Tang, M.J. Regulation of proximal tubular cell differentiation and proliferation in primary culture by matrix stiffness and ECM components. Am. J. Physiol. Ren. Physiol. 2014, 307, F695–F707. [Google Scholar] [CrossRef]
- Beamish, J.A.; Chen, E.; Putnam, A.J. Engineered extracellular matrices with controlled mechanics modulate renal proximal tubular cell epithelialization. PLoS ONE 2017, 12, e0181085. [Google Scholar] [CrossRef]
- Leight, J.L.; Wozniak, M.A.; Chen, S.; Lynch, M.L.; Chen, C.S. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 2012, 23, 781–791. [Google Scholar] [CrossRef]
- Love, H.D.; Ao, M.; Jorgensen, S.; Swearingen, L.; Ferrell, N.; Evans, R.; Gewin, L.; Harris, R.C.; Zent, R.; Roy, S.; et al. Substrate elasticity governs differentiation of renal tubule cells in prolonged culture. Tissue Eng. Part A 2019, 25, 1013–1022. [Google Scholar] [CrossRef]
- Mahmood, A.; Napoli, C.; Aldahmash, A. In vitro differentiation and maturation of human embryonic stem cell into multipotent cells. Stem Cells Int. 2011, 2011, 735420. [Google Scholar] [CrossRef] [PubMed]
- Casellas, C.P.; Rookmaaker, M.B.; Verhaar, M.C. Controlling cellular plasticity to improve in vitro models for kidney regeneration. Curr. Opin. Biomed. Eng. 2021, 20, 100345. [Google Scholar] [CrossRef]
- Yu, S.-M.; Oh, J.M.; Lee, J.; Lee-Kwon, W.; Jung, W.; Amblard, F.; Granick, S.; Cho, Y.-K. Substrate curvature affects the shape, orientation, and polarization of renal epithelial cells. Acta Biomater. 2018, 77, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Korolj, A.; Laschinger, C.; James, C.; Hu, E.; Velikonja, C.; Smith, N.; Gu, I.; Ahadian, S.; Willette, R.; Radisic, M. Curvature facilitates podocyte culture in a biomimetic platform. Lab Chip 2018, 18, 3112–3128. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Vrana, N.E.; Ghaemmaghami, A.M.; Huri, P.Y.; McGiuness, G.B. Basement membrane properties and their recapituation in organ-on-chip applications. Mater. Today Bio 2022, 15, 100301. [Google Scholar] [CrossRef]
- Wang, D.; Sant, S.; Ferrell, N. A biomimetic in vitro model of the kidney filtration barrier using tissue-derived glomerular basement membrane. Adv. Healthc. Mater. 2021, 10, 2002275. [Google Scholar] [CrossRef]
- Lin, N.Y.C.; Homan, K.A.; Robinson, S.S.; Kolesky, D.B.; Duarte, N.; Moisan, A.; Lewis, J.A. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl. Acad. Sci. USA 2019, 116, 5399–5404. [Google Scholar] [CrossRef]
- Paul, C.D.; Hung, W.-C.; Wirtz, D.; Konstantopoulos, K. Engineered models of confined cell migration. Annu. Rev. Biomed. Eng. 2016, 18, 159. [Google Scholar] [CrossRef]
- Wong, I.Y.; Javaid, S.; Wong, E.A.; Perk, S.; Haber, D.A.; Toner, M.; Irimia, D. Collective and individual migration following the epithelial–mesenchymal transition. Nat. Mater. 2014, 13, 1063–1071. [Google Scholar] [CrossRef]
- Bosch-Fortea, M.; Rodriguez-Fraticelli, A.E.; Herranz, G.; Hachimi, M.; Barea, M.D.; Young, J.; Ladoux, B.; Martin-Belmonte, F. Micropattern-based platform as a physiologically relevant model to study epithelial morphogenesis and nephrotoxicity. Biomaterials 2019, 218, 119339. [Google Scholar] [CrossRef]
- Xi, W.; Sonam, S.; Beng Saw, T.; Ladoux, B.; Teck Lim, C. Emergent patterns of collective cell migration under tubular confinement. Nat. Commun. 2017, 8, 1517. [Google Scholar] [CrossRef] [PubMed]
- Englezakis, A.; Gozalpour, E.; Kamran, M.; Fenner, K.; Mele, E.; Coopman, K. Development of a hollow fibre-based renal module for active transport studies. J. Artif. Organs 2021, 24, 473–484. [Google Scholar] [CrossRef]
- Oo, Z.Y.; Deng, R.; Hu, M.; Ni, M.; Kandasamy, K.; Ibrahim, M.S.b.; Ying, J.Y.; Zink, D. The performance of primary human renal cells in hollow fiber bioreactors for bioartificial kidneys. Biomaterials 2011, 32, 8806–8815. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Dai, H.; Heruth, D.P.; Alon, U.S.; Garola, R.E.; Zhou, J.; Duncan, R.S.; El-Meanawy, A.; McCarthy, E.T.; Sharma, R. Mechanotransduction signaling in podocytes from fluid flow shear stress. Am. J. Physiol. Ren. Physiol. 2018, 314, F22–F34. [Google Scholar] [CrossRef]
- Weinbaum, S.; Duan, Y.; Stalin, L.M.; Wang, T.; Weinstein, A. Mechanotransduction in the renal tubule. Am. J. Physiol. Ren. Physiol. 2010, 299, F1220–F1236. [Google Scholar] [CrossRef] [PubMed]
- Carrisoza-Gaytan, R.; Liu, Y.; Flores, D.; Else, C.; Lee, H.G.; Rhodes, G.; Sandoval, R.M.; Kleyman, T.R.; Lee, F.Y.-I.; Molitoris, B.; et al. Effects of biomechanical forces on signalling in the cortical collecting duct (CCD). Am. J. Physiol. Ren. Physiol. 2014, 307, F195–F204. [Google Scholar] [CrossRef]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. 1998, 54, S100–S108. [Google Scholar] [CrossRef]
- Raghavan, V.; Weisz, O. Discerning the role of mechanosensors in regulating proximal tubule function. Am. J. Physiol. Ren. Physiol. 2016, 310, F1–F5. [Google Scholar] [CrossRef][Green Version]
- Rennke, H.G.; Cotran, R.S.; Venkatachalam, M.A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J. Cell Biol. 1975, 67, 638–646. [Google Scholar] [CrossRef]
- Huang, C.; Bruggeman, L.A.; Hydo, L.M.; Miller, R.T. Shear stress induces cell apoptosis via a c-Src-phospholipase D-mTOR signaling pathway in cultured podocytes. Exp. Cell Res. 2012, 318, 1075–1085. [Google Scholar] [CrossRef]
- Yang, S.H.; Choi, J.W.; Huh, D.; Jo, H.A.; Kim, S.; Lim, C.S.; Lee, J.C.; Kim, H.C.; Kwon, H.M.; Jeong, C.W. Roles of fluid shear stress and retinoic acid in the differentiation of primary cultured human podocytes. Exp. Cell Res. 2017, 354, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Joshi, T.; Jiang, Y.; Heruth, D.P.; Rezaiekhaligh, M.H.; Novak, J.; Staggs, V.S.; Alon, U.S.; Garola, R.E.; El-Meanawy, A. Upregulated proteoglycan-related signaling pathways in fluid flow shear stress-treated podocytes. Am. J. Physiol. Ren. Physiol. 2020, 319, F312–F322. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; McCarthy, E.T.; Sharma, R.; Cudmore, P.A.; Sharma, M.; Johnson, M.L.; Bonewald, L.F. Prostaglandin E2 is crucial in the response of podocytes to fluid flow shear stress. J. Cell Commun. Signal. 2010, 4, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Eng, E.; Ballermann, B.J. Diminished NF-kB activation and PDGF-B expression in glomerular endothelial cells subjected to chronic shear stress. Microvasc. Res. 2003, 65, 137–144. [Google Scholar] [CrossRef]
- Slater, S.C.; Ramnath, R.D.; Uttridge, K.; Saleem, M.A.; Cahill, P.A.; Mathieson, P.W.; Welsh, G.I.; Satchell, S.C. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int. J. Biochem. Cell Biol. 2012, 44, 1482–1490. [Google Scholar] [CrossRef]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2021, 37, 973–982. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiologic, pathologic and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Zhuo, J.L.; Li, X.C. Proximal nephron. Compr. Physiol. 2013, 3, 1079. [Google Scholar] [CrossRef]
- Duan, Y.; Gotoh, N.; Yan, Q.; Du, Z.; Weinstein, A.M.; Wang, T.; Weinbaum, S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 11418–11423. [Google Scholar] [CrossRef]
- Jang, K.-J.; Cho, H.S.; Kang, D.H.; Bae, W.G.; Kwon, T.-H.; Suh, K.-Y. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr. Biol. 2011, 3, 134–141. [Google Scholar] [CrossRef]
- Long, K.R.; Shipman, K.E.; Rbaibi, Y.; Menshikova, E.V.; Ritov, V.B.; Eshbach, M.L.; Jiang, Y.; Jackson, E.K.; Baty, C.J.; Weisz, O.A. Proximal tubule apical endocytosis is modulated by fluid shear stress via an mTOR-dependent pathway. Mol. Biol. Cell 2017, 28, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, V.; Rbaibi, Y.; Pastor-Soler, N.M.; Carattino, M.D.; Weisz, O.A. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc. Natl. Acad. Sci. USA 2014, 111, 8506–8511. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Gliozzi, M.L.; Rittenhouse, N.L.; Edmunds, L.R.; Rbaibi, Y.; Locker, J.D.; Poholek, A.C.; Jurczak, M.J.; Baty, C.J.; Weisz, O.A. Shear stress and oxygen availability drive differential changes in opossum kidney proximal tubule cell metabolism and endocytosis. Traffic 2019, 20, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Rein, J.L.; Heja, S.; Flores, D.; Carrisoza-Gaytán, R.; Lin, N.Y.; Homan, K.A.; Lewis, J.A.; Satlin, L.M. Effect of luminal flow on doming of mpkCCD cells in a 3D perfusable kidney cortical collecting duct model. Am. J. Physiol. Cell Physiol. 2020, 319, C136–C147. [Google Scholar] [CrossRef]
- Kimura, H.; Nishikawa, M.; Yanagawa, N.; Nakamura, H.; Miyamoto, S.; Hamon, M.; Hauser, P.; Zhao, L.; Jo, O.D.; Komeya, M. Effect of fluid shear stress on in vitro cultured ureteric bud cells. Biomicrofluidics 2018, 12, 44107. [Google Scholar] [CrossRef]
- Ferrell, N.; Cheng, J.; Miao, S.; Roy, S.; Fissell, W.H. Orbital shear stress regulates differentiation and barrier function of primary renal tubular epithelial cells. ASAIO J. 2018, 64, 766. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S. Kidney-on-a-chip: A new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr. Drug Metab. 2018, 19, 577–583. [Google Scholar] [CrossRef]
- Ajay, A.K. Functional drug screening using kidney cells on-a-chip: Advances in disease modeling and development of biomarkers. Kidney360 2022, 3, 194. [Google Scholar] [CrossRef]
- Fukuda, Y.; Kaishima, M.; Ohnishi, T.; Tohyama, K.; Chisaki, I.; Nakayama, Y.; Ogasawara-Shimizu, M.; Kawamata, Y. Fluid shear stress stimulates MATE2-K expression via Nrf2 pathway activation. Biochem. Biophys. Res. Commun. 2017, 484, 358–364. [Google Scholar] [CrossRef]
- Jayagopal, A.; Brakeman, P.R.; Soler, P.; Ferrell, N.; Fissell, W.; Kroetz, D.L.; Roy, S. Apical shear stress enhanced organic cation transport in human OCT2/MATE1-transfected madin-darby canine kidney cells involves ciliary sensing. J. Pharmacol. Exp. Ther. 2019, 369, 523–530. [Google Scholar] [CrossRef]
- Yin, L.; Du, G.; Zhang, B.; Zhang, H.; Yin, R.; Zhang, W.; Yang, S.-M. Efficient drug screening and nephrotoxicity assessment on co-culture microfluidic kidney chip. Sci. Rep. 2020, 10, 6568. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Peters, J.G.; Nieskens, T.T.; Škovroňová, R.; Blaimschein, N.; Schmidts, M.; Roepman, R.; Schirris, T.J.; Russel, F.G.; Masereeuw, R. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129433. [Google Scholar] [CrossRef] [PubMed]
- Rennke, H.; Venkatachalam, M. Glomerular permeability of macromolecules. Effect of molecular configuration on the fractional clearance of uncharged dextran and neutral horseradish peroxidase in the rat. J. Clin. Investig. 1979, 63, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H.; Miner, J.H. What is the glomerular ultrafiltration barrier? J. Am. Soc. Nephrol. 2018, 29, 2262–2264. [Google Scholar] [CrossRef]
- Jarad, G.; Miner, J.H. Update on the glomerular filtration barrier. Curr. Opin. Nephrol. Hypertens. 2009, 18, 226–232. [Google Scholar] [CrossRef]
- Hunt, J.L.; Pollock, M.R.; Denker, B.M. Cultured podocytes establish a size-selective barrier regulated by specific signaling pathways and demonstrate synchronized barrier assembly in a calcium switch model of junction formation. J. Am. Soc. Nephrol. 2005, 16, 1593–1602. [Google Scholar] [CrossRef]
- Lee, E.Y.; Chung, C.H.; Khoury, C.C.; Yeo, T.K.; Pyagay, T.E.; Wang, A.; Chen, S. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am. J. Physiol. Ren. Physiol. 2009, 297, F85–F94. [Google Scholar] [CrossRef]
- Xie, R.; Korolj, A.; Liu, C.; Song, X.; Lu, R.X.Z.; Zhang, B.; Ramachandran, A.; Liang, Q.; Radisic, M. h-FIBER: Microfluidic topographical hollow fiber for studies of glomerular filtration barrier. ACS Cent. Sci. 2020, 6, 903–912. [Google Scholar] [CrossRef]
- Endlich, N.; Kress, K.R.; Reiser, J.; Uttenweiler, D.; Kriz, W.; Mundel, P.; Endlich, K. Podocytes respond to mechanical stress in vitro. J. Am. Soc. Nephrol. 2001, 12, 413–422. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, J.-S.; Ko, Y.-C.; Chen, J.-W.; Chu, H.-Y.; Lu, C.-S.; Chu, C.-W.; Hsu, H.-H.; Tseng, F.-G. A microfluidic platform for investigating transmembrane pressure-induced glomerular leakage. Micromachines 2018, 9, 228. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Douville, N.J.; Tung, Y.-C.; Li, R.; Wang, J.D.; El-Sayed, M.E.; Takayama, S. Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal. Chem. 2010, 82, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, A.; Schavemaker, F.; Kosim, K.; Kurek, D.; Haarmans, M.; Bulst, M.; Lee, K.; Wegner, S.; Hankemeier, T.; Joore, J. High throughput transepithelial electrical resistance (TEER) measurements on perfused membrane-free epithelia. Lab Chip 2021, 21, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Kim, K.H.; Jabbar, F.; Kim, S.; Choi, K.H. Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule. Microfluid. Nanoflu. 2020, 24, 43. [Google Scholar] [CrossRef]
- Cohen, A.; Ioannidis, K.; Ehrlich, A.; Regenbaum, S.; Cohen, M.; Ayyash, M.; Tikva, S.S.; Nahmias, Y. Mechanism and reversal of drug-induced nephrotoxicity on a chip. Sci. Transl. Med. 2021, 13, eabd6299. [Google Scholar] [CrossRef]
- Thomas, S.C.; Blantz, R.C. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J. Am. Soc. Nephrol. 2008, 19, 2272–2275. [Google Scholar] [CrossRef]
- Gewin, L.; Zent, R.; Pozzi, A. Progression of chronic kidney disease: Too much cellular talk causes damage. Kidney Int. 2017, 91, 552–560. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M. Intravital multiphoton microscopy of dynamic renal processes. Am. J. Physiol. Ren. Physiol. 2005, 288, F1084–F1089. [Google Scholar] [CrossRef]
- Kang, J.J.; Toma, I.; Sipos, A.; McCulloch, F.; Peti-Peterdi, J. Quantitative imaging of basic function in renal (patho) physiology. Am. J. Physiol. Ren. Physiol. 2006, 297, F495–F502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Gust, M.; Ferrell, N. Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration. Sensors 2022, 22, 6889. https://doi.org/10.3390/s22186889
Wang D, Gust M, Ferrell N. Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration. Sensors. 2022; 22(18):6889. https://doi.org/10.3390/s22186889
Chicago/Turabian StyleWang, Dan, Matthew Gust, and Nicholas Ferrell. 2022. "Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration" Sensors 22, no. 18: 6889. https://doi.org/10.3390/s22186889
APA StyleWang, D., Gust, M., & Ferrell, N. (2022). Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration. Sensors, 22(18), 6889. https://doi.org/10.3390/s22186889





