Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals’ Analysis: A Proof-of-Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Experimental Pain
2.2.2. Baseline Measures, Participant at Rest
2.2.3. Active Measures, Participant Executing a Visuo-Motor Task
2.2.4. Task for Chronic Fibromyalgia Pain Group
2.2.5. Task Performance in the Chronic Fibromyalgia Pain Group
2.2.6. Task for the Healthy Group
2.2.7. Task Performance for the Healthy Group
2.3. EEG Data Acquisition
2.3.1. EEG Data Preprocessing Analysis
2.3.2. EEG Frequency Sub-Bands Retained for Signal Analysis
2.3.3. EEG Data Normalization
2.3.4. Calculation of the Coefficient of Variation of Upper Envelope (CVUE)
- (1)
- The first step was the calculation of the amplitude of upper envelope (AUE) of EEG signals for frequency sub-bands (), defined as the absolute value of the Hilbert transform, as follows
- (2)
- The second step was the assessment of the coefficient of variation of upper amplitude in alpha (CVUEα), beta (CVUEβ) and gamma (CVUEɣ) EEG frequency bands. To this end, the mean and standard deviation (std) of AUE were computed in each epoch to obtain CVUEα, CVUEβ and CVUEɣ as follows
2.4. Statistical Analysis
3. Results
3.1. Pain Intensity
3.2. Task Performance
3.3. EEG Baseline Measures: Coefficient of Variation of Upper Envelope from Alpha (CVUEα), Beta (CVUEβ) and Gamma (CVUEγ) EEG Frequency Bands, at Rest
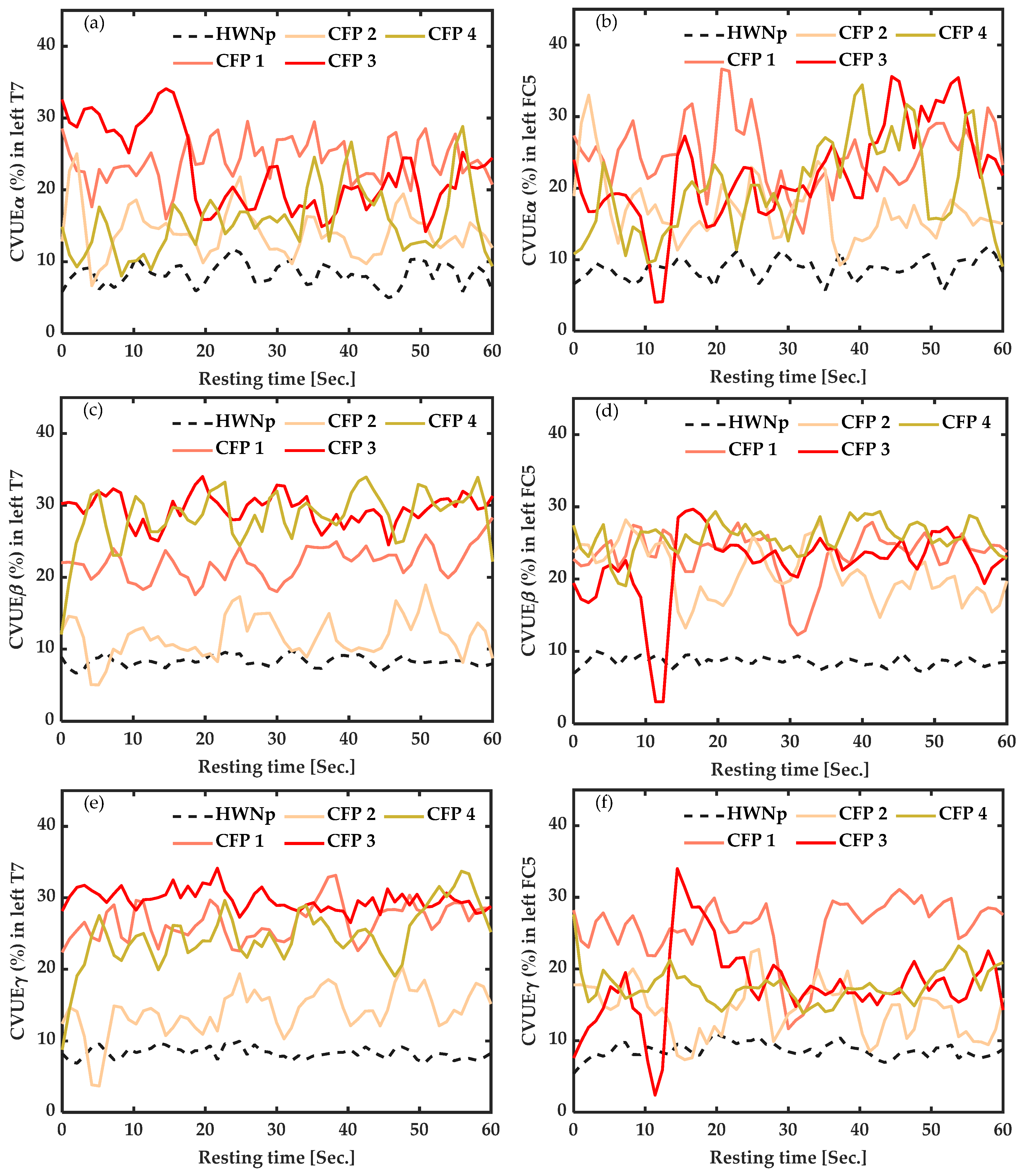
3.3.1. In Participants with Chronic Pain vs. Healthy with “No Pain” (HWNp) at Rest
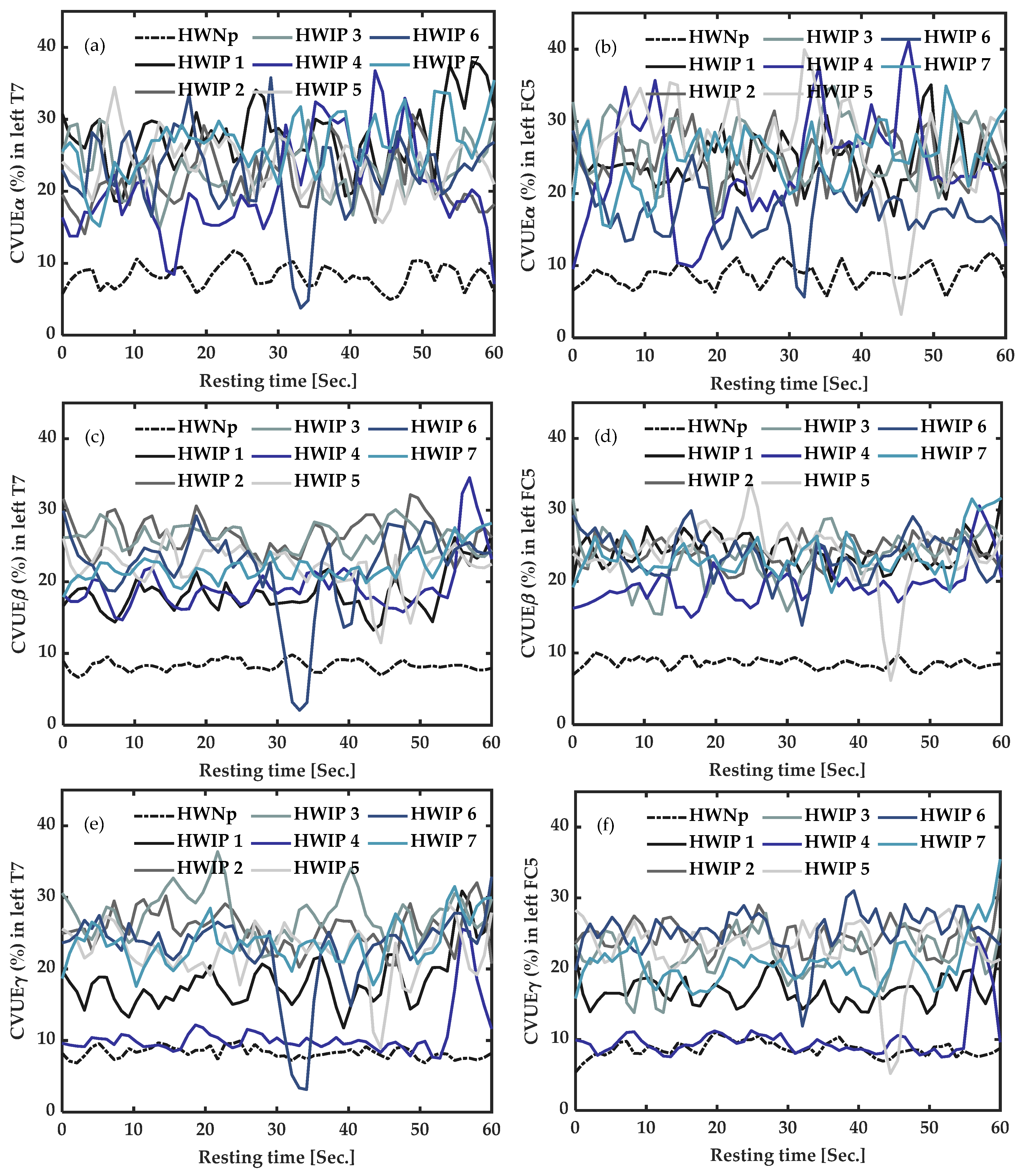
3.3.2. In Healthy Participants with Induced Pain (HWIP) vs. Healthy with “No Pain” (HWNp) at Rest
3.4. EEG Active Measures: Coefficient of Variation of Upper Envelope from Alpha (CVUEα), Beta (CVUEβ) and Gamma (CVUEγ) EEG Frequency Bands, during Task Execution
3.4.1. In Participants with Chronic Fibromyalgia Pain (CFP) vs. Healthy with “No Pain” (HWNp) during Task Execution
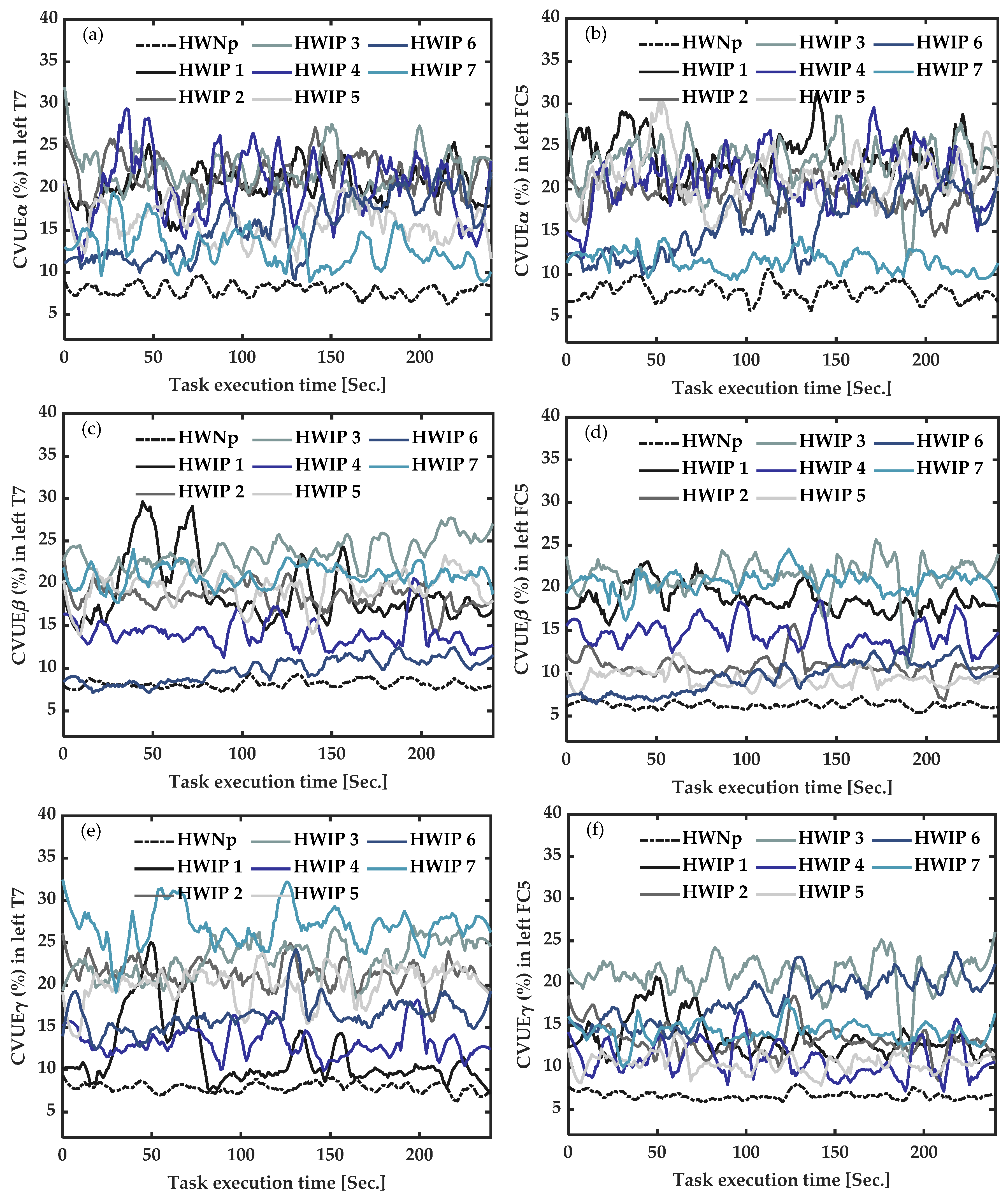
3.4.2. In Healthy Participants with Induced Pain (HWIP) vs. Healthy with “No Pain” (HWNp) during Task Execution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lynch, M.E.; Campbell, F.; Clark, A.J.; Dunbar, M.J.; Goldstein, D.H.; Peng, P.; Stinson, J.; Tupper, H. A systematic review of the effect of waiting for treatment for chronic pain. Pain 2008, 136, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Roll, J.M.; Schraudner, T.; Murphy, S.; McPherson, S. Prevalence of persistent pain in the US adult population: New data from the 2010 national health interview survey. J. Pain 2014, 15, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Turk, D.C.; Melzack, R. The Measurement of Pain and the Assessment of People Experiencing Pain. In Handbook of Pain Assessment; Turk, D.C., Melzack, R., Eds.; The Guilford Press: New York, NY, USA, 2011; pp. 3–16. [Google Scholar]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Reid, K.J.; Harker, J.; Bala, M.M.; Truyers, C.; Kellen, E.; Bekkering, G.E.; Kleijnen, J. Epidemiology of chronic non-cancer pain in Europe: Narrative review of prevalence, pain treatments and pain impact. Curr. Med. Res. Opin. 2011, 27, 449–462. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheumatol. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Williams, A.D.; Gracely, H.R. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res. Ther. 2007, 8, 224. [Google Scholar] [CrossRef][Green Version]
- Shieh, J.-S.; Dai, C.-Y.; Wen, Y.-R.; Sun, W.-Z. A novel fuzzy pain demand index derived from patient-controlled analgesia for postoperative pain. IEEE Trans. Biomed. Eng. 2007, 54, 2123–2132. [Google Scholar] [CrossRef]
- Kamdar, M.M. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2010. [Google Scholar]
- Walsh, T. Practical problems in pain measurements. Pain 1984, 19, 96–98. [Google Scholar] [CrossRef]
- Schnakers, C.; Zasler, N.D. Pain assessment and management in disorders of consciousness. Curr. Opin. Neurol. 2007, 20, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Herr, K.; Coyne, P.J.; McCaffery, M.; Manworren, R.; Merkel, S. Pain assessment in the patient unable to self-report: Position statement with clinical practice recommendations. Pain Manag. Nurs. 2011, 12, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Ploner, M.; May, E.S. Electroencephalography and magnetoencephalography in pain research—Current state and future perspectives. Pain 2018, 159, 206–211. [Google Scholar] [CrossRef]
- Ploner, M.; Sorg, C.; Gross, J. Brain rhythms of pain. Trends Cogn. Sci. 2017, 21, 100–110. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, P.; Hung, Y.; Iannetti, G.; Zhang, Z.; Hu, L. A novel approach to predict subjective pain perception from single-trial laser-evoked potentials. Neuroimage 2013, 81, 283–293. [Google Scholar] [CrossRef]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.-W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef]
- Kumbhare, D.; Elzibak, A.H.; Noseworthy, M.D. Evaluation of chronic pain using magnetic resonance (MR) neuroimaging approaches. Clin. J. Pain 2017, 33, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.-R.; Sinai, A.; Raz, E.; Sprecher, E.; Yarnitsky, D. Pain assessment by continuous EEG: Association between subjective perception of tonic pain and peak frequency of alpha oscillations during stimulation and at rest. Brain Res. 2010, 1344, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.-R.; Sinai, A.; Moont, R.; Harari, E.; Yarnitsky, D. Tonic pain and continuous EEG: Prediction of subjective pain perception by alpha-1 power during stimulation and at rest. Clin. Neurophysiol. 2012, 123, 605–612. [Google Scholar] [CrossRef]
- Hu, L.; Iannetti, G. Neural indicators of perceptual variability of pain across species. Proc. Natl. Acad. Sci. USA 2019, 116, 1782–1791. [Google Scholar] [CrossRef]
- May, E.S.; Nickel, M.M.; Dinh, S.T.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019, 40, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-S.; Keller, C.J.; Li, J.; Lin, Y.-P.; Nakanishi, M.; Wagner, J.; Wu, W.; Zhang, Y.; Jung, T.-P. Editorial: Inter-and Intra-subject Variability in Brain Imaging and Decoding. Front. Comput. Neurosci. 2021, 15, 791129. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.R.; Voytek, B. Brain oscillations and the importance of waveform shape. Trends Cogn. Sci. 2017, 21, 137–149. [Google Scholar] [CrossRef]
- Díaz, J.; Razeto-Barry, P.; Letelier, J.-C.; Caprio, J.; Bacigalupo, J. Amplitude modulation patterns of local field potentials reveal asynchronous neuronal populations. J. Neurosci. 2007, 27, 9238–9245. [Google Scholar] [CrossRef]
- Díaz, J.; Arancibia, J.M.; Bassi, A.; Vivaldi, E.A. Envelope analysis of the airflow signal to improve polysomnographic assessment of sleep disordered breathing. Sleep 2014, 37, 199–208. [Google Scholar] [CrossRef]
- Romaine, J.; Martín, M.P.; Ortiz, J.S.; Crespo, J.M. EEG—Single-Channel Envelope Synchronisation and Classification for Seizure Detection and Prediction. Brain Sci. 2021, 11, 516. [Google Scholar] [CrossRef]
- Katz, J.S. Scale-Independent Measures: Theory and Practice. In Proceedings of the 17th International Conference on Science and Technology Indicators, Montréal, QC Canada, 5–8 September 2012; pp. 1–19. [Google Scholar]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheumatol. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Edlin, J.M.; Leppanen, M.; Fain, R.J.; Hackländer, R.P.; Hanaver-Torrez, S.D.; Lyle, K.B. On the use (and misuse?) of the Edinburgh Handedness Inventory. Brain Cogn. 2015, 94, 44–51. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. Hoboken 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, J.; Bouyer, L.J.; Roy, J.-S.; Mercier, C. Pain induced during both the acquisition and retention phases of locomotor adaptation does not interfere with improvements in motor performance. Neural Plast. 2016, 2016, 8539096. [Google Scholar] [CrossRef] [PubMed]
- Lomond, K.V.; Côté, J.N. Movement timing and reach to reach variability during a repetitive reaching task in persons with chronic neck/shoulder pain and healthy subjects. Exp. Brain Res. 2010, 206, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Flake, G.W.; Baum, E.B. Rush Hour is PSPACE-complete, or “Why you should generously tip parking lot attendants”. Theor. Comput. Sci. 2002, 270, 895–911. [Google Scholar]
- Athif, M.; Ren, H. WaveCSP: A robust motor imagery classifier for consumer EEG devices. Australas. Phys. Eng. Sci. Med. 2019, 42, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Chipchase, L.; Schabrun, S. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur. J. Pain 2016, 20, 1203–1213. [Google Scholar] [CrossRef]
- Bouffard, J.; Bouyer, L.J.; Roy, J.-S.; Mercier, C. Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. J. Neurosci. 2014, 34, 9190–9195. [Google Scholar] [CrossRef]
- Segning, C.; Ezzaidi, H.; da Silva, R.; Ngomo, S. A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG. Int. J. Environ. Res. Public Health 2021, 18, 2001. [Google Scholar] [CrossRef]
- Power, J.D.; Lynch, C.J.; Dubin, M.J.; Silver, B.M.; Martin, A.; Jones, R.M. Characteristics of respiratory measures in young adults scanned at rest, including systematic changes and “missed” deep breaths. Neuroimage 2020, 204, 116234. [Google Scholar] [CrossRef]
- Liberati, G.; Klöcker, A.; Algoet, M.; Mulders, D.; Safronova, M.M.; Santos, S.F.; Vaz, J.-G.R.; Raftopoulos, C.; Mouraux, A. Gamma-band oscillations preferential for nociception can be recorded in the human insula. Cereb. Cortex 2018, 28, 3650–3664. [Google Scholar] [CrossRef]
- Shao, S.; Shen, K.; Yu, K.; Wilder-Smith, E.; Li, X. Frequency-domain EEG source analysis for acute tonic cold pain perception. Clin. Neurophysiol. 2012, 123, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Aboalayon, K.A.I.; Almuhammadi, W.S.; Faezipour, M. A Comparison of Different Machine Learning Algorithms Using Single Channel EEG Signal for Classifying Human Sleep Stages. In Proceedings of the 2015 Long Island Systems, Applications and Technology, Farmingdale, NY, USA, 1 May 2015; pp. 1–6. [Google Scholar]
- Zhang, X.; Yao, L.; Zhang, D.; Wang, X.; Sheng, Q.Z.; Gu, T. Multi-person brain activity recognition via comprehensive EEG signal analysis. In Proceedings of the 14th EAI International Conference on Mobile and Ubiquitous Systems: Computing, Networking and Services, New York, NY, USA, 7–10 November 2017; pp. 28–37. [Google Scholar]
- Zhao, Y.; Cen, Y. Data Mining Applications with R; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Cohen, M.X. MATLAB for Brain and Cognitive Scientists; MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Yousif, E.S.; Abdulbaqi, A.S.; Hameed, A.Z.; Al-din, S. Electroencephalogram Signals Classification Based on Feature Normalization. Dans IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 032028. [Google Scholar] [CrossRef]
- Boashash, B. Time-Frequency and Instantaneous Frequency Concepts. In Dans Time-Frequency Signal Analysis and Processing: A Comprehensive Reference; Academic Press: Cambridge, MA, USA, 2015; pp. 31–64. [Google Scholar]
- Sanei, S.; Chambers, J.A. Fundamentals of EEG Signal Processing. In Dans EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 35–125. [Google Scholar]
- Park, I.; Díaz, J.; Matsumoto, S.; Iwayama, K.; Nabekura, Y.; Ogata, H.; Kayaba, M.; Aoyagi, A.; Yajima, K.; Satoh, M.; et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zamm, A.; Debener, S.; Bauer, A.-K.R.; Bleichner, M.G.; Demos, A.P.; Palmer, C. Amplitude envelope correlations measure synchronous cortical oscillations in performing musicians. Ann. N. Y. Acad. Sci. 2018, 1423, 251–263. [Google Scholar] [CrossRef]
- Díaz, J.; Bassi, A.; Coolen, A.; Vivaldi, E.A.; Letelier, J.-C. Envelope analysis links oscillatory and arrhythmic EEG activities to two types of neuronal synchronization. Neuroimage 2018, 172, 575–585. [Google Scholar] [CrossRef]
- Charier, D.J.; Zantour, D.; Pichot, V.; Chouchou, F.; Barthelemy, J.-C.M.; Roche, F.; Molliex, S.B. Assessing pain using the variation coefficient of pupillary diameter. J. Pain 2017, 18, 1346–1353. [Google Scholar] [CrossRef]
- Mani, S.; Sharma, S.; Singh, D.K. Concurrent validity and reliability of telerehabilitation-based physiotherapy assessment of cervical spine in adults with non-specific neck pain. J. Telemed. Telecare 2019, 27, 88–97. [Google Scholar] [CrossRef]
- Maquet, D.; Croisier, J.-L.; Demoulin, C.; Crielaard, J.-M. Pressure pain thresholds of tender point sites in patients with fibromyalgia and in healthy controls. Eur. J. Pain 2004, 8, 111–117. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Volz, M.S.; Medeiros, L.F.; Tarragô, M.D.G.; Vidor, L.P.; Dall’agnol, L.; Deitos, A.; Brietzke, A.; Rozisky, J.R.; Rispolli, B.; Torres, I.L.; et al. The Relationship between Cortical Excitability and Pain Catastrophizing in Myofascial Pain. J. Pain 2013, 14, 1140–1147. [Google Scholar] [CrossRef]
- Vidor, L.P.; Torres, I.L.; Medeiros, L.F.; Dussán-Sarria, J.A.; Dall’Agnol, L.; Deitos, A.; Brietzke, A.; Laste, G.; Rozisky, J.R.; Fregni, F.; et al. Association of anxiety with intracortical inhibition and descending pain modulation in chronic myofascial pain syndrome. BMC Neurosci. 2014, 15, 1–13. [Google Scholar] [CrossRef]
- Caumo, W.; Deitos, A.; Carvalho, S.; Leite, J.; Carvalho, F.; Dussan-Sarria, J.; Tarragó, M.D.G.L.; Souza, A.; Torres, I.L.; Fregni, F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front. Hum. Neurosci. 2016, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.L.; Morales-Quezada, L.; Rozisky, R.J.; Brietzke, P.A.; Torres, L.I.; Deitos, A.; Caumo, W. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front. Hum. Neurosci. 2016, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yuan, Q. Structural homeostasis in the nervous system: A balancing act for wiring plasticity and stability. Front. Cell. Neurosci. 2015, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Terkelsen, A.J.; Mølgaard, H.; Hansen, J.; Andersen, O.K.; Jensen, T.S. Acute pain increases heart rate: Differential mechanisms during rest and mental stress. Auton. Neurosci. 2005, 121, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability and pain: Associations of two interrelated homeostatic processes. Biol. Psychol. 2008, 77, 174–182. [Google Scholar] [CrossRef]
- Saccò, M.; Meschi, M.; Regolisti, G.; Detrenis, S.; Bianchi, L.; Bertorelli, M.; Pioli, S.; Magnano, A.; Spagnoli, F.; Giuri, P.G.; et al. The relationship between blood pressure and pain. J. Clin. Hypertens. 2013, 15, 600–605. [Google Scholar] [CrossRef]
- Panavaranan, P.; Wongsawat, Y. EEG-Based Pain Estimation via Fuzzy Logic and Polynomial Kernel Support Vector Machine. In Proceedings of the 6th 2013 Biomedical Engineering International Conference, Amphur Muang, Thailand, 23–25 October 2013; pp. 1–4. [Google Scholar]
- Vatankhah, M.; Asadpour, V.; Fazel-Rezai, R. Perceptual pain classification using ANFIS adapted RBF kernel support vector machine for therapeutic usage. Appl. Soft Comput. 2013, 13, 2537–2546. [Google Scholar] [CrossRef]
- Alazrai, R.; Momani, M.; Abu Khudair, H.; Daoud, M.I. EEG-based tonic cold pain recognition system using wavelet transform. Neural Comput. Appl. 2019, 31, 3187–3200. [Google Scholar] [CrossRef]
- Frot, M.; Feine, J.S.; Bushnell, M.C. Sex differences in pain perception and anxiety. A psychophysical study with topical capsaicin. Pain 2004, 108, 230–236. [Google Scholar] [CrossRef]
- Ramírez-Maestre, C.; Esteve, R. The role of sex/gender in the experience of pain: Resilience, fear, and acceptance as central variables in the adjustment of men and women with chronic pain. J. Pain 2014, 15, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Nickel, M.; May, E.S.; Tiemann, L.; Schmidt, P.; Postorino, M.; Dinh, S.T.; Gross, J.; Ploner, M. Brain oscillations differentially encode noxious stimulus intensity and pain intensity. Neuroimage 2017, 148, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Arendsen, L.J.; Guggenberger, R.; Zimmer, M.; Weigl, T.; Gharabaghi, A. Peripheral electrical stimulation modulates cortical beta-band activity. Front. Neurosci. 2021, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.F.; Chen, T.W.; Liu, Y.H.; Wang, F.Y.; Chen, P.S.; Lai, L.K.; Hope Pan, L.; Coppola, G.; Wang, S.J. Migraine chronification is associated with beta-band connectivity within the pain-related cortical regions: A magnetoencephalographic study. Pain 2021, 162, 2590–2598. [Google Scholar] [CrossRef]
- Kim, J.A.; Bosma, R.L.; Hemington, K.S.; Rogachov, A.; Osborne, N.R.; Cheng, J.C.; Oh, J.; Crawley, A.P.; Dunkley, B.T.; Davis, K.D. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. Pain 2019, 160, 187–197. [Google Scholar] [CrossRef]
- Wei, M.; Liao, Y.; Liu, J.; Li, L.; Huang, G.; Huang, J.; Li, D.; Xiao, L.; Zhang, Z. EEG beta-band spectral entropy can predict the effect of drug treatment on pain in patients with herpes zoster. J. Clin. Neurophysiol. 2022, 39, 166–173. [Google Scholar] [CrossRef]
- Hargrove, J.B.; Bennett, R.; Simons, D.G.; Smith, S.J.; Nagpal, S.; Deering, D.E. Quantitative electroencephalographic abnormalities in fibromyalgia patients. Clin. EEG Neurosci. 2010, 41, 132–139. [Google Scholar] [CrossRef]

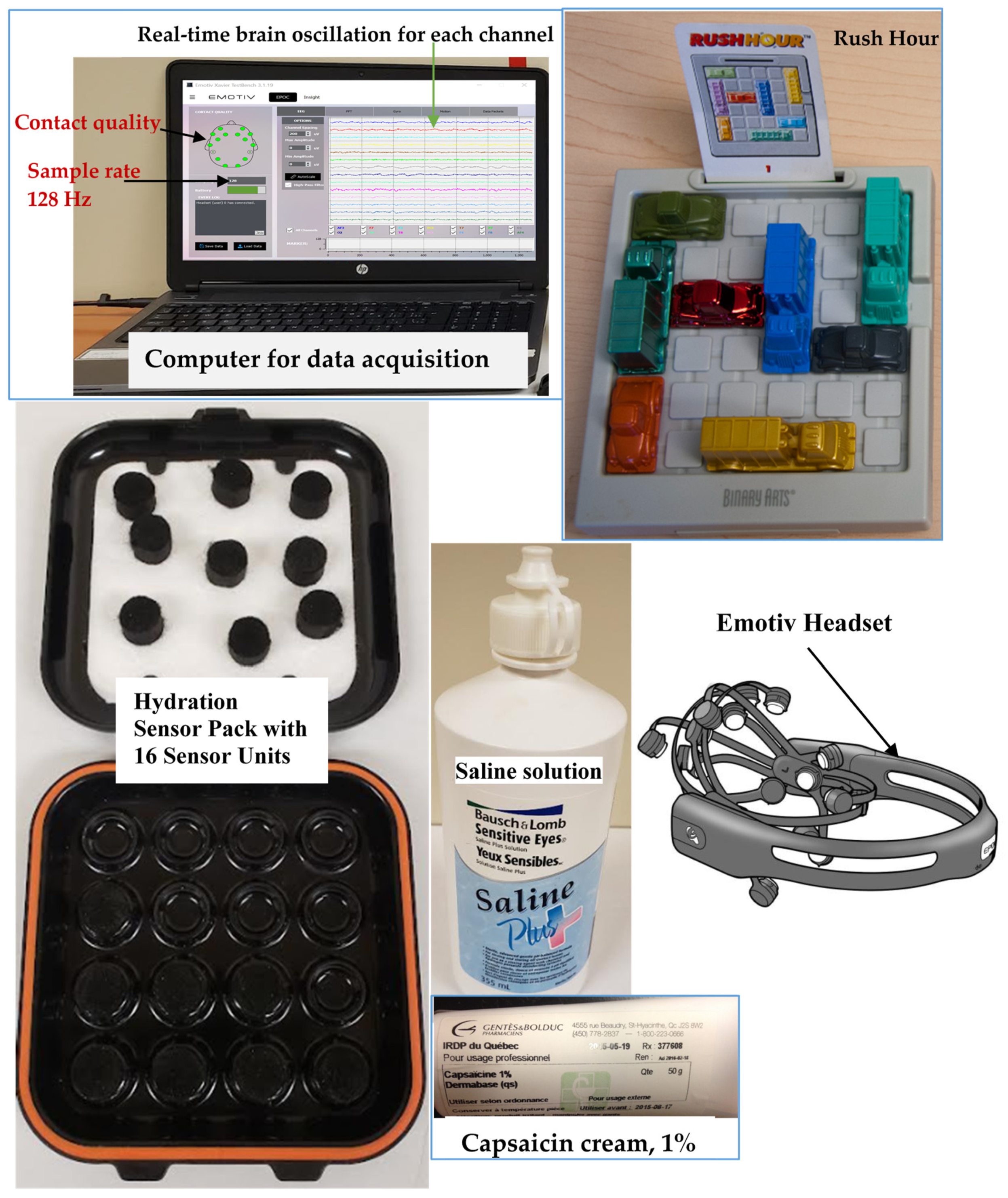


| Chronic Fibromyalgia Pain Group (n = 4 Women) | Healthy Volunteers’ Group (n = 7) | ||||||
|---|---|---|---|---|---|---|---|
| Men (n = 6) | Woman (n = 1) | Mean | |||||
| Age (years) | 42.75 | 28.67 | 45 | 36.83 | |||
| Pain duration (years) | 19.5 | - | - | - | |||
| Pain intensity (NRS: 0–10) | 4.75 | 3.83 | 5 | 4 | |||
| WPI (/19 body pain sides) | 13.75 | - | - | - | |||
| Task Performance Index (/1) | 0.02 | with “No pain” | with induced pain | with “No pain” | with induced pain | with “No pain” | with induced pain |
| 0.59 | 0.63 | 0.39 | 0.39 | 0.56 | 0.59 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segning, C.M.; Harvey, J.; Ezzaidi, H.; Fernandes, K.B.P.; da Silva, R.A.; Ngomo, S. Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals’ Analysis: A Proof-of-Concept. Sensors 2022, 22, 6272. https://doi.org/10.3390/s22166272
Segning CM, Harvey J, Ezzaidi H, Fernandes KBP, da Silva RA, Ngomo S. Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals’ Analysis: A Proof-of-Concept. Sensors. 2022; 22(16):6272. https://doi.org/10.3390/s22166272
Chicago/Turabian StyleSegning, Colince Meli, Jessica Harvey, Hassan Ezzaidi, Karen Barros Parron Fernandes, Rubens A. da Silva, and Suzy Ngomo. 2022. "Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals’ Analysis: A Proof-of-Concept" Sensors 22, no. 16: 6272. https://doi.org/10.3390/s22166272
APA StyleSegning, C. M., Harvey, J., Ezzaidi, H., Fernandes, K. B. P., da Silva, R. A., & Ngomo, S. (2022). Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals’ Analysis: A Proof-of-Concept. Sensors, 22(16), 6272. https://doi.org/10.3390/s22166272






