Design and Validation of a Custom-Made Laboratory Hyperspectral Imaging System for Biomedical Applications Using a Broadband LED Light Source
Abstract
:1. Introduction
2. Materials and Methods
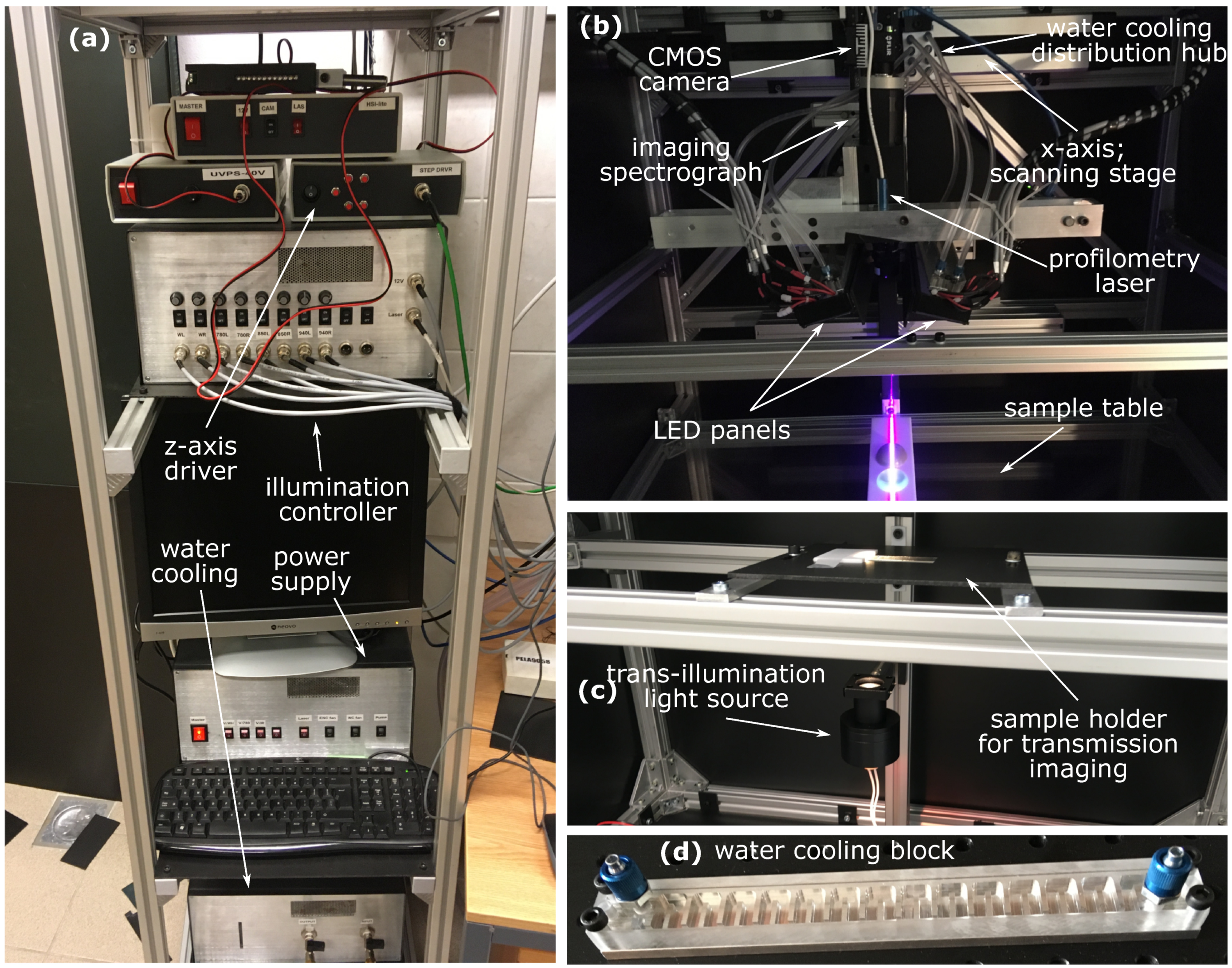
2.1. HSI System
- The system should be able to image samples of the size of a human hand with a field-of-view (FOV) of 20–30 cm and with the spatial resolution of approximately 100 µm. Additionally, it should be possible to vary both FOV and spatial resolution to suit a particular application.
- The system should offer a spectral range from 400 nm to 1000 nm (dictated by tissue native chromophores, i.e., tissue components absorbing light), while the spectral resolution should be below 10 nm and preferably close to 1 nm in order to study the fine spectral features of chromophores.
- The illumination system should not heat samples, while the object’s shape and thickness information should also be obtained to enable quantitative imaging in transmission and reflection geometry using corrections for sample curvature and thickness.
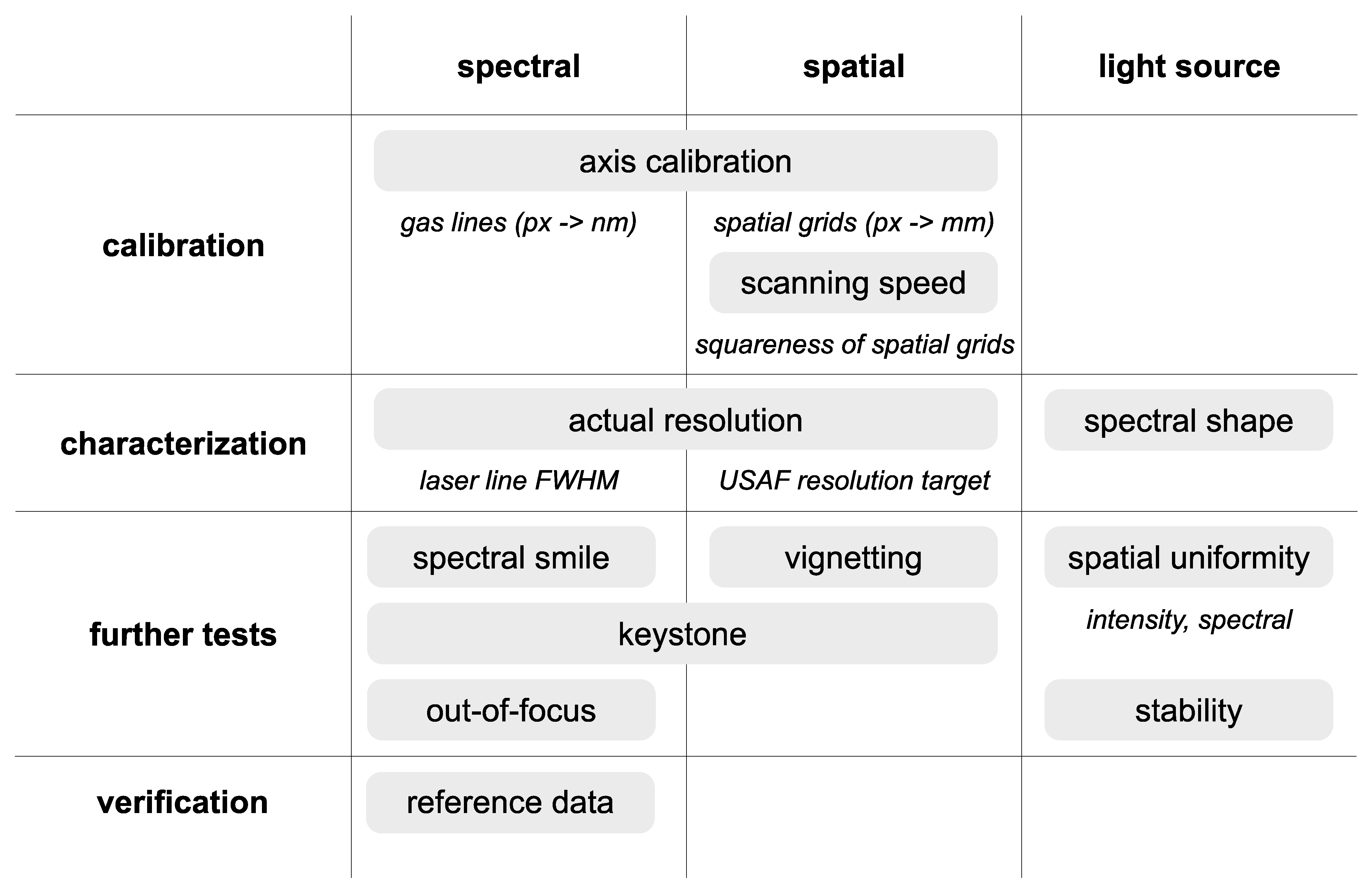
2.2. Verification and Calibration Protocols
3. Results
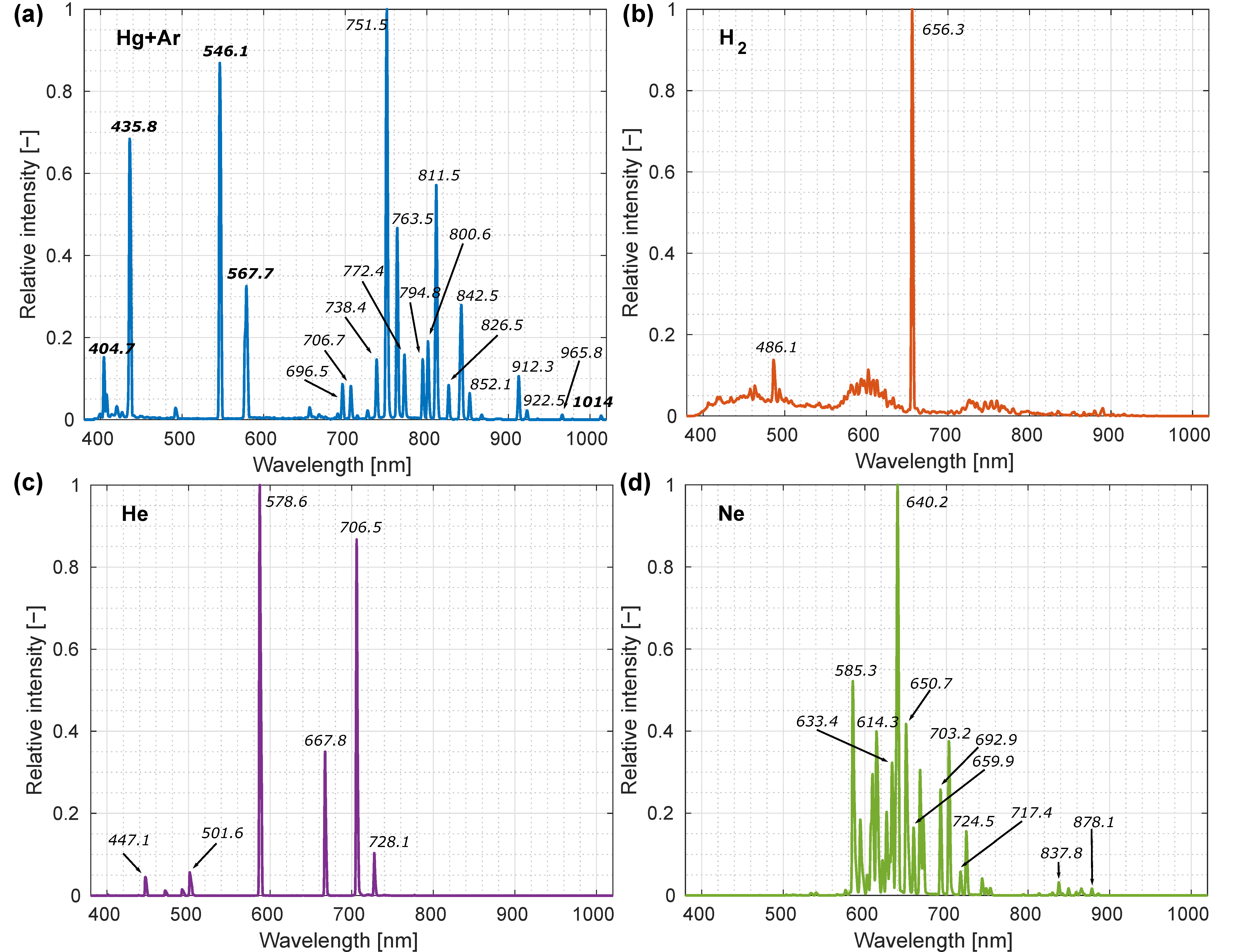
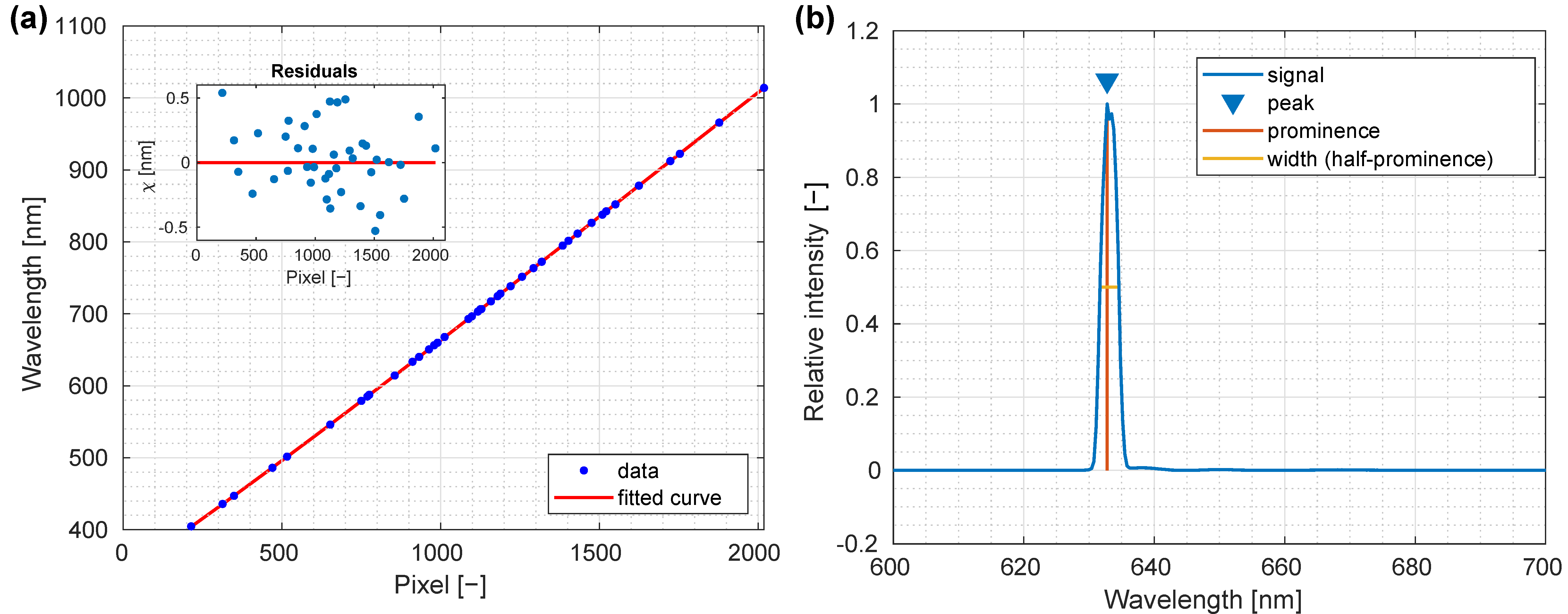
3.1. Spectral Calibration and Characterization
3.2. Spatial Calibration and Characterization
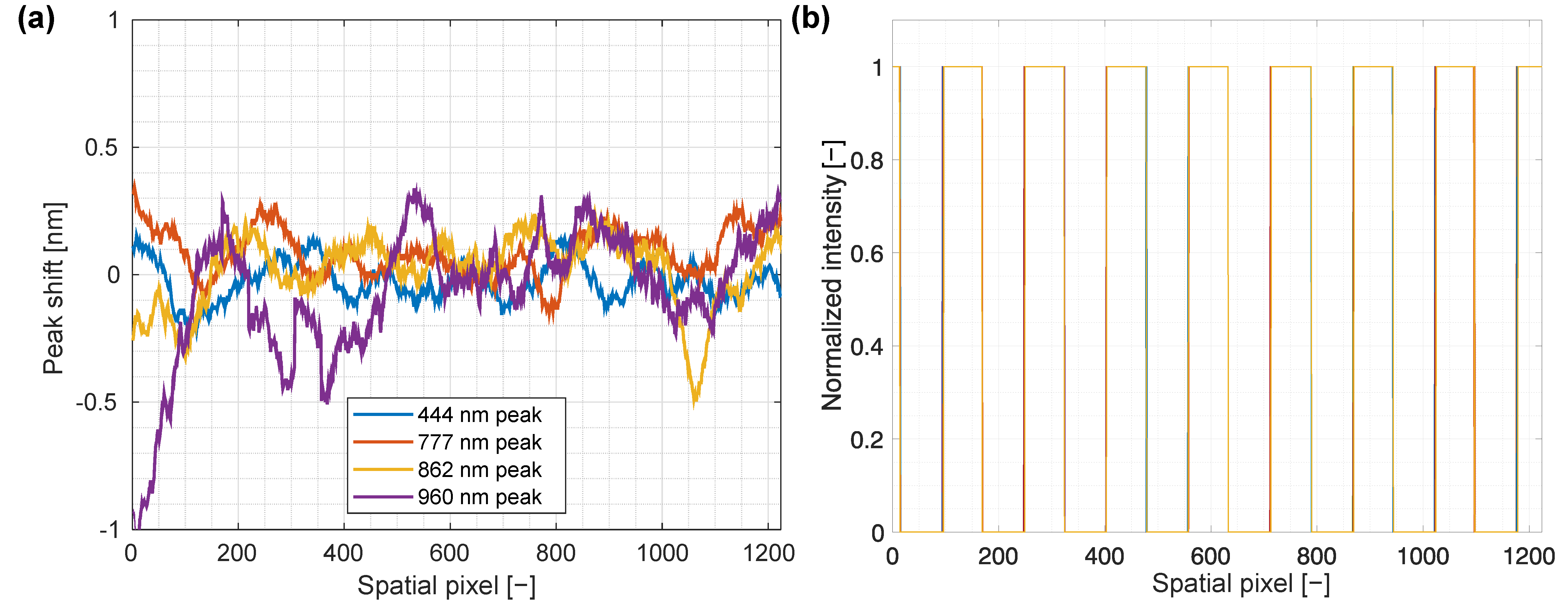
3.3. Spectral Smile and Keystone Analysis
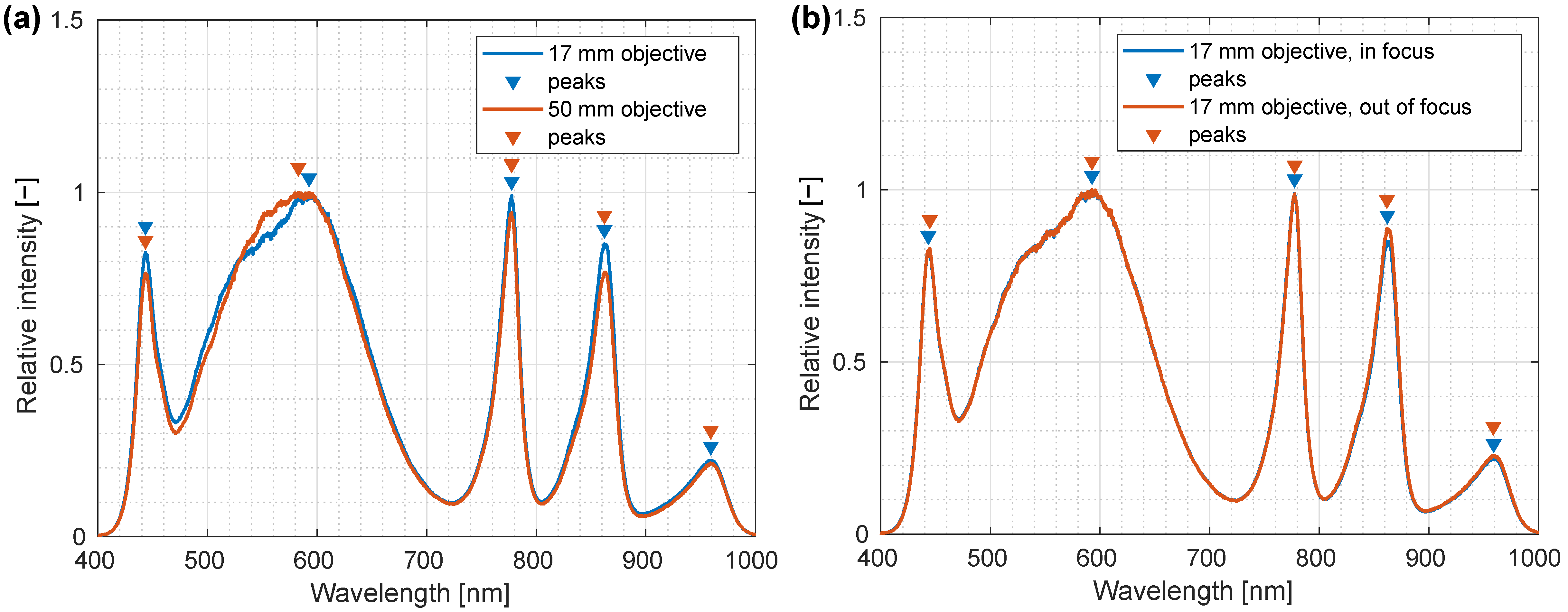
3.4. Testing Lens and Out-of-Focus Effects
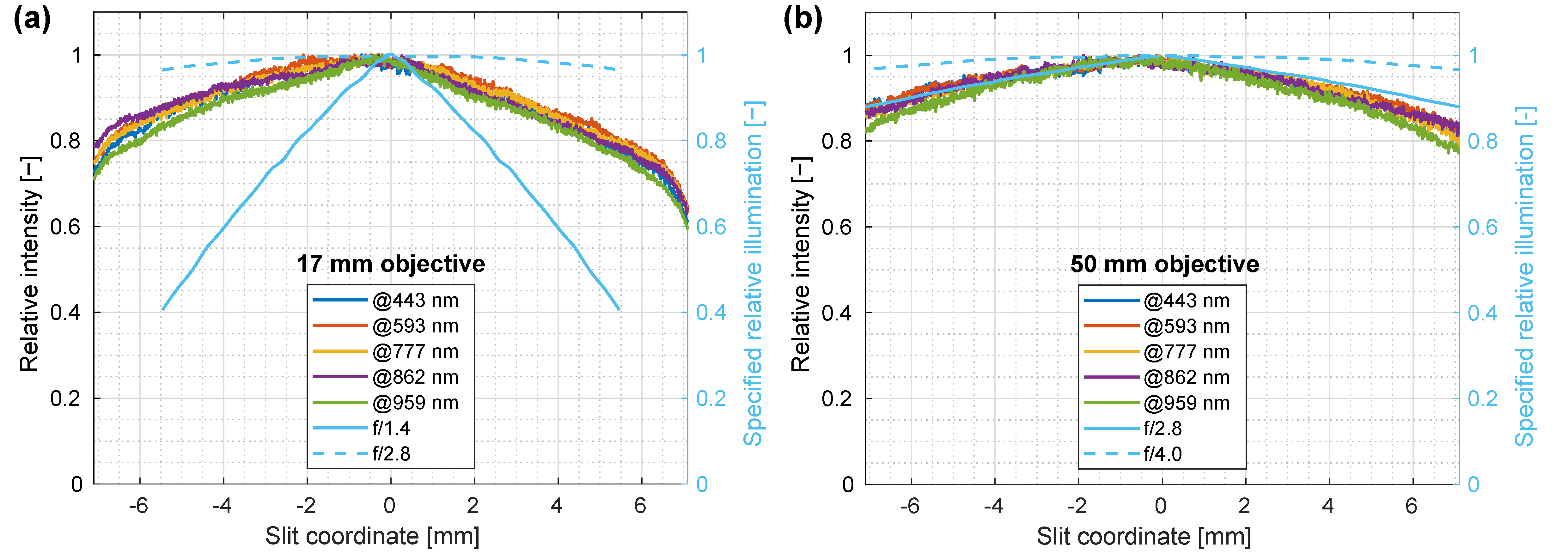
3.5. Spatial Homogeneity of Illumination
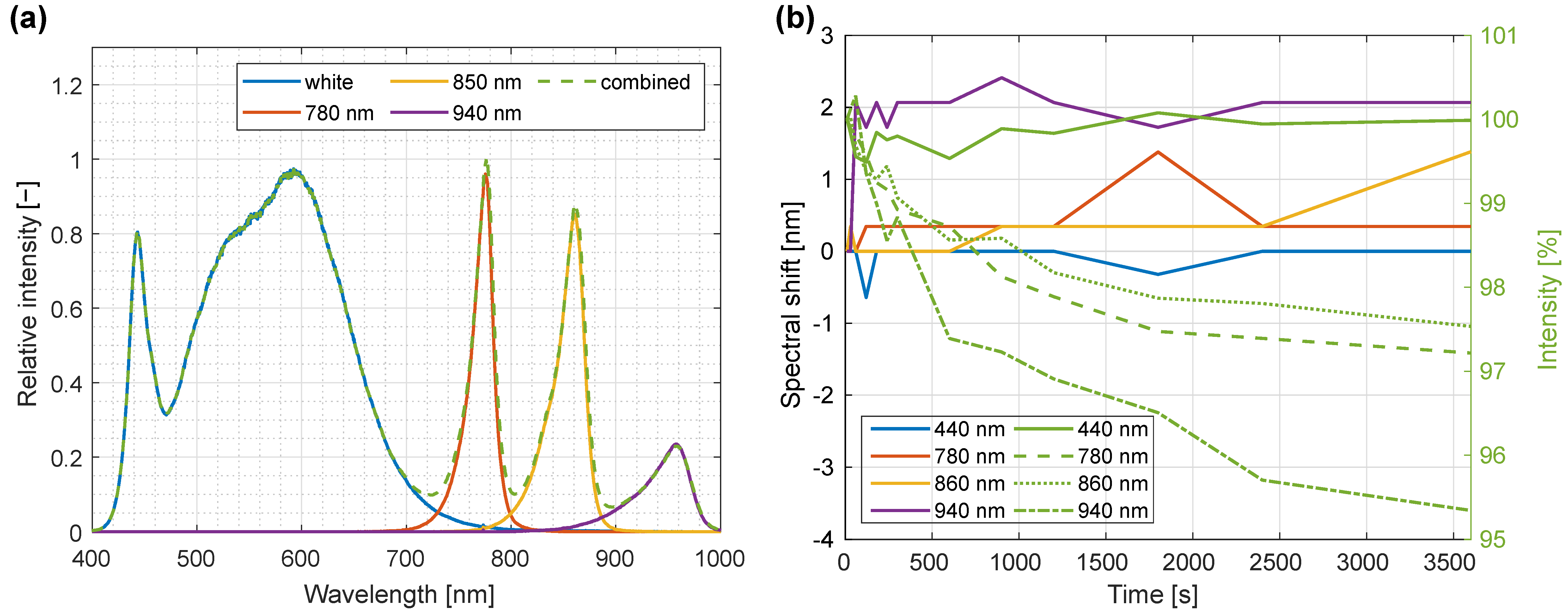
3.6. Illumination Composition and Temporal Spectral Stability of LED Light Source
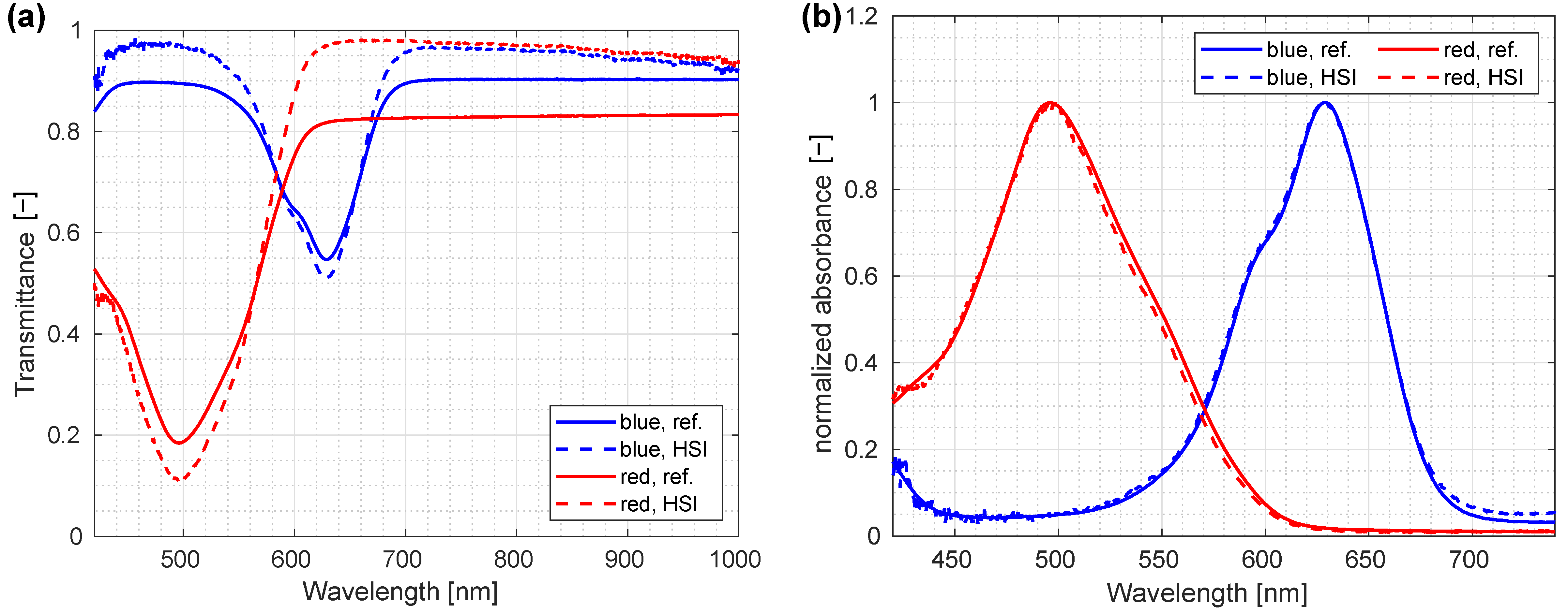
3.7. Verification against a Reference Instrument
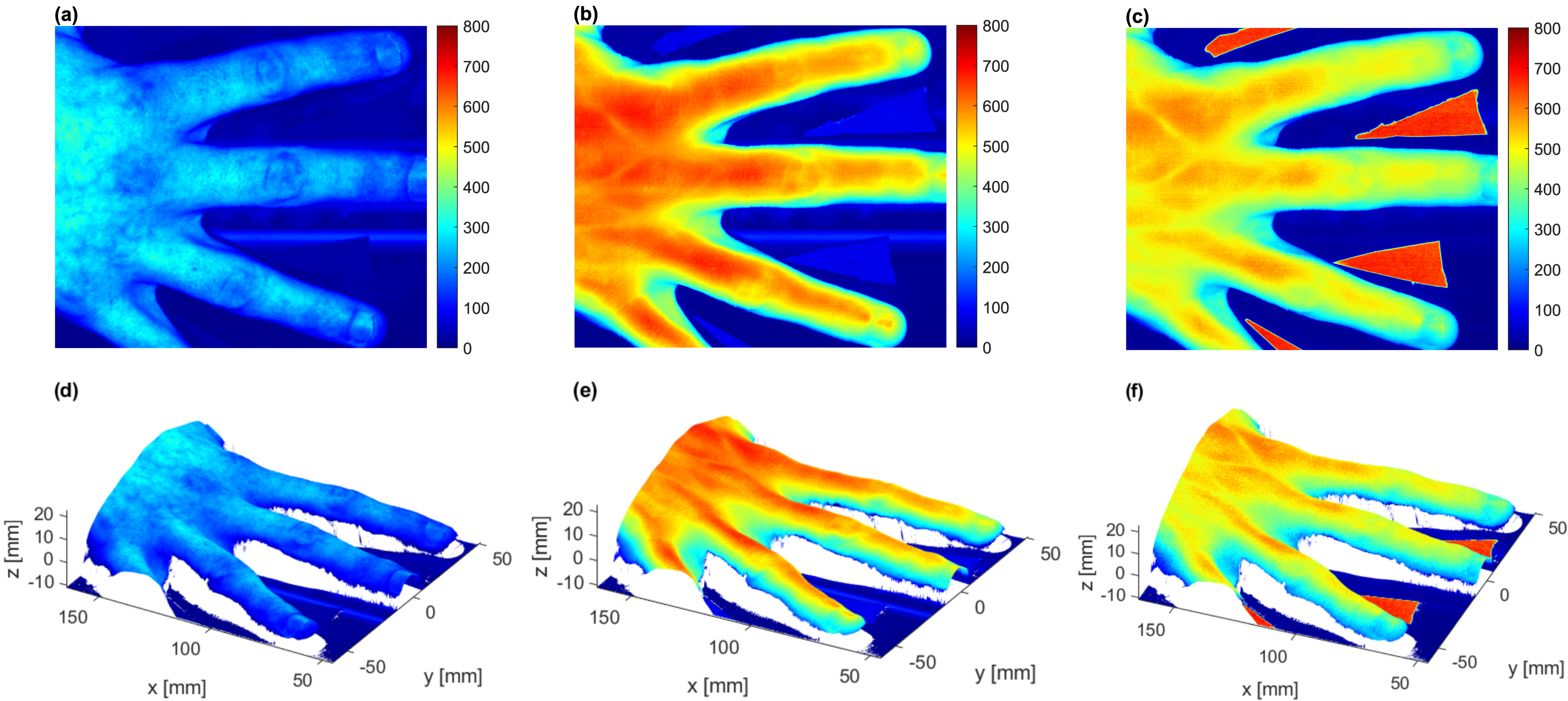
3.8. Example #1 of HSI-System Application in the Biomedical Field: Imaging of a Human Hand
3.9. Example #2 of HSI-System Application in the Biomedical Field: Murine Tumor Model
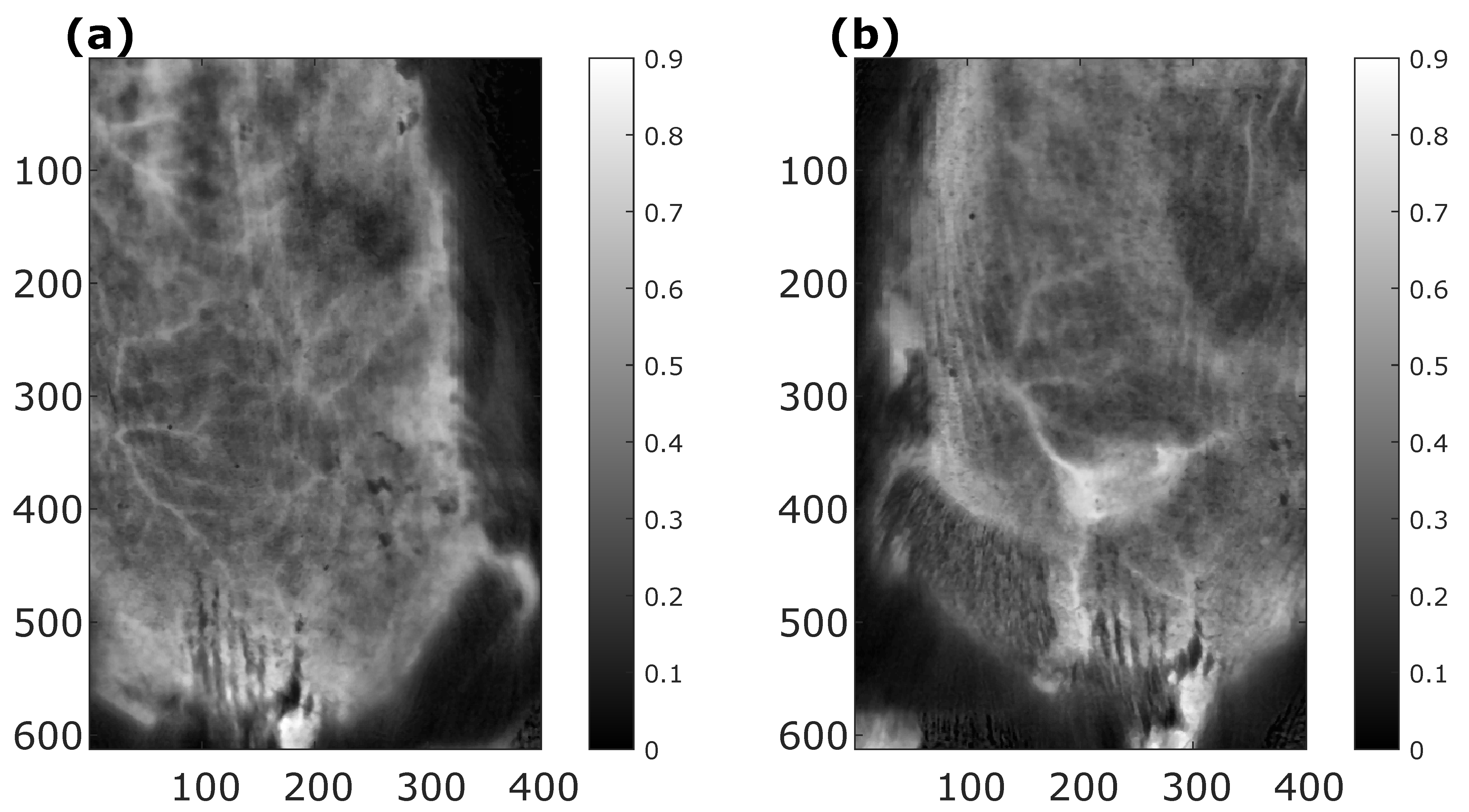
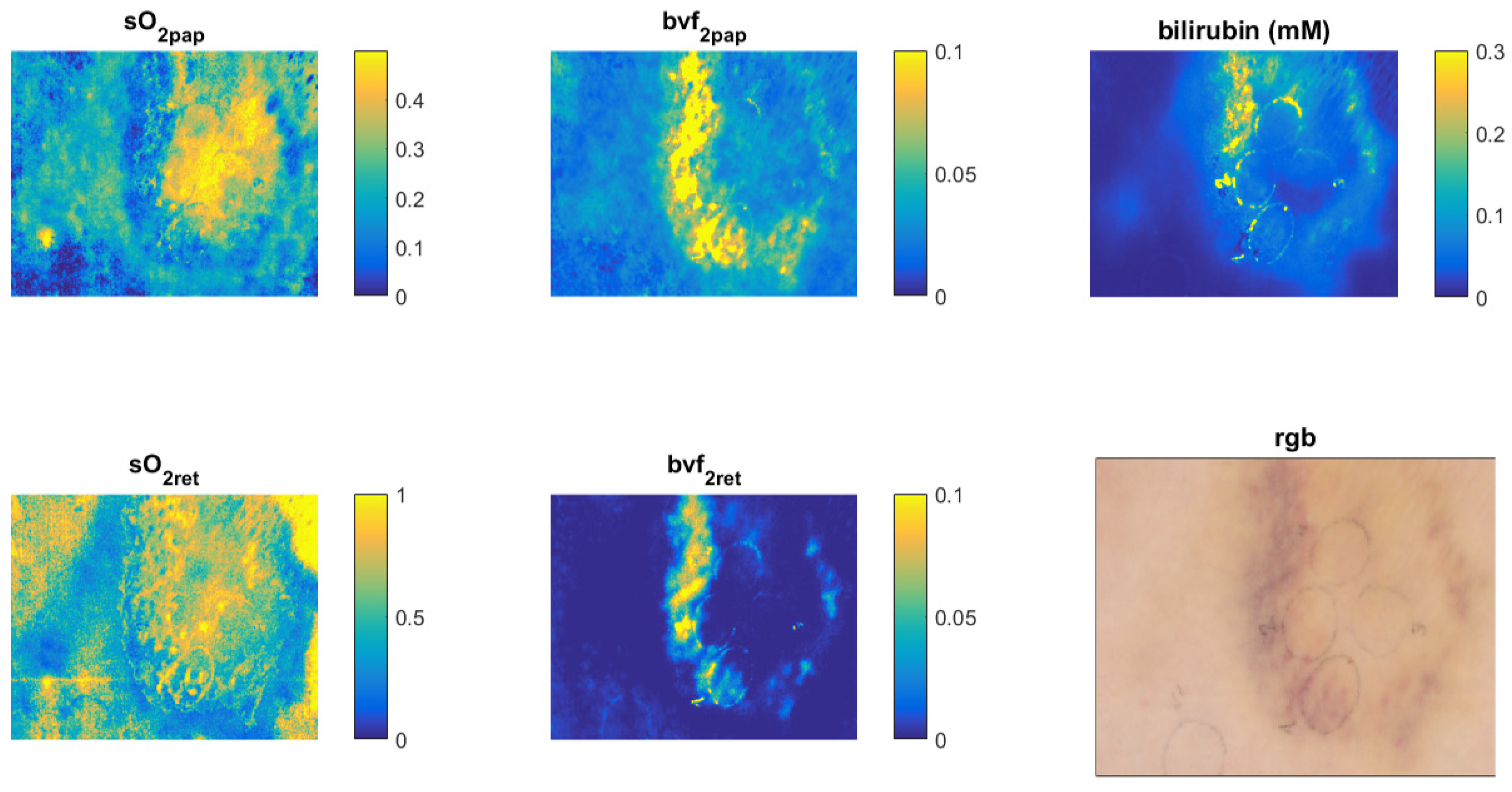
3.10. Example #3 of HSI-System Application in the Biomedical Field: Bruise Imaging
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FOV—field of view |
| FWHM—full width at half maximum |
| HNL—helium-neon laser |
| HSI—hyperspectral imaging |
| LED—light emitting diode |
| MP—megapixel |
| NIR—near infrared |
| PCB—printed circuit board |
| PSU—power supply unit |
| PTFE—polytetrafluoroethylene |
| RGB—red, green, and blue |
| USAF—United States Air Force |
| USB—universal serial bus |
| UV—ultraviolet |
| VIS—visible |
Appendix A. Development of the Custom-Made Laboratory HSI System
Appendix A.1. Imaging Components
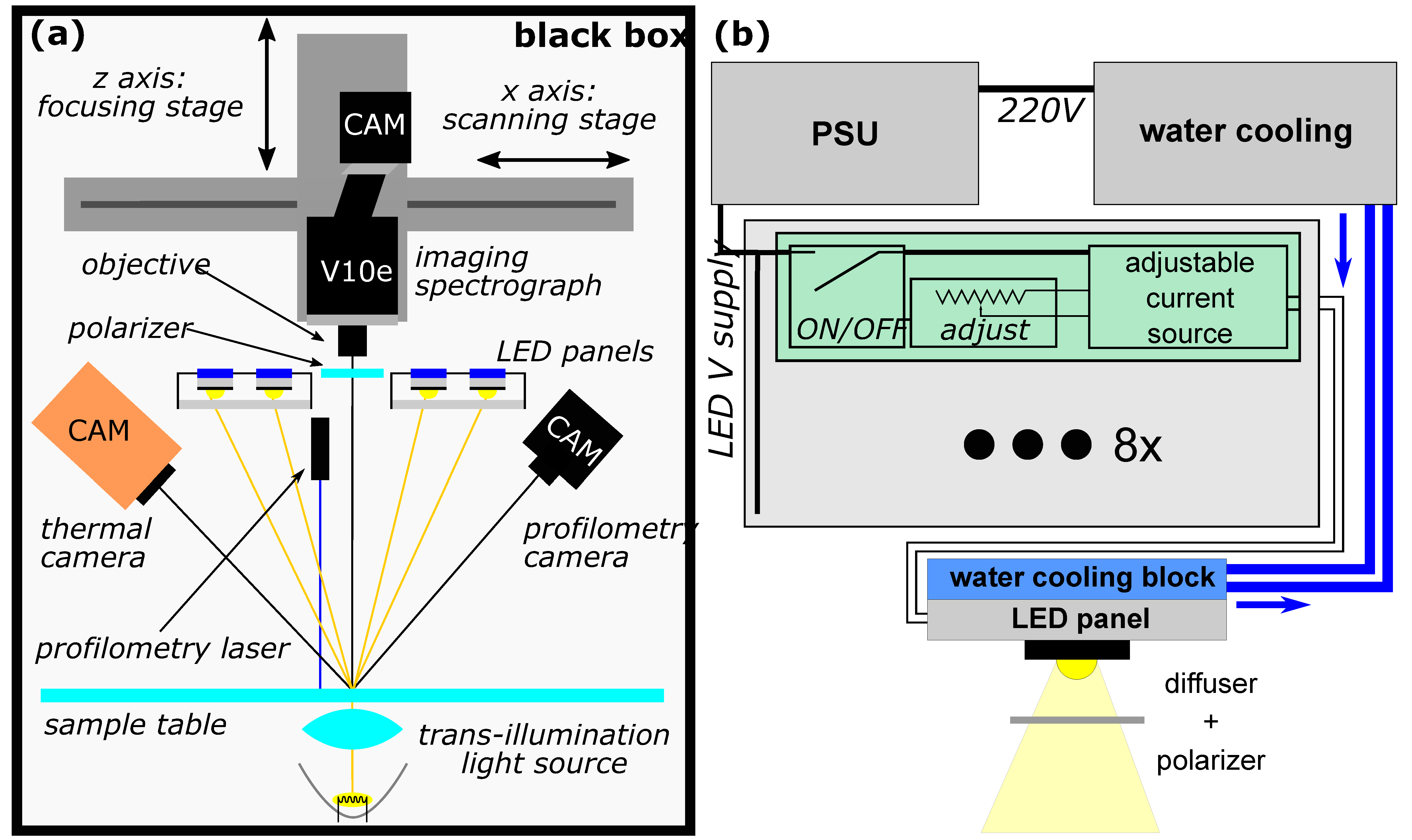
Appendix A.2. The Light Source
Appendix A.3. Specular Reflection Minimization
Appendix A.4. Additional Components
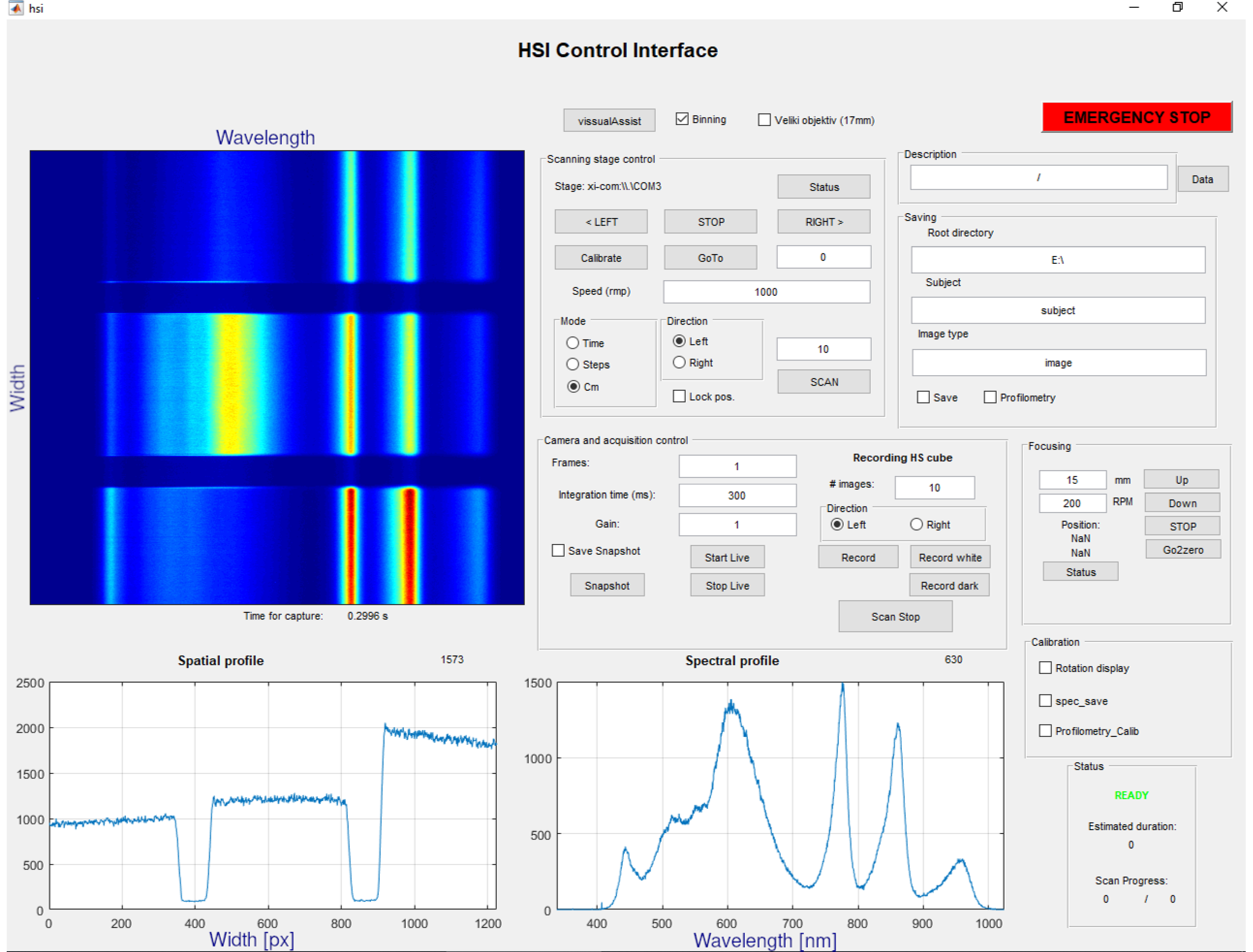
Appendix A.5. The Software
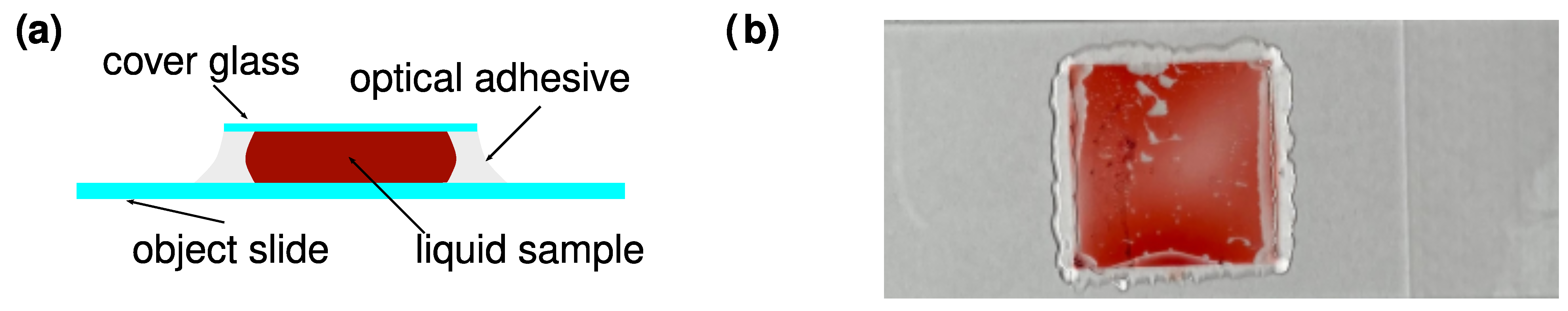
Appendix B. Preparation of Sample Cells
References
- Goetz, A.F.H.; Vane, G.; Solomon, J.E.; Rock, B.N. Imaging Spectrometry for Earth Remote Sensing. Science 1985, 228, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Takalkar, M.A.; Xu, M.; Hoang, D.T.; Truong, H.A.; Dutkiewicz, E.; Perry, S. Airborne Object Detection Using Hyperspectral Imaging: Deep Learning Review. In Computational Science and Its Applications—ICCSA 2019; Misra, S., Gervasi, O., Murgante, B., Stankova, E., Korkhov, V., Torre, C., Rocha, A.M.A.C., Taniar, D., Apduhan, B.O., Tarantino, E., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Germany, 2019; Volume 11619, pp. 306–321. ISBN 978-3-030-24288-6. [Google Scholar]
- Selci, S. The Future of Hyperspectral Imaging. J. Imaging 2019, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, M.; Chetty, K.; Bulcock, H. A Review of Hypersspectral Remote Sensing and Its Application in Vegetation and Water Resource Studies. WSA 2007, 33, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Castro-Esau, K. Discrimination of Lianas and Trees with Leaf-Level Hyperspectral Data. Remote Sens. Environ. 2004, 90, 353–372. [Google Scholar] [CrossRef]
- Caballero, D.; Calvini, R.; Amigo, J.M. Chapter 3.3—Hyperspectral Imaging in Crop Fields: Precision Agriculture. In Data Handling in Science and Technology; Amigo, J.M., Ed.; Hyperspectral Imaging; Elsevier: Amsterdam, The Netherlands, 2019; Volume 32, pp. 453–473. [Google Scholar]
- Schimleck, L.; Ma, T.; Inagaki, T.; Tsuchikawa, S. Review of near Infrared Hyperspectral Imaging Applications Related to Wood and Wood Products. Appl. Spectrosc. Rev. 2022, 1–25. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Ngadi, M. Recent Developments in Hyperspectral Imaging for Assessment of Food Quality and Safety. Sensors 2014, 14, 7248–7276. [Google Scholar] [CrossRef] [Green Version]
- Gowen, A.; Odonnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral Imaging—An Emerging Process Analytical Tool for Food Quality and Safety Control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Soni, A.; Dixit, Y.; Reis, M.M.; Brightwell, G. Hyperspectral Imaging and Machine Learning in Food Microbiology: Developments and Challenges in Detection of Bacterial, Fungal, and Viral Contaminants. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3717–3745. [Google Scholar] [CrossRef]
- Balas, C.; Epitropou, G.; Tsapras, A.; Hadjinicolaou, N. Hyperspectral Imaging and Spectral Classification for Pigment Identification and Mapping in Paintings by El Greco and His Workshop. Multimed. Tools Appl. 2018, 77, 9737–9751. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Legan, L.; Retko, K.; Kavčič, M.; Kosel, J.; Poohphajai, F.; Diaz, R.H.; Ponnuchamy, V.; Sajinčič, N.; et al. Nondestructive Evaluation of Heritage Object Coatings with Four Hyperspectral Imaging Systems. Coatings 2021, 11, 244. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical Hyperspectral Imaging: A Review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Halig, L.; Wang, D.; Qin, X.; Chen, Z.G.; Fei, B. Spectral-Spatial Classification for Noninvasive Cancer Detection Using Hyperspectral Imaging. J. Biomed. Opt. 2014, 19, 106004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuzak, K.J.; Naik, S.C.; Alexandrakis, G.; Hawkins, D.; Behbehani, K.; Livingston, E.H. Characterization of a Near-Infrared Laparoscopic Hyperspectral Imaging System for Minimally Invasive Surgery. Anal. Chem. 2007, 79, 4709–4715. [Google Scholar] [CrossRef]
- Barberio, M.; Benedicenti, S.; Pizzicannella, M.; Felli, E.; Collins, T.; Jansen-Winkeln, B.; Marescaux, J.; Viola, M.G.; Diana, M. Intraoperative Guidance Using Hyperspectral Imaging: A Review for Surgeons. Diagnostics 2021, 11, 2066. [Google Scholar] [CrossRef]
- Nasr-Esfahani, S.; Muthukumar, V.; Regentova, E.; Taghva, K.; Trabia, M. Hyperspectral Methods in Microscopy Image Analysis: A Survey. In Proceedings of the 18th International Conference on Signal Processing and Multimedia Applications, Online Streaming, 6–8 July 2021; SciTePress—Science and Technology Publications: Setúbal, Portugal, 2021; pp. 111–119. [Google Scholar]
- Chalopin, C.; Nickel, F.; Pfahl, A.; Köhler, H.; Maktabi, M.; Thieme, R.; Sucher, R.; Jansen-Winkeln, B.; Studier-Fischer, A.; Seidlitz, S.; et al. Künstliche Intelligenz und hyperspektrale Bildgebung zur bildgestützten Assistenz in der minimal-invasiven Chirurgie. Chirurgie 2022. [Google Scholar] [CrossRef]
- Aloupogianni, E.; Ishikawa, M.; Kobayashi, N.; Obi, T. Hyperspectral and Multispectral Image Processing for Gross-Level Tumor Detection in Skin Lesions: A Systematic Review. J. Biomed. Opt. 2022, 27, 060901. [Google Scholar] [CrossRef]
- Aggarwal, L.P.; Papay, F.A. Applications of Multispectral and Hyperspectral Imaging in Dermatology. Exp. Dermatol. 2022, 31, 1128–1135. [Google Scholar] [CrossRef]
- Wu, F.; Li, S.; Hong, W.; Yue, S.; Wang, P. Hyperspectral Coherent Raman Scattering and Its Applications. Laser Optoelectron. Prog. 2022, 59, 0617003. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, J.A.; Pardo, A.; Real, E.; López-Higuera, J.M.; Conde, O.M. Custom Scanning Hyperspectral Imaging System for Biomedical Applications: Modeling, Benchmarking, and Specifications. Sensors 2019, 19, 1692. [Google Scholar] [CrossRef] [Green Version]
- Dolenec, R.; Rogelj, L.; Stergar, J.; Milanic, M. Modular Multi-Wavelength LED Based Light Source for Hyperspectral Imaging. In Proceedings of the Novel Biophotonics Techniques and Applications V, Munich, Germany, 22 July 2019; Amelink, A., Nadkarni, S.K., Eds.; SPIE: Munich, Germany, 2019; p. 56. [Google Scholar]
- Pavlovcic, U.; Stergar, J.; Rogelj, L.; Kosir, J.; Jezersek, M.; Milanic, M. 3D Profilomer Combined with Hyperspectral Camera for Simplified Rheumatoid Arthritis Diagnostics. In Proceedings of the 3DBODY.TECH 2018-9th International Conference and Exhibition on 3D Body Scanning and Processing Technologies, Lugano, Switzerland, 16–17 October 2018; Hometrica Consulting—Dr. Nicola D’Apuzzo: Lugano, Switzerland, 2018; pp. 31–35. [Google Scholar]
- Pavlovčič, U.; Diaci, J.; Možina, J.; Jezeršek, M. Wound Perimeter, Area, and Volume Measurement Based on Laser 3D and Color Acquisition. BioMed. Eng. OnLine 2015, 14, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogelj, L.; Pavlovčič, U.; Stergar, J.; Jezeršek, M.; Simončič, U.; Milanič, M. Curvature and Height Corrections of Hyperspectral Images Using Built-in 3D Laser Profilometry. Appl. Opt. 2019, 58, 9002. [Google Scholar] [CrossRef] [PubMed]
- Stergar, J.; Dolenec, R.; Kojc, N.; Lakota, K.; Perše, M.; Tomšič, M.; Milanic, M. Hyperspectral Evaluation of Peritoneal Fibrosis in Mouse Models. Biomed. Opt. Express 2020, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, L.; Pavlovčič, U.; Jezeršek, M.; Milanic, M.; Simončič, U. Reducing Object Curvature and Height Variation Effects in Hyperspectral Images. In Proceedings of the Diffuse Optical Spectroscopy and Imaging VII, Munich, Germany, 11 July 2019; Dehghani, H., Wabnitz, H., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 85. [Google Scholar]
- Kosec, M.; Bürmen, M.; Tomaževič, D.; Pernuš, F.; Likar, B. Characterization of a Spectrograph Based Hyperspectral Imaging System. Opt. Express 2013, 21, 12085. [Google Scholar] [CrossRef]
- Polder, G.; van der Heijden, G.W.A.M.; Keizer, L.C.P.; Young, I.T. Calibration and Characterisation of Imaging Spectrographs. J. Near Infrared Spectrosc. 2003, 11, 193–210. [Google Scholar] [CrossRef]
- Fisher, J.; Baumback, M.M.; Bowles, J.H.; Grossmann, J.M.; Antoniades, J.A. Comparison of Low-Cost Hyperspectral Sensors; Descour, M.R., Shen, S.S., Eds.; SPIE: San Diego, CA, USA, 1998; p. 23. [Google Scholar]
- Sansonetti, J. Handbook of Basic Atomic Spectroscopic Data; NIST Standard Reference Database: Gaithersburg, MD, USA, 2003; p. 108. [Google Scholar]
- Stergar, J.; Osterman, N. Thermophoretic Tweezers for Single Nanoparticle Manipulation. Beilstein J. Nanotechnol. 2020, 11, 1126–1133. [Google Scholar] [CrossRef]
- Dolenec, R.; Laistler, E.; Milanic, M. Assessing Spectral Imaging of the Human Finger for Detection of Arthritis. Biomed. Opt. Express 2019, 10, 6555. [Google Scholar] [CrossRef]
- Tomanic, T.; Rogelj, L.; Stergar, J.; Markelc, B.; Bozic, T.; Kranjc Brezar, S.; Sersa, G.; Milanic, M. Estimating Quantitative Physiological and Morphological Tissue Parameters of Murine Tumor Models Using Hyperspectral Imaging and Optical Profilometry. J. Biophotonics 2022. [Google Scholar] [CrossRef]
- Diffey, B.L.; Oliver, R.J.; Farr, P.M. A Portable Instrument for Quantifying Erythema Induced by Ultraviolet Radiation. Br. J. Dermatol. 1984, 111, 663–672. [Google Scholar] [CrossRef]
- Stam, B.; van Gemert, M.J.C.; van Leeuwen, T.G.; Teeuw, A.H.; van der Wal, A.C.; Aalders, M.C.G. Can Color Inhomogeneity of Bruises Be Used to Establish Their Age? J. Biophoton. 2011, 4, 759–767. [Google Scholar] [CrossRef]
- Naglič, P.; Vidovič, L.; Milanič, M.; Randeberg, L.L.; Majaron, B. Suitability of Diffusion Approximation for an Inverse Analysis of Diffuse Reflectance Spectra from Human Skin in Vivo. OSA Contin. 2019, 2, 905. [Google Scholar] [CrossRef]
- Modir, N.; Shahedi, M.; Dormer, J.D.; Ma, L.; Ghaderi, M.; Sirsi, S.; Cheng, Y.-S.L.; Fei, B. LED-Based Hyperspectral Endoscopic Imaging. In Proceedings of the Optical Biopsy XX: Toward Real-Time Spectroscopic Imaging and Diagnosis, San Francisco, CA, USA, 2 March 2022; Alfano, R.R., Demos, S.G., Seddon, A.B., Eds.; SPIE: San Francisco, CA, USA, 2022; p. 26. [Google Scholar]
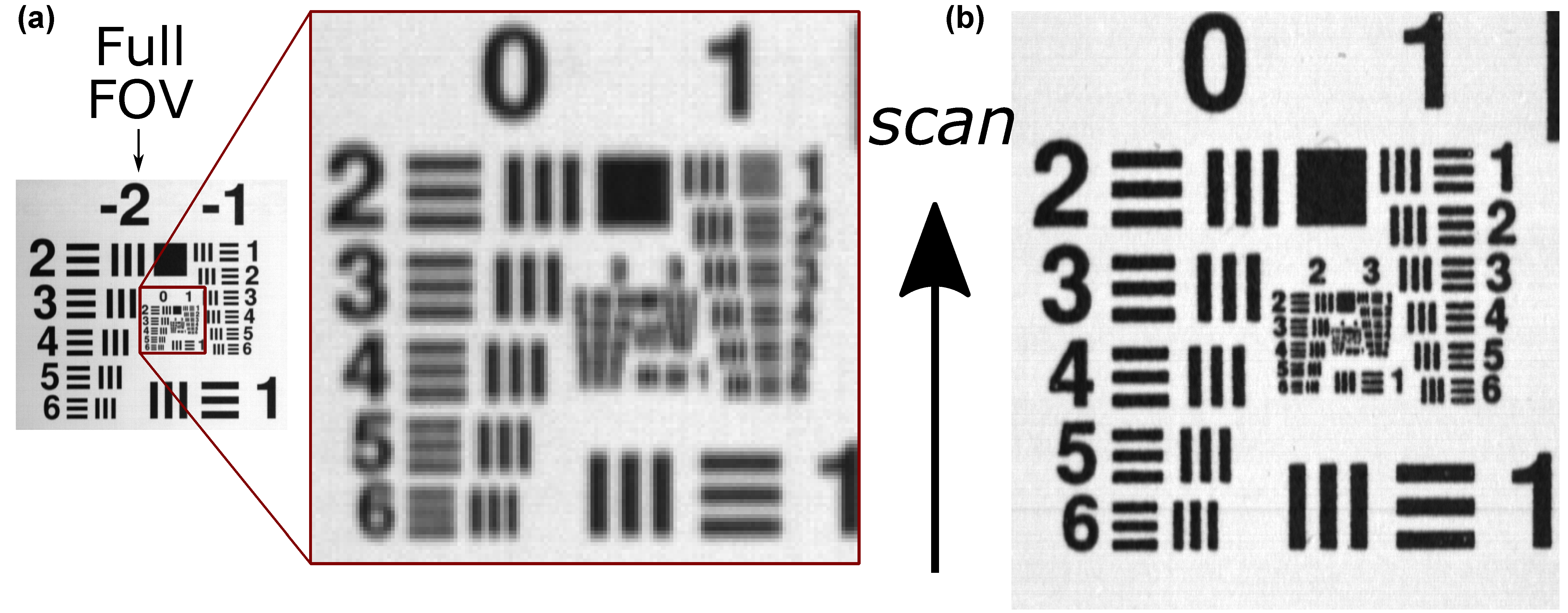
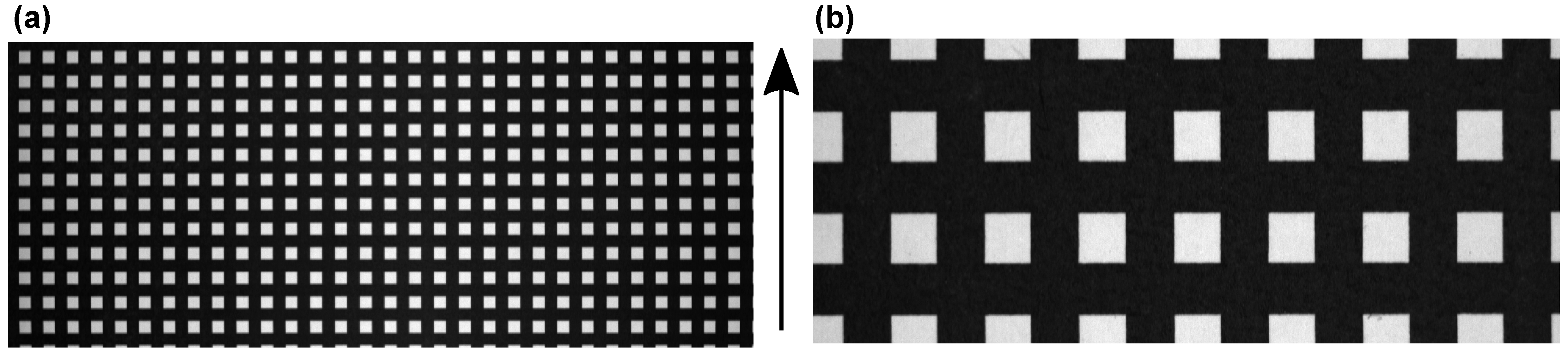
| Objective | x (mm) | n (px) | Δx (mm) |
|---|---|---|---|
| 17 mm (1.0 lp/mm) | 50 | 401 | 0.12 |
| 17 mm (0.5 lp/mm) | 50 | 401 | 0.12 |
| 50 mm (1.0 lp/mm) | 38 | 1173 | 0.03 |
| 50 mm (0.5 lp/mm) | 39 | 1205 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stergar, J.; Hren, R.; Milanič, M. Design and Validation of a Custom-Made Laboratory Hyperspectral Imaging System for Biomedical Applications Using a Broadband LED Light Source. Sensors 2022, 22, 6274. https://doi.org/10.3390/s22166274
Stergar J, Hren R, Milanič M. Design and Validation of a Custom-Made Laboratory Hyperspectral Imaging System for Biomedical Applications Using a Broadband LED Light Source. Sensors. 2022; 22(16):6274. https://doi.org/10.3390/s22166274
Chicago/Turabian StyleStergar, Jošt, Rok Hren, and Matija Milanič. 2022. "Design and Validation of a Custom-Made Laboratory Hyperspectral Imaging System for Biomedical Applications Using a Broadband LED Light Source" Sensors 22, no. 16: 6274. https://doi.org/10.3390/s22166274
APA StyleStergar, J., Hren, R., & Milanič, M. (2022). Design and Validation of a Custom-Made Laboratory Hyperspectral Imaging System for Biomedical Applications Using a Broadband LED Light Source. Sensors, 22(16), 6274. https://doi.org/10.3390/s22166274







