High-Sensitivity Optical Fiber-Based Glucose Sensor Using Helical Intermediate-Period Fiber Grating
Abstract
:1. Introduction
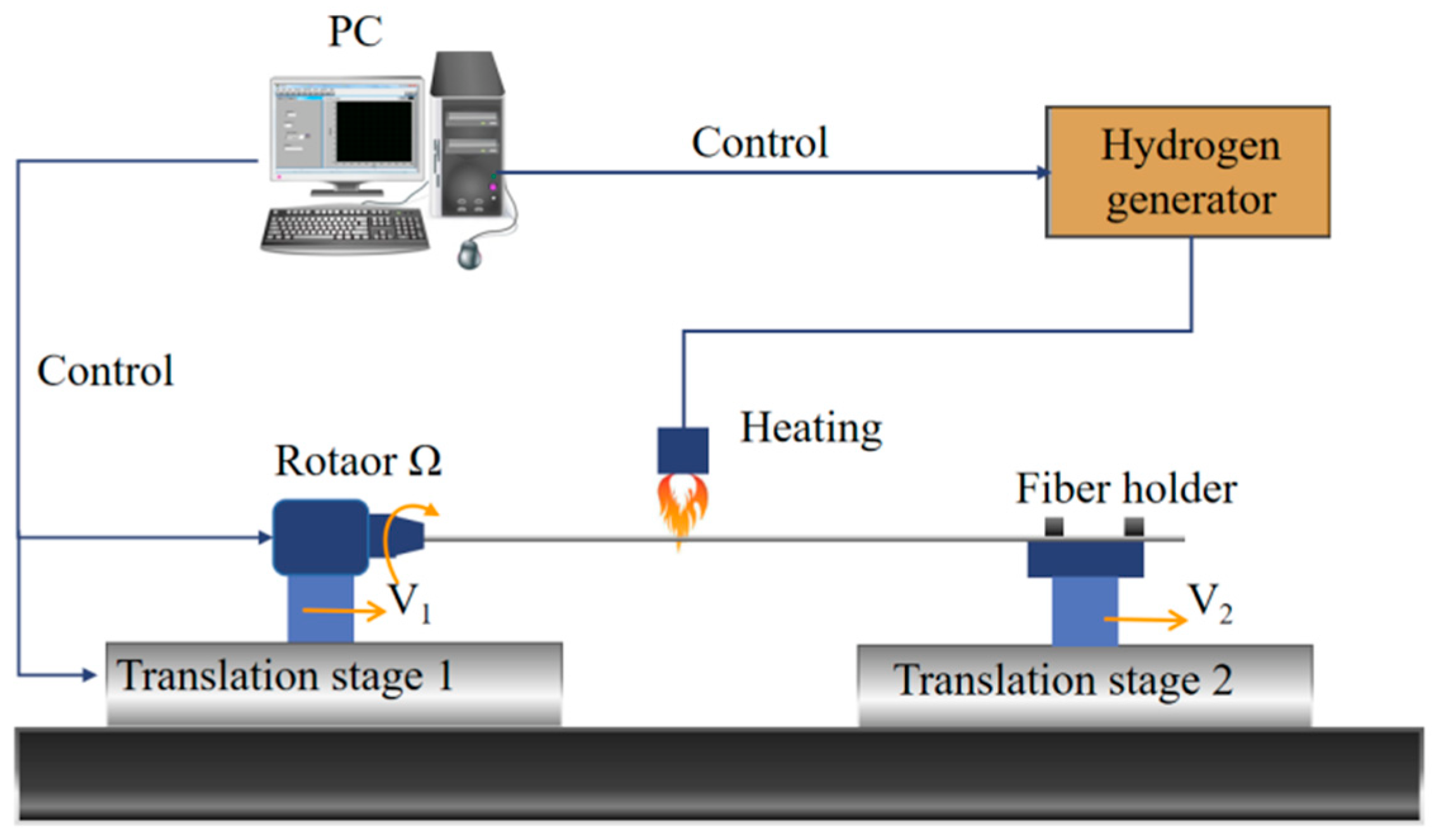
2. Materials and Methods
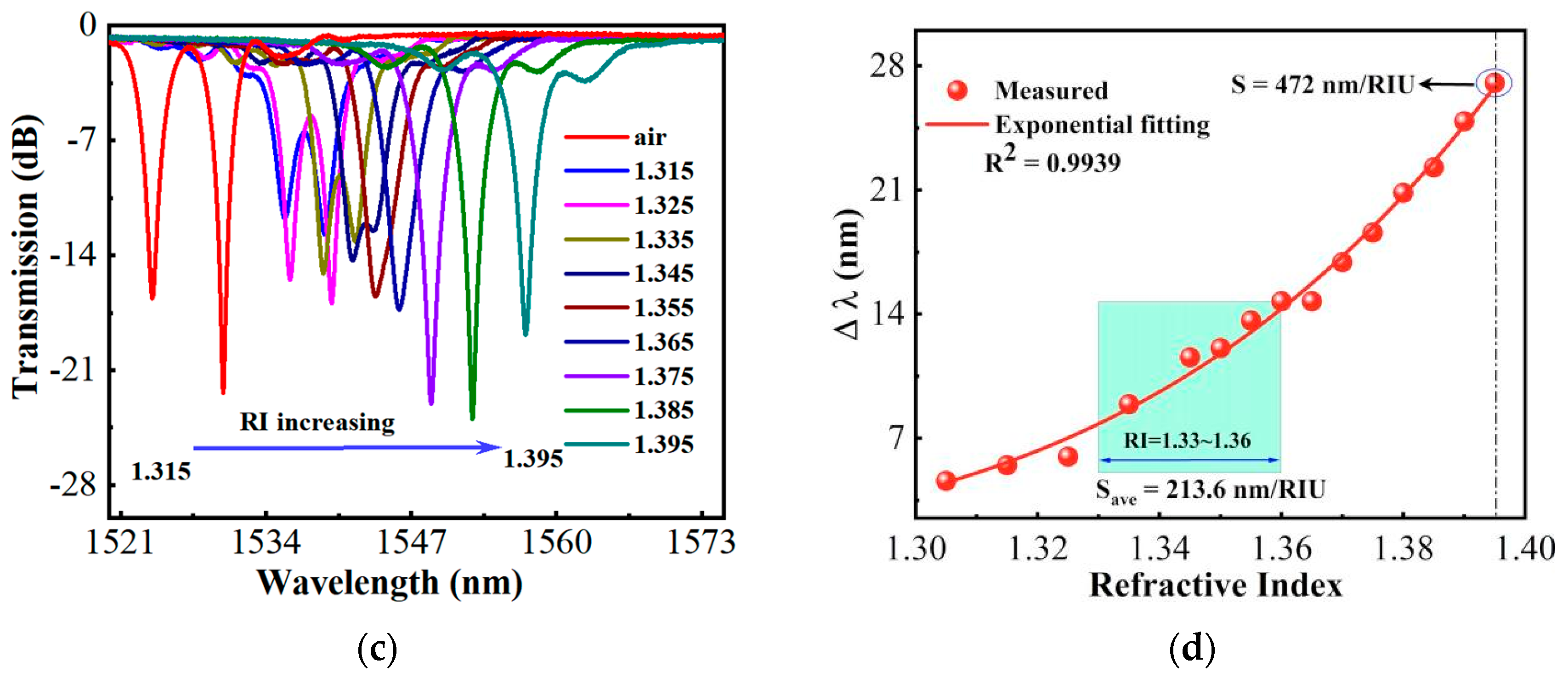
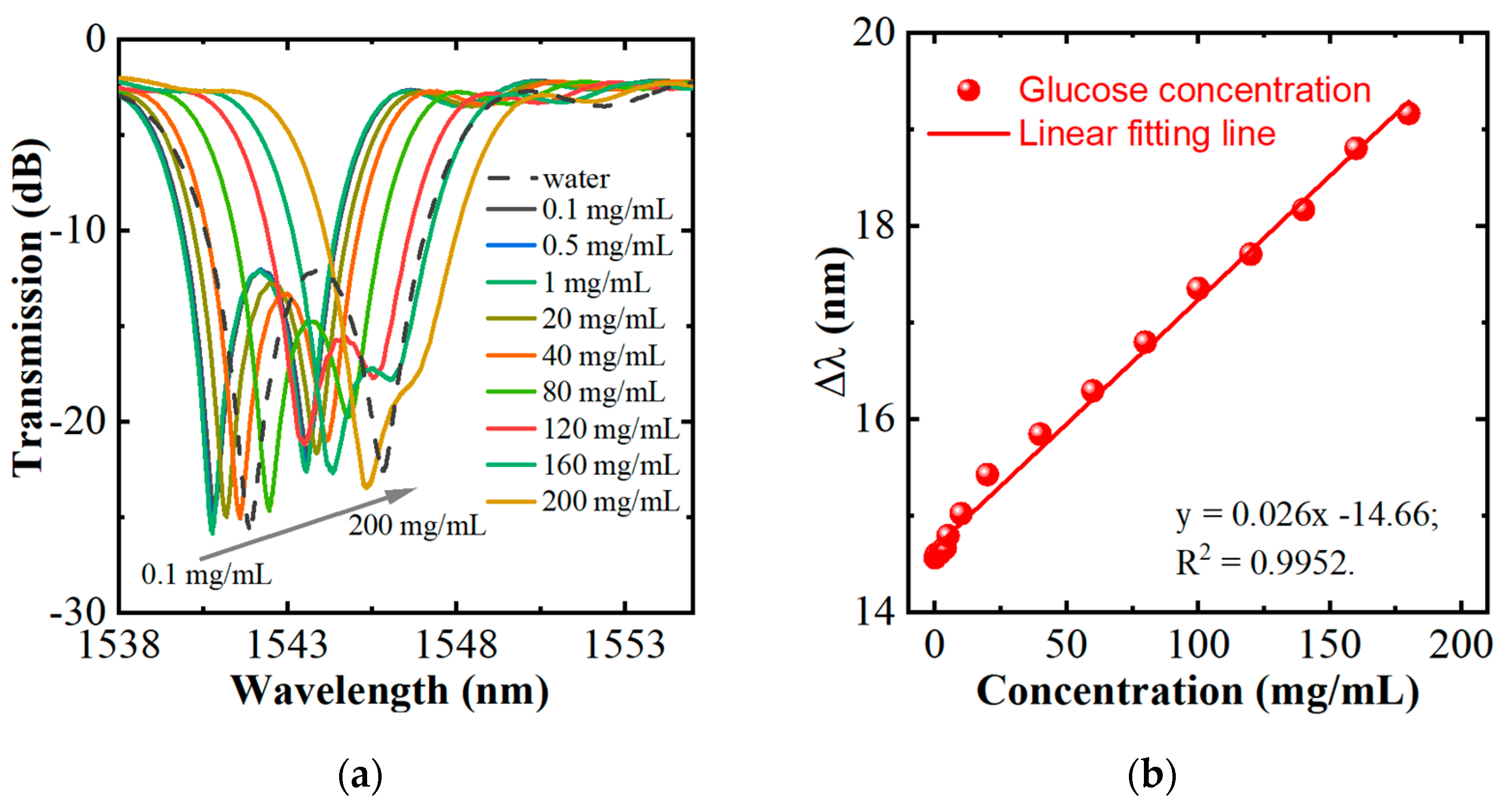
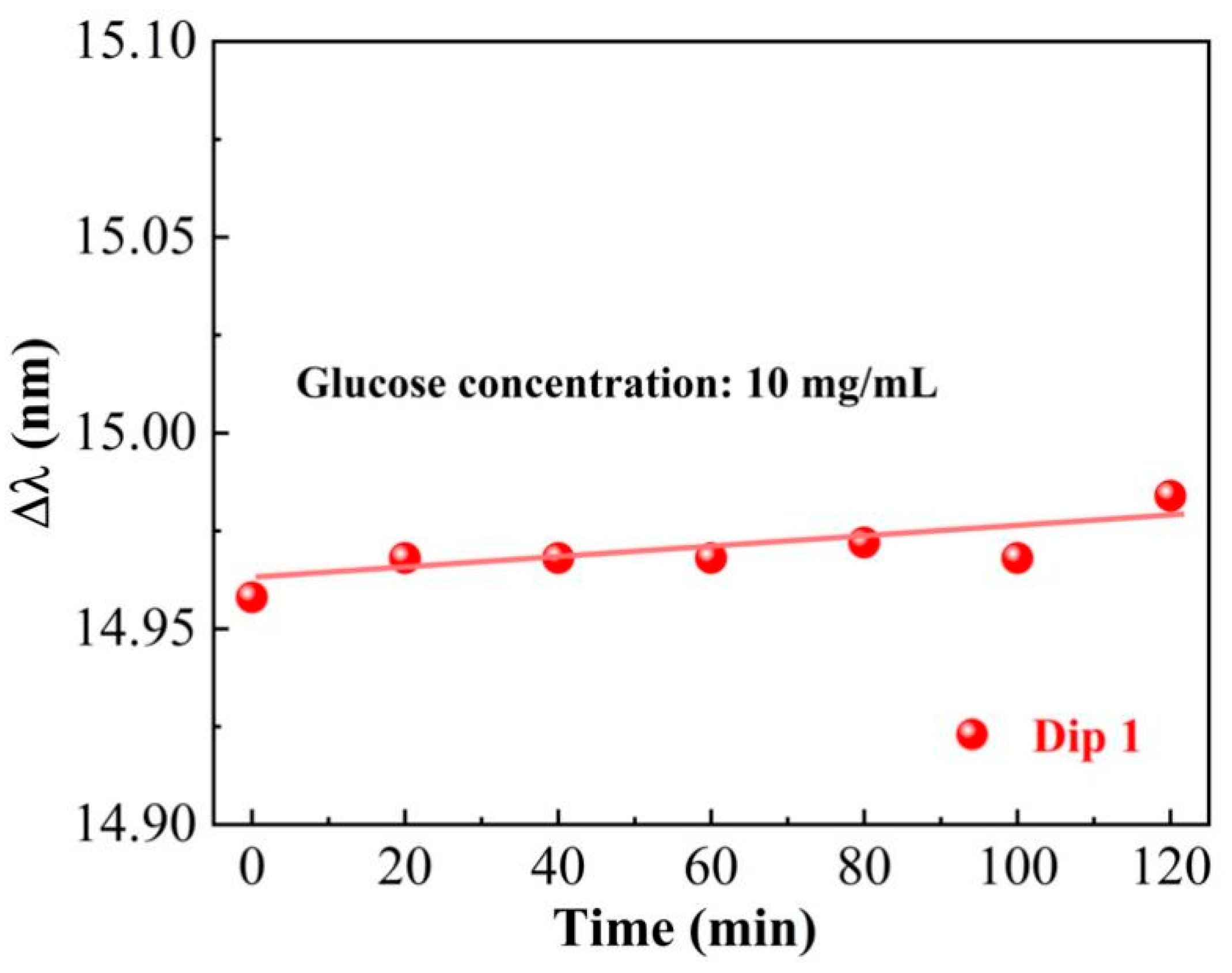
3. Glucose-Sensing Properties of the HIPFG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, K.; Zhang, D.; Wu, D.; Wei, H.; Lu, G.M. Design of a breath analysis system for diabetes screening and blood glucose level prediction. IEEE Trans. Biomed. Eng. 2014, 61, 2787–2795. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.J.; Yang, D.W.; Xiao, H.L.; Fu, Y.C.; Tan, Y.M.; Yao, S.Z. Recent advances in electrochemical glucose biosensors: A review. Rsc Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Yu, J.H.; Ge, L.; Huang, J.D.; Wang, S.M.; Ge, S.G. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Li, H.; Zhao, Q.; Zhu, R.; Yang, Y.; Jia, Q.; Bian, B.; Zhuo, L. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of porphyrin-Fe3O4 nanocomposites. Mater. Sci. Eng. C 2014, 41, 142–151. [Google Scholar] [CrossRef]
- Binu, S.; Pillai, V.P.M.; Pradeepkumar, V.; Padhy, B.B.; Joseph, C.S.; Chandrasekaran, N. Fibre optic glucose sensor. Mater. Sci. Eng. C 2009, 29, 183–186. [Google Scholar] [CrossRef]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Badmos, A.A.; Sun, Q.Z.; Sun, Z.Y.; Zhang, J.X.; Yan, Z.J.; Lutsyk, P.; Rozhin, A.; Zhang, L. Enzyme-functionalized thin-cladding long-period fiber grating in transition mode at dispersion turning point for sugar-level and glucose detection. J. Biomed. Opt. 2007, 22, 027003. [Google Scholar] [CrossRef]
- Yang, R.Z.; Dong, W.F.; Meng, X.; Zhang, X.L.; Sun, Y.L.; Hao, Y.W.; Guo, J.C.; Zhang, W.Y.; Yu, Y.S.; Song, J.F.; et al. Nanoporous TiO2/polyion thin-film-coated long-period grating sensors for the direct measurement of low-molecular-weight analytes. Langmuir 2012, 28, 8814–8821. [Google Scholar] [CrossRef]
- Novais, S.; Ferreira, C.I.A.; Ferreira, M.S.; Pinto, A.J.L. Optical fiber tip sensor for the measurement of glucose aqueous solutions. IEEE Photonics J. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Huang, J.; Ding, L.Y.; Cai, J. Graphene oxide-functionalized long period fiber grating for ultrafast label-free glucose biosensor. Mater. Sci. Eng. C 2020, 107, 110329. [Google Scholar] [CrossRef]
- Wu, C.W. S-shaped long period fiber grating glucose concentration biosensor based on immobilized glucose oxidase. Optik 2020, 203, 163960. [Google Scholar] [CrossRef]
- Luo, B.B.; Yan, Z.J.; Sun, Z.Y.; Li, J.F.; Zhang, L. Novel glucose sensor based on enzyme-immobilized 81 tilted fiber grating. Opt. Express 2014, 22, 30571–30578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.Q.; Zhou, K.M.; Wang, C.L.; Sun, Q.Z.; Yin, G.L.; Tai, Z.J.; Wilsone, K.; Zhao, J.L.; Zhang, L. Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sens. Actuator B Chem. 2018, 254, 1033–1039. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Abdulhalim, I. Spectral interrogation based SPR sensor for blood glucose detection with improved sensitivity and stability. Biosens. Bioelectron. 2015, 6, 10–12. [Google Scholar]
- Li, Y.P.; Ma, H.; Gan, L.; Liu, Q.; Yan, Z.J.; Liu, D.M.; Sun, Q.Z. Immobilized optical fiber microprobe for selective and high sensitive glucose detection. Sens. Actuators B 2018, 255, 3004–3010. [Google Scholar] [CrossRef]
- Lidiya, A.E.; Raja, R.V.J.; Ngo, M.Q.; Vigneswaran, D. Detecting hemoglobin content blood glucose using surface plasmon resonance in D-shaped photonic crystal fiber. Opt. Fiber Technol. 2019, 50, 132–138. [Google Scholar] [CrossRef]
- Brice, I.; Grundsteins, K.; Atvars, A.; Alnis, J.; Viter, R.; Ramanavicius, A. Whispering gallery mode resonator and glucose oxidase based glucose biosensor. Sens. Actuators B Chem. 2020, 318, 128004. [Google Scholar] [CrossRef]
- Kopp, V.I.; Churikov, V.M.; Singer, J.; Chao, N.; Neugroschl, D.; Genack, A.Z. Chiral fiber gratings. Science 2004, 305, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Zhong, J.L.; Liu, S.; Zhu, G.X.; Zhao, Y.Y.; Luo, J.X.; Lu, S.Z.; Zhang, Q.; He, J.; Bai, Z.Y.; et al. Helical Intermediate-period Fiber Grating for Refractive Index Measurements with Low-Sensitive Temperature and Torsion Response. J. Light. Technol. 2021, 39, 6678–6685. [Google Scholar] [CrossRef]
- Zhong, J.L.; Liu, S.; Zou, T.; Yan, W.Q.; Zhou, M.; Liu, B.N.; Rao, X.; Wang, Y.; Sun, Z.Y.; Wang, A.P. All Fiber-Optic Immunosensors Based on Elliptical Core Helical Intermediate-Period Fiber Grating with Low-Sensitivity to Environmental Disturbances. Biosensors 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Fu, C.L.; Bai, Z.Y.; Li, Z.L.; Liao, C.R.; Wang, Y.; He, J.; Liu, Y.; Wang, Y.P. Temperature insensitivity polarization-controlled orbital angular momentum mode converter based on an LPFG induced in four-mode fiber. Sensors 2018, 18, 1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Y.; Liu, S.; Luo, J.X.; Chen, Y.P.; Fu, C.L.; Xiong, C.; Wang, Y.; Jing, S.Y.; Bai, Z.Y.; Liao, C.R.; et al. Torsion, Refractive Index, and Temperature Sensors Based on An Improved Helical Long Period Fiber Grating. J. Lightwave Technol. 2020, 38, 2504–2510. [Google Scholar] [CrossRef]
- Fu, C.L.; Wang, Y.P.; Liu, S.; Bai, Z.Y.; Tang, J.; Shao, L.P.; Liu, X.Y. Transverse-load, strain, temperature, and torsion sensors based on a helical photonic crystal fiber. Opt. Lett. 2019, 44, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, W.Q.; Zhong, J.L.; Zou, T.; Zhou, M.; Chen, P.J.; Xiao, H.; Liu, B.N.; Bai, Z.Y.; Wang, Y.P. Compact breath monitoring based on helical intermediate-period fiber grating. Sens. Actuator B Chem. 2022, 369, 132372. [Google Scholar] [CrossRef]
- Yan, Z.J.; Wang, H.S.; Wang, C.L.; Sun, Z.Y.; Yin, G.L.; Zhou, K.M.; Wang, Y.S.; Zhao, W.; Zhang, L. Theoretical and experimental analysis of excessively tilted fiber gratings. Opt. Express 2016, 24, 12107–12115. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.Y.; Li, Z.H.; Zheng, H.R.; Jiang, B.Q.; Albert, J.; Zhao, J.L. Strong cladding mode excitation in ultrathin fiber inscribed Bragg grating with ultraviolet photosensitivity. Opt. Express 2020, 30, 25936–25945. [Google Scholar] [CrossRef]
- Xuewen, S.; Lin, Z.; Bennion, I. Sensitivity characteristics of long-period fiber gratings. J. Lightwave Technol. 2002, 20, 255–266. [Google Scholar] [CrossRef]
- Kurmoo, Y.; Hook, A.L.; Harvey, D.; Dubern, J.F.; Williams, P.; Morgan, S.P.; Korposh, S.; Alexander, M.R. Real time monitoring of biofilm formation on coated medical devices for the reduction and interception of bacterial infections. Biomater. Sci. 2020, 8, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.Q.; Li, X.G.; Zhou, X.; Zhang, Y.N.; Chen, N.; Wang, S.K.; Zhang, S.Q.; Zhao, Y. Optical fiber sensors for glucose concentration measurement: A review. Opt. Laser Technol. 2021, 139, 106981. [Google Scholar] [CrossRef]
- Yasin, M.; Irawati, N.; Harun, S.W.; Ahmad, F.; Khasana, M. Sodium nitrate (NaNO3) sensor based on graphene coated microfiber. Measurement 2019, 146, 208–214. [Google Scholar] [CrossRef]



| Sensor Type | External Material | SRI Sensitivity | Sensing Range | Glucose Sensitivity | Temperature Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| TFG | GO 1 and GOD 2 | 128 nm/RIU | 0–8 mM | 1.33 nm/(mg/mL) | - | [13] |
| LPG | GOD | - | 0–1.2 mg/mL | 0.77 nm/(mg/mL) | - | [10] |
| S-shape LPG | GOD | - | 0–1% | 0.64 dB/(mg/mL) | - | [11] |
| HIPFG | Not required | 472 nm/RIU | 0–200 mg/mL | 0.026 nm/(mg/mL) | 3.67 pm/°C | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Liu, S.; Zou, T.; Yan, W.; Chen, P.; Liu, B.; Sun, Z.; Wang, Y. High-Sensitivity Optical Fiber-Based Glucose Sensor Using Helical Intermediate-Period Fiber Grating. Sensors 2022, 22, 6824. https://doi.org/10.3390/s22186824
Zhong J, Liu S, Zou T, Yan W, Chen P, Liu B, Sun Z, Wang Y. High-Sensitivity Optical Fiber-Based Glucose Sensor Using Helical Intermediate-Period Fiber Grating. Sensors. 2022; 22(18):6824. https://doi.org/10.3390/s22186824
Chicago/Turabian StyleZhong, Junlan, Shen Liu, Tao Zou, Wenqi Yan, Peijing Chen, Bonan Liu, Zhongyuan Sun, and Yiping Wang. 2022. "High-Sensitivity Optical Fiber-Based Glucose Sensor Using Helical Intermediate-Period Fiber Grating" Sensors 22, no. 18: 6824. https://doi.org/10.3390/s22186824
APA StyleZhong, J., Liu, S., Zou, T., Yan, W., Chen, P., Liu, B., Sun, Z., & Wang, Y. (2022). High-Sensitivity Optical Fiber-Based Glucose Sensor Using Helical Intermediate-Period Fiber Grating. Sensors, 22(18), 6824. https://doi.org/10.3390/s22186824







