Improving EEG-Based Driver Distraction Classification Using Brain Connectivity Estimators
Abstract
:1. Introduction
2. Materials and Methods
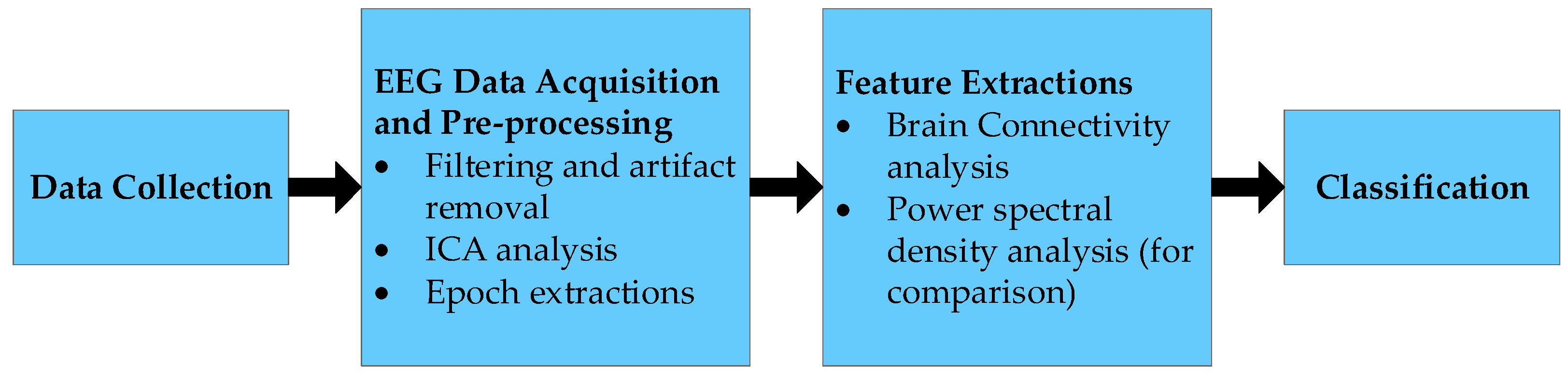
2.1. General Structure
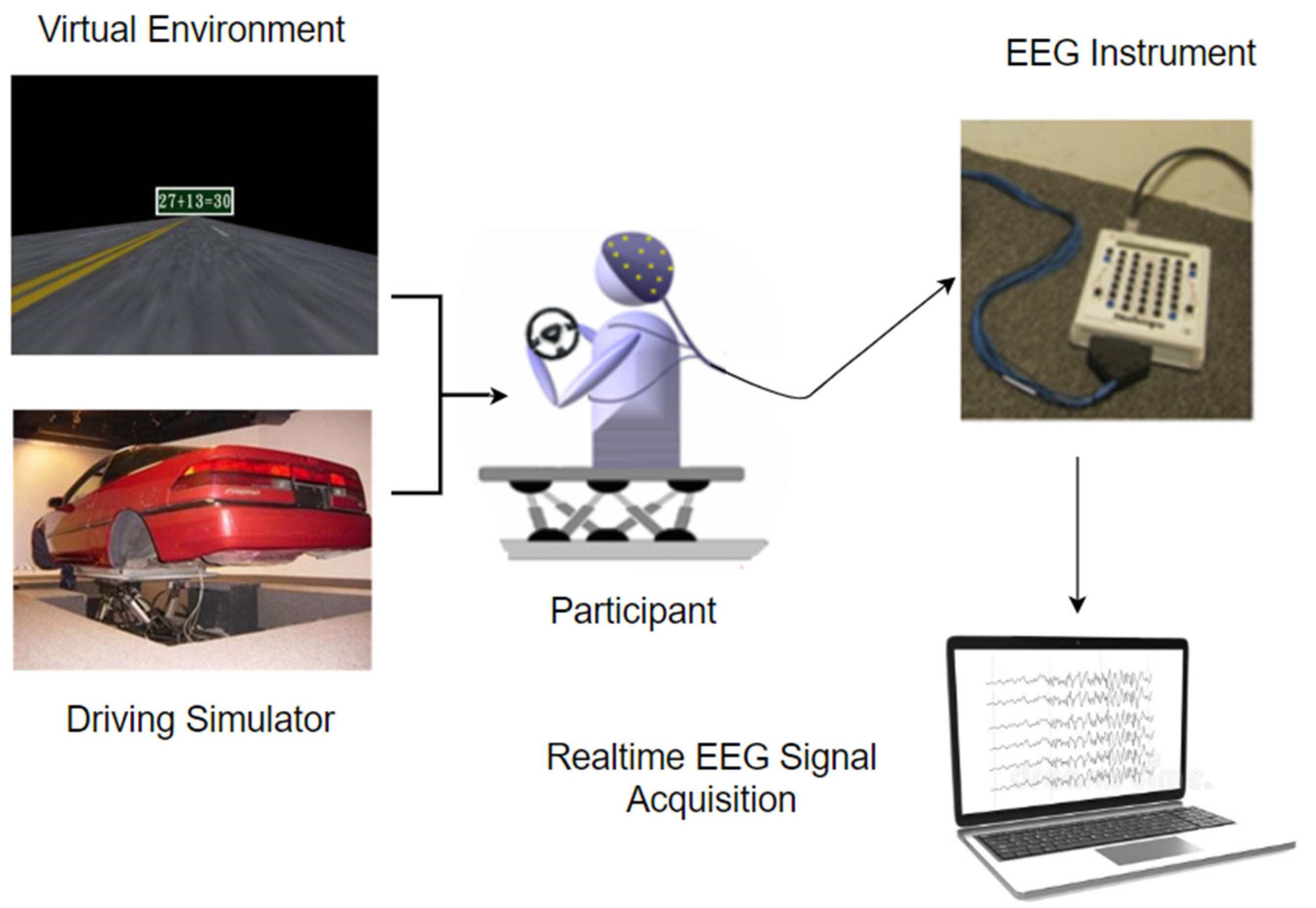
2.2. Data Collection
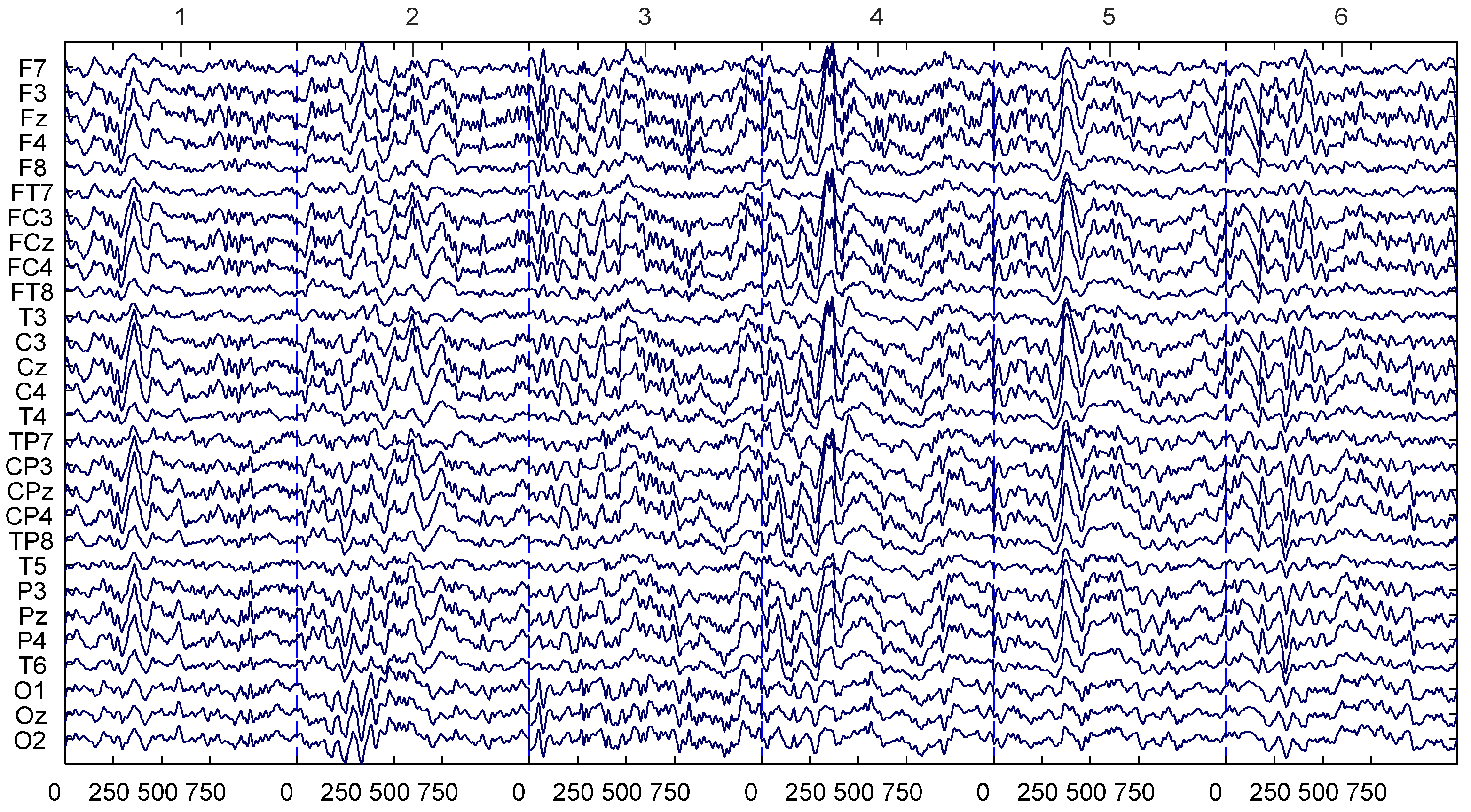
2.3. EEG Data Acquisition and Preprocessing
2.4. Preprocessing: Independent Component Analysis
2.5. Feature Extractions: Power Spectral Density Analysis
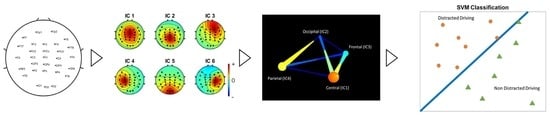
2.6. Feature Extractions: Brain Connectivity Analysis Structure
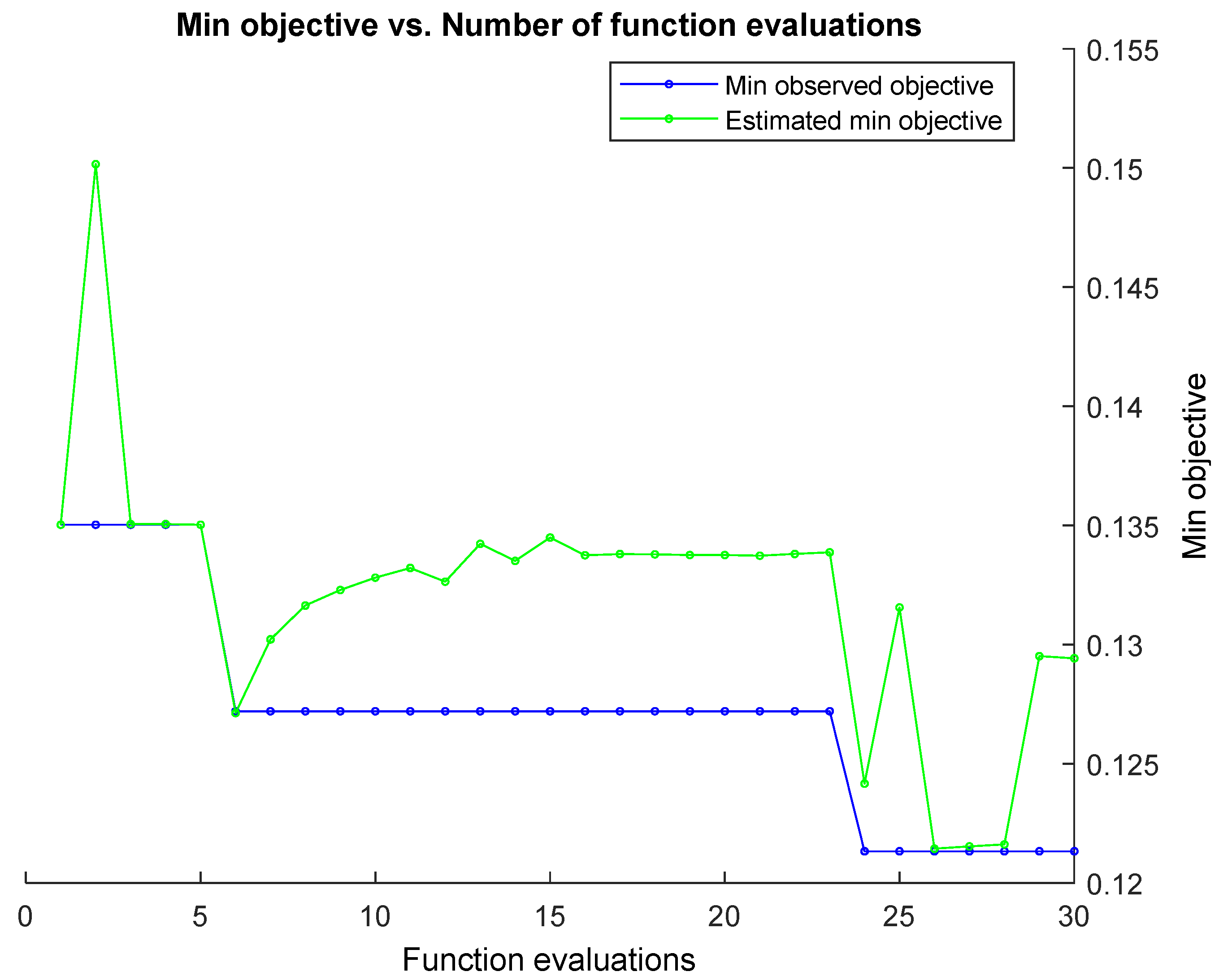
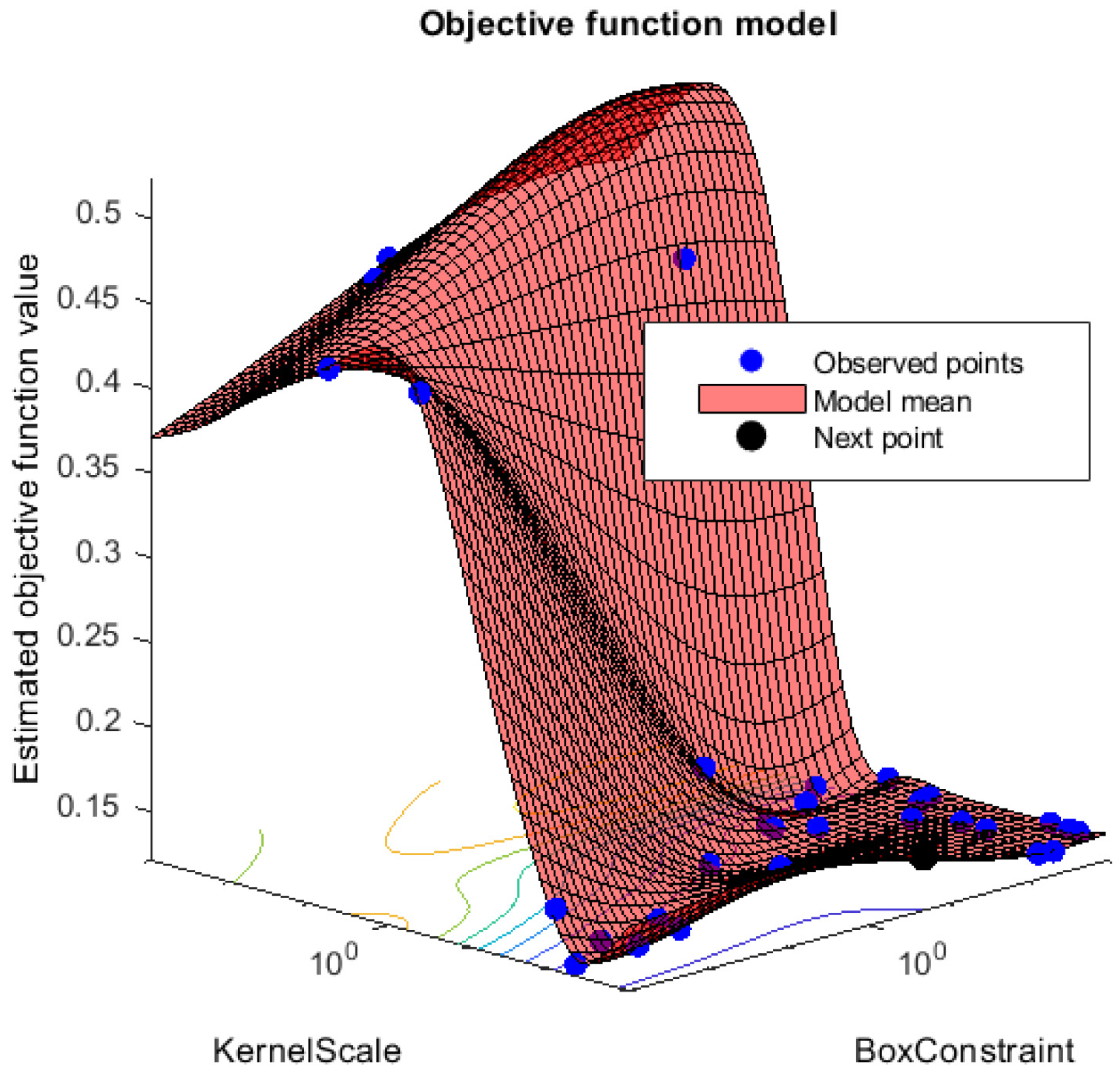
2.7. Classification and Optimization
3. Results
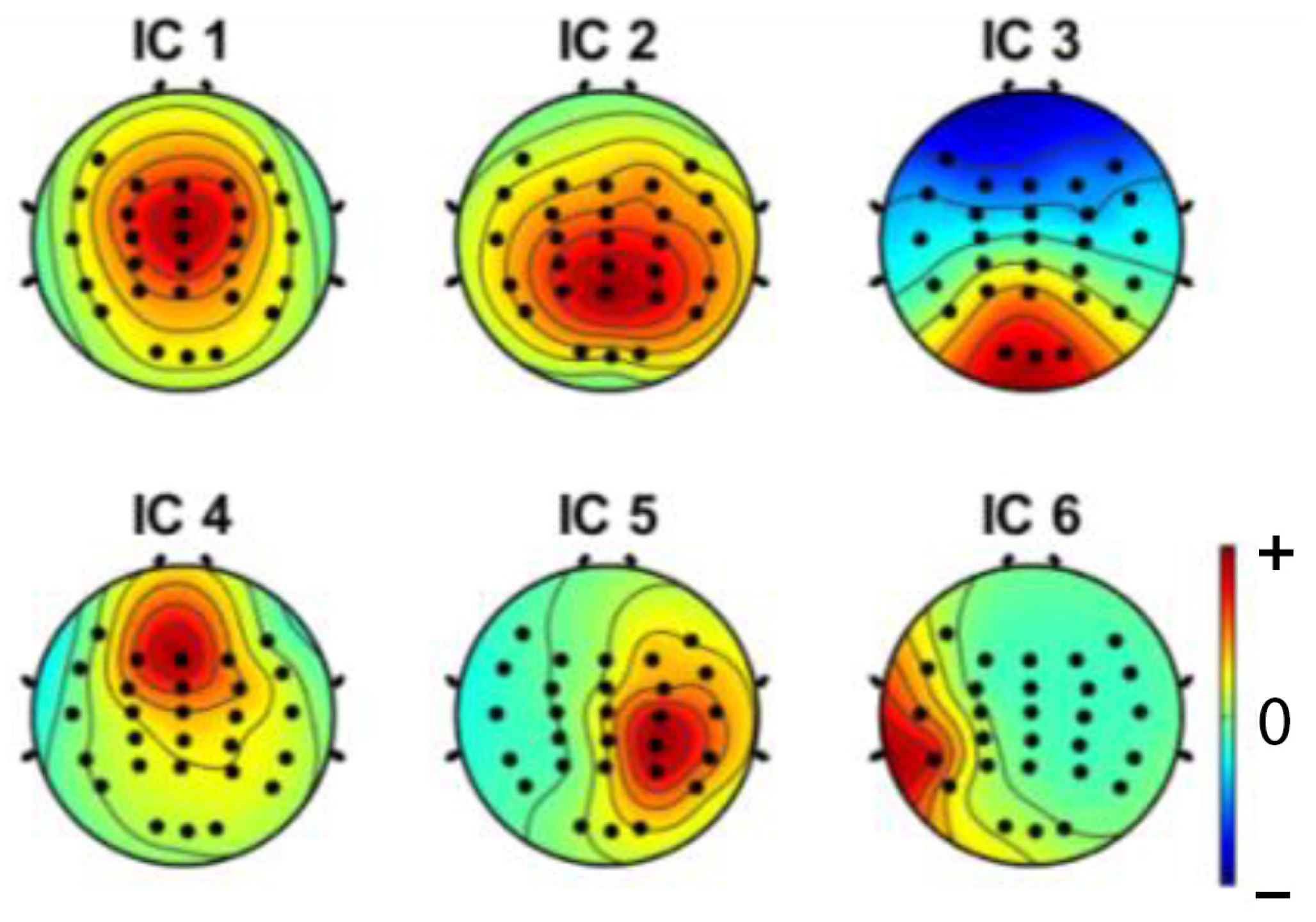
3.1. Independent Component Analysis
3.2. Model Order Calculation
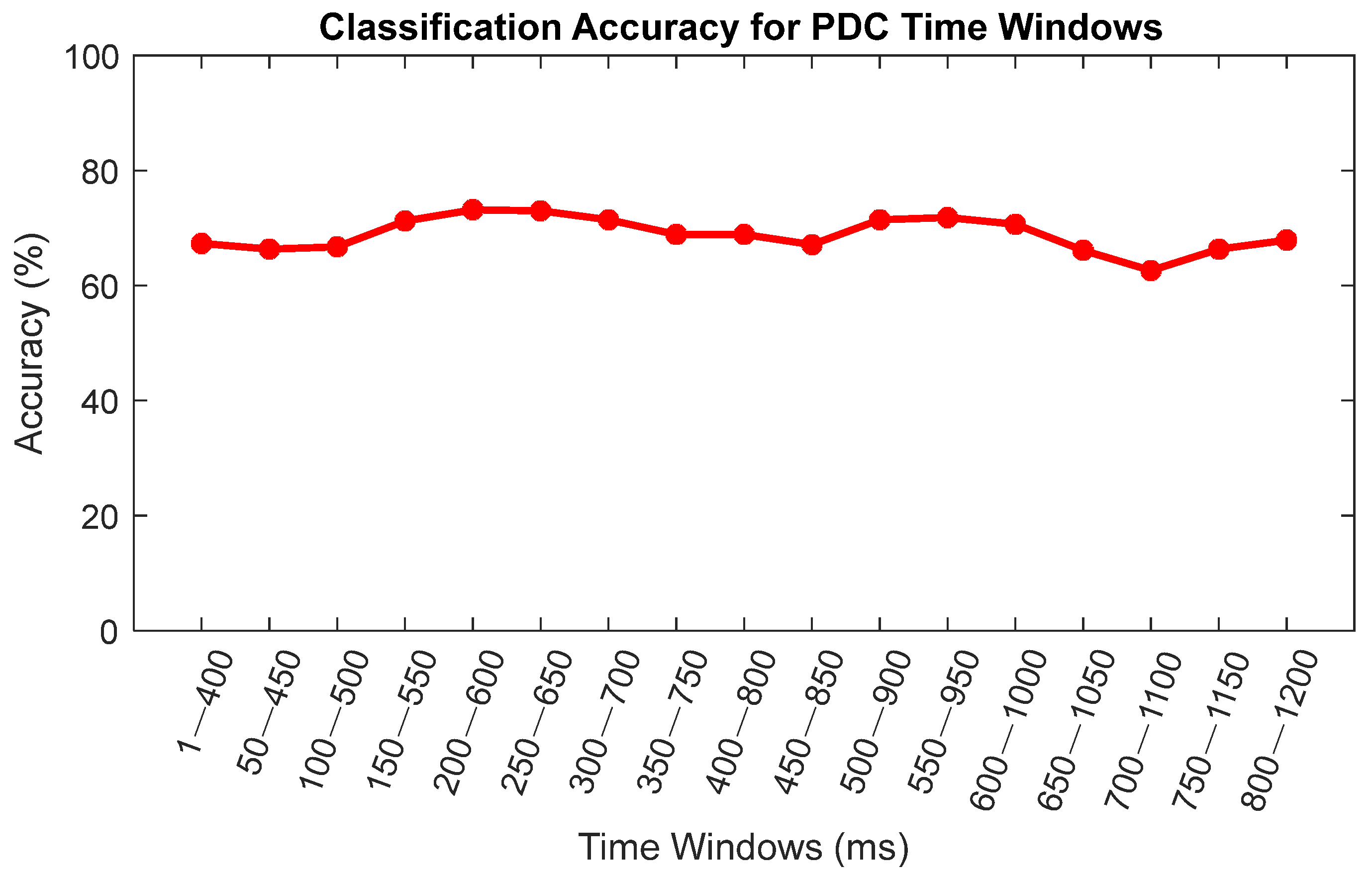
3.3. Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gazder, U.; Assi, K.J. Determining driver perceptions about distractions and modeling their effects on driving behavior at different age groups. J. Traffic Transp. Eng. 2022, 9, 33–43. [Google Scholar] [CrossRef]
- Young, K.L.; Charlton, J.; Koppel, S.; Grzebieta, R.H.; Williamson, A.; Woollery, J.; Senserrick, T.M. Distraction and older drivers: An emerging problem? J. Australas. Coll. Road Saf. 2018, 29, 18–29. [Google Scholar]
- Kashevnik, A.; Shchedrin, R.; Kaiser, C.; Stocker, A. Driver Distraction Detection Methods: A Literature Review and Framework. IEEE Access 2021, 9, 60063–60076. [Google Scholar] [CrossRef]
- Lee, J.D. Driving Safety. Rev. Hum. Factors Ergon. 2005, 1, 172–218. [Google Scholar] [CrossRef]
- Papakostas, M.; Riani, K.; Gasiorowski, A.B.; Sun, Y.; Abouelenien, M.; Mihalcea, R.; Burzo, M. Understanding Driving Distractions: A Multimodal Analysis on Distraction Characterization. In Proceedings of the 26th International Conference on Intelligent User Interfaces, College Station, TX, USA, 14–17 April 2021; pp. 377–386. [Google Scholar]
- Ke, J.; Du, J.; Luo, X. The effect of noise content and level on cognitive performance measured by electroencephalography (EEG). Autom. Constr. 2021, 130, 103836. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, C.; Guo, Y.; Yuan, W.; Fu, R. Research on a Cognitive Distraction Recognition Model for Intelligent Driving Systems Based on Real Vehicle Experiments. Sensors 2020, 20, 4426. [Google Scholar] [CrossRef]
- Botta, M.; Cancelliere, R.; Ghignone, L.; Tango, F.; Gallinari, P.; Luison, C. Real-time detection of driver distraction: Random projections for pseudo-inversion-based neural training. Knowl. Inf. Syst. 2019, 60, 1549–1564. [Google Scholar] [CrossRef]
- Aljasim, M.; Kashef, R. E2DR: A Deep Learning Ensemble-Based Driver Distraction Detection with Recommendations Model. Sensors 2022, 22, 1858. [Google Scholar] [CrossRef]
- Chai, R.; Naik, G.R.; Nguyen, T.N.; Ling, S.H.; Tran, Y.; Craig, A.; Nguyen, H.T. Driver Fatigue Classification with Independent Component by Entropy Rate Bound Minimization Analysis in an EEG-Based System. IEEE J. Biomed. Health Inform. 2017, 21, 715–724. [Google Scholar] [CrossRef]
- Tran, Y.; Craig, A.; Craig, R.; Chai, R.; Nguyen, H. The influence of mental fatigue on brain activity: Evidence from a systematic review with meta-analyses. Psychophysiology 2020, 57, e13554. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Nguyen, H. Regional brain wave activity changes associated with fatigue. Psychophysiology 2012, 49, 574–582. [Google Scholar] [CrossRef]
- Chai, R.; Ling, S.H.; San, P.P.; Naik, G.R.; Nguyen, T.N.; Tran, Y.; Craig, A.; Nguyen, H.T. Improving EEG-Based Driver Fatigue Classification Using Sparse-Deep Belief Networks. Front. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef]
- Chai, R.; Tran, Y.; Naik, G.R.; Nguyen, T.N.; Ling, S.H.; Craig, A.; Nguyen, H.T. Classification of EEG based-mental fatigue using principal component analysis and Bayesian neural network. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4654–4657. [Google Scholar]
- Shah, S.M.; Sun, Z.; Zaman, K.; Hussain, A.; Shoaib, M.; Pei, L. A Driver Gaze Estimation Method Based on Deep Learning. Sensors 2022, 22, 3959. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Wang, J.; Cao, Y.; Kato, N. When Machine Learning Meets Privacy in 6G: A Survey. IEEE Commun. Surv. Tutor. 2020, 22, 2694–2724. [Google Scholar] [CrossRef]
- Thomas, K.P.; Robinson, N.; Prasad, V.A. Separability of Motor Imagery Directions Using Subject-Specific Discriminative EEG Features. IEEE Trans. Hum.-Mach. Syst. 2021, 51, 544–553. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kim, J.-W.; Haufe, S.; Lee, S.-W. Detection of braking intention in diverse situations during simulated driving based on EEG feature combination. J. Neural Eng. 2014, 12, 016001. [Google Scholar] [CrossRef]
- Gonzalez-Trejo, E.; Mögele, H.; Pfleger, N.; Hannemann, R.; Strauss, D.J. Electroencephalographic Phase–Amplitude Coupling in Simulated Driving with Varying Modality-Specific Attentional Demand. IEEE Trans. Hum.-Mach. Syst. 2019, 49, 589–598. [Google Scholar] [CrossRef]
- Zhang, H.; Chavarriaga, R.; Khaliliardali, Z.; Gheorghe, L.; Iturrate, I.; Millán, J.d.R. EEG-based decoding of error-related brain activity in a real-world driving task. J. Neural Eng. 2015, 12, 066028. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Jung, T.-P.; Lin, C.-T. Theta and Alpha Oscillations in Attentional Interaction during Distracted Driving. Front. Behav. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef]
- Wang, Y.K.; Jung, T.P.; Lin, C.T. EEG-Based Attention Tracking During Distracted Driving. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1085–1094. [Google Scholar] [CrossRef]
- Huang, K.-C.; Huang, T.-Y.; Chuang, C.-H.; King, J.-T.; Wang, Y.-K.; Lin, C.-T.; Jung, T.-P. An EEG-Based Fatigue Detection and Mitigation System. Int. J. Neural Syst. 2016, 26, 1650018. [Google Scholar] [CrossRef]
- Li, G.; Chung, W.Y. Combined EEG-Gyroscope-tDCS Brain Machine Interface System for Early Management of Driver Drowsiness. IEEE Trans. Hum.-Mach. Syst. 2018, 48, 50–62. [Google Scholar] [CrossRef]
- Monteiro, T.G.; Skourup, C.; Zhang, H. Using EEG for Mental Fatigue Assessment: A Comprehensive Look into the Current State of the Art. IEEE Trans. Hum.-Mach. Syst. 2019, 49, 599–610. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel multi-modal machine learning based approach for automatic classification of EEG recordings in dementia. Neural Netw. 2020, 123, 176–190. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Functional brain imaging based on ERD/ERS. Vis. Res. 2001, 41, 1257–1260. [Google Scholar] [CrossRef]
- Kim, C.; Sun, J.; Liu, D.; Wang, Q.; Paek, S. An effective feature extraction method by power spectral density of EEG signal for 2-class motor imagery-based BCI. Med. Biol. Eng. Comput. 2018, 56, 1645–1658. [Google Scholar] [CrossRef]
- Hamedi, M.; Salleh, S.; Noor, A.M. Electroencephalographic Motor Imagery Brain Connectivity Analysis for BCI: A Review. Neural Comput. 2016, 28, 999–1041. [Google Scholar] [CrossRef]
- Ambrosen, K.S.; Eskildsen, S.F.; Hinne, M.; Krug, K.; Lundell, H.; Schmidt, M.N.; van Gerven, M.A.J.; Mørup, M.; Dyrby, T.B. Validation of structural brain connectivity networks: The impact of scanning parameters. NeuroImage 2020, 204, 116207. [Google Scholar] [CrossRef] [PubMed]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. A Review on Mental Stress Assessment Methods Using EEG Signals. Sensors 2021, 21, 5043. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Astolfi, L.; Valdés-Sosa, P.A.; Marinazzo, D.; Palva, S.O.; Bénar, C.G.; Michel, C.M.; Koenig, T. Electrophysiological Brain Connectivity: Theory and Implementation. IEEE Trans. Biomed. Eng. 2019, 66, 2115–2137. [Google Scholar] [CrossRef] [PubMed]
- Samdin, S.B.; Ting, C.; Ombao, H.; Salleh, S. A Unified Estimation Framework for State-Related Changes in Effective Brain Connectivity. IEEE Trans. Biomed. Eng. 2017, 64, 844–858. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J.-M. A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Kong, W.; Lin, W.; Babiloni, F.; Hu, S.; Borghini, G. Investigating Driver Fatigue versus Alertness Using the Granger Causality Network. Sensors 2015, 15, 19181–19198. [Google Scholar] [CrossRef]
- Wang, D.; Ren, D.; Li, K.; Feng, Y.; Ma, D.; Yan, X.; Wang, G. Epileptic Seizure Detection in Long-Term EEG Recordings by Using Wavelet-Based Directed Transfer Function. IEEE Trans. Biomed. Eng. 2018, 65, 2591–2599. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhang, R.; Zhang, J.; Guo, X. EEG-Based Emotion Recognition Using Partial Directed Coherence Dense Graph Propagation. In Proceedings of the 2022 14th International Conference on Measuring Technology and Mechatronics Automation (ICMTMA), Changsha, China, 15–16 January 2022; pp. 610–617. [Google Scholar]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Analysis of Default Mode Network in Social Anxiety Disorder: EEG Resting-State Effective Connectivity Study. Sensors 2021, 21, 4098. [Google Scholar] [CrossRef]
- Cho, J.-H.; Vorwerk, J.; Wolters, C.H.; Knösche, T.R. Influence of the head model on EEG and MEG source connectivity analyses. NeuroImage 2015, 110, 60–77. [Google Scholar] [CrossRef]
- Sameshima, K.; Baccala, L.A.; Astolfi, L. Methods in Brain CONNECTIVITY Inference through Multivariate Time Series Analysis; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Perera, D.; Wang, Y.K.; Lin, C.T.; Zheng, J.; Nguyen, H.T.; Chai, R. Statistical Analysis of Brain Connectivity Estimators during Distracted Driving. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3208–3211. [Google Scholar]
- Xie, Y.; Oniga, S. A Review of Processing Methods and Classification Algorithm for EEG Signal. Carpathian J. Electron. Comput. Eng. 2020, 13, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Chen, P.; Yang, Y.; Ma, C.; Gao, Z. A gradient-based automatic optimization CNN framework for EEG state recognition. J. Neural Eng. 2022, 19, 016009. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Lim, J.; de Souza, J.J.; Bezerianos, A.; Sun, Y. Task-Independent Mental Workload Classification Based Upon Common Multiband EEG Cortical Connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1940–1949. [Google Scholar] [CrossRef]
- Mathur, A.; Foody, G.M. Multiclass and Binary SVM Classification: Implications for Training and Classification Users. IEEE Geosci. Remote Sens. Lett. 2008, 5, 241–245. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Wang, R. Recognition of Mental Workload Levels under Complex Human–Machine Collaboration by Using Physiological Features and Adaptive Support Vector Machines. IEEE Trans. Hum.-Mach. Syst. 2015, 45, 200–214. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Chen, S.-A.; Lin, C.-T. An EEG-based brain–computer interface for dual task driving detection. Neurocomputing 2014, 129, 85–93. [Google Scholar] [CrossRef]
- Gorjan, D.; Gramann, K.; De Pauw, K.; Marusic, U. Removal of movement-induced EEG artifacts: Current state of the art and guidelines. J. Neural Eng. 2022, 19, 011004. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar] [CrossRef]
- Pagnotta, M.F.; Plomp, G. Time-varying MVAR algorithms for directed connectivity analysis: Critical comparison in simulations and benchmark EEG data. PLoS ONE 2018, 13, e0198846. [Google Scholar] [CrossRef]
- Ding, M.; Bressler, S.L.; Yang, W.; Liang, H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: Data preprocessing, model validation, and variability assessment. Biol. Cybern. 2000, 83, 35–45. [Google Scholar] [CrossRef]
- Delorme, A.; Mullen, T.; Kothe, C.; Acar, Z.A.; Bigdely-Shamlo, N.; Vankov, A.; Makeig, S. EEGLAB, SIFT, NFT, BCILAB, and ERICA: New tools for advanced EEG processing. Intell. Neurosci. 2011, 2011, 130714. [Google Scholar] [CrossRef]
- Wang, F.; Wu, S.; Ping, J.; Xu, Z.; Chu, H. EEG Driving Fatigue Detection with PDC-Based Brain Functional Network. IEEE Sens. J. 2021, 21, 10811–10823. [Google Scholar] [CrossRef]
- Snoek, J.; Larochelle, H.; Adams, R.P. Practical Bayesian Optimization of Machine Learning Algorithms. arXiv 2012, arXiv:1206.2944. [Google Scholar]

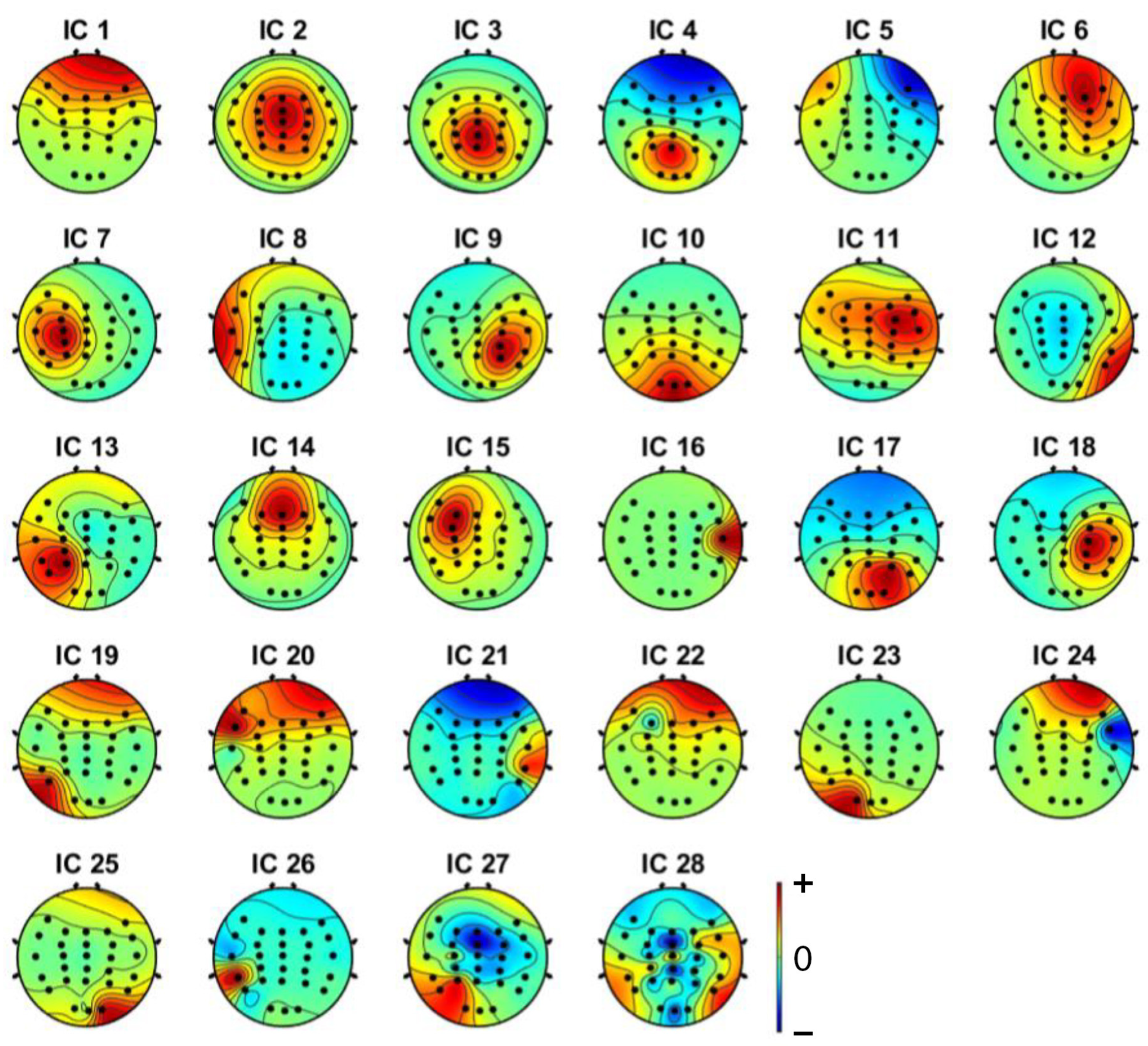

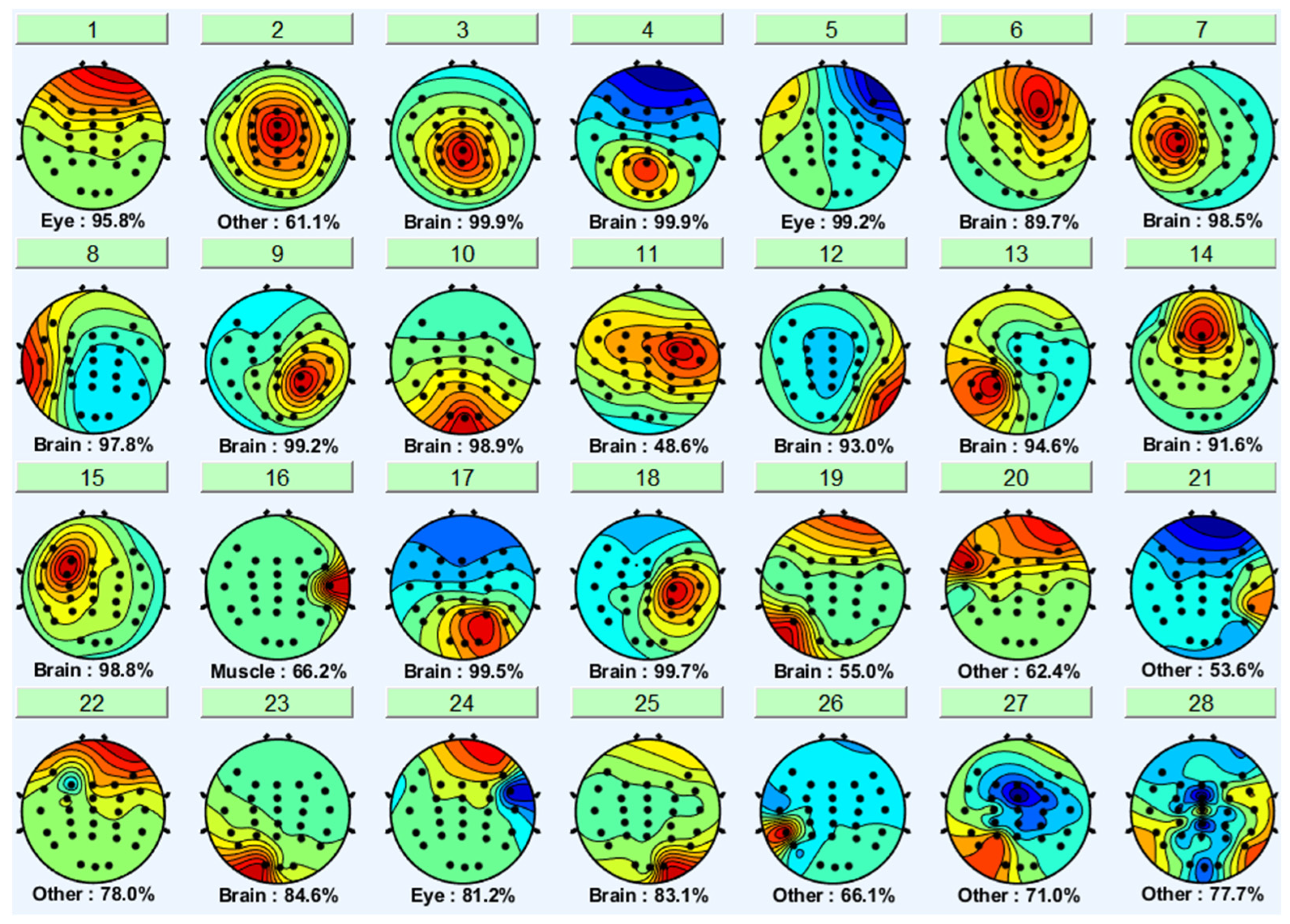
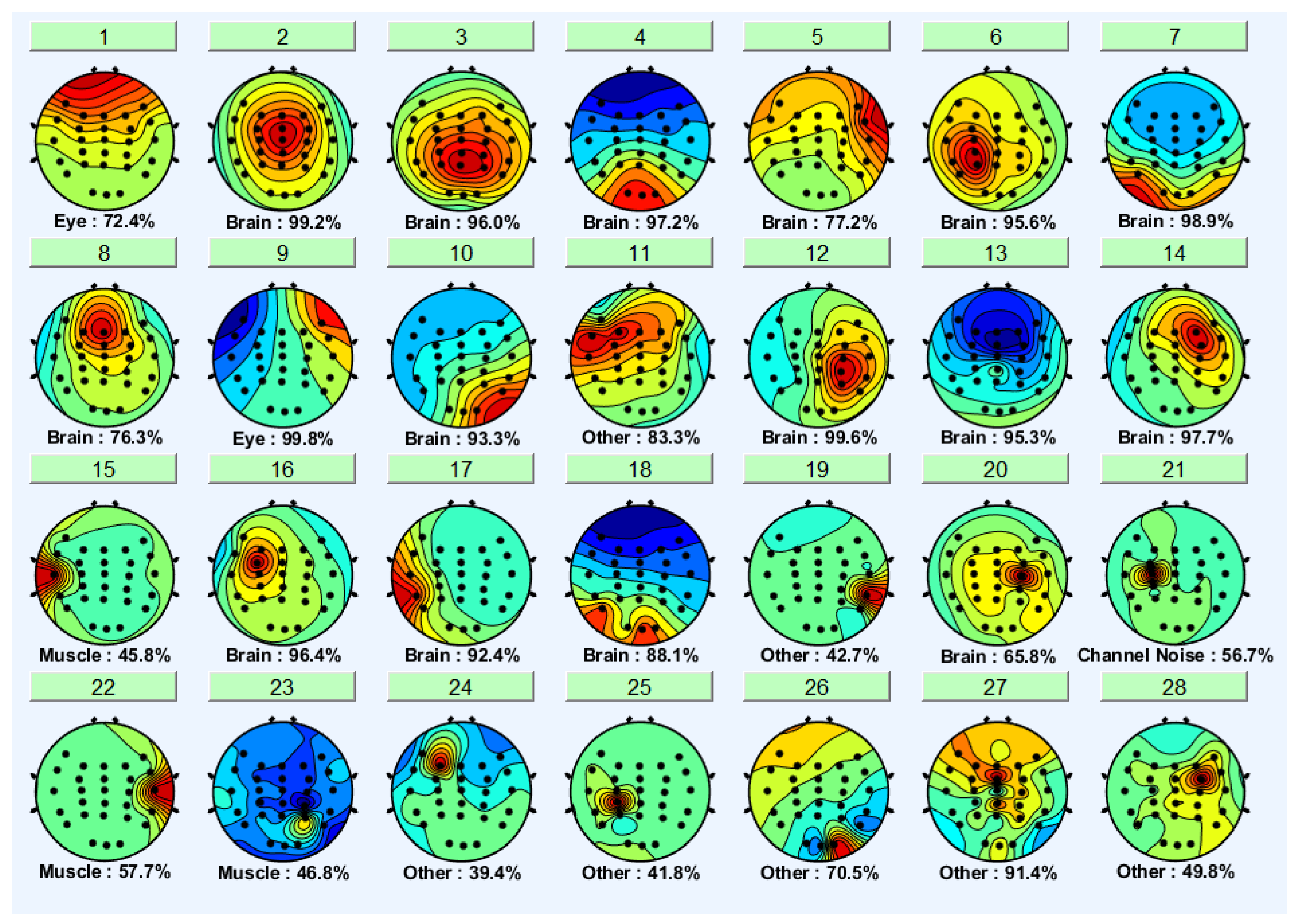
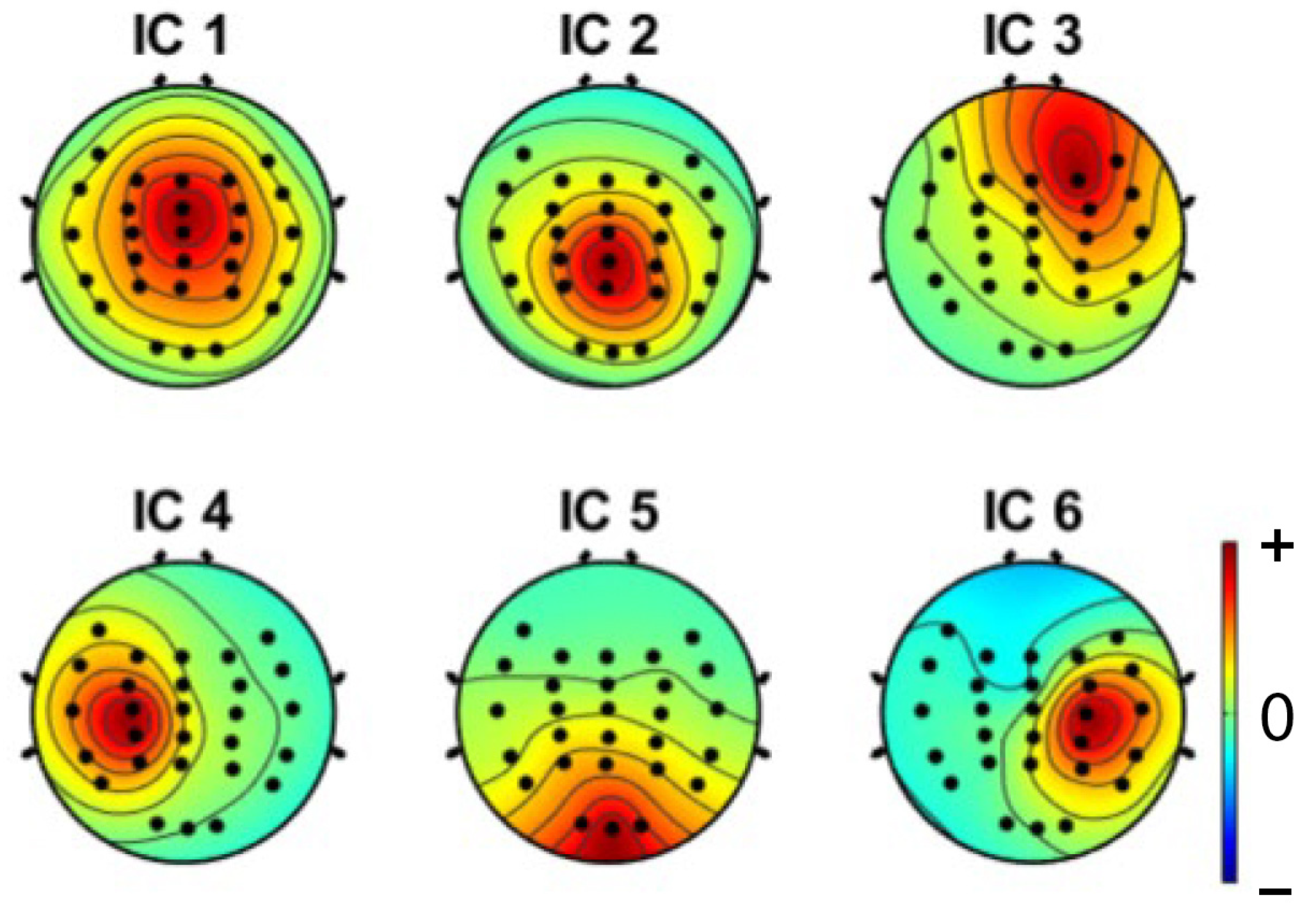

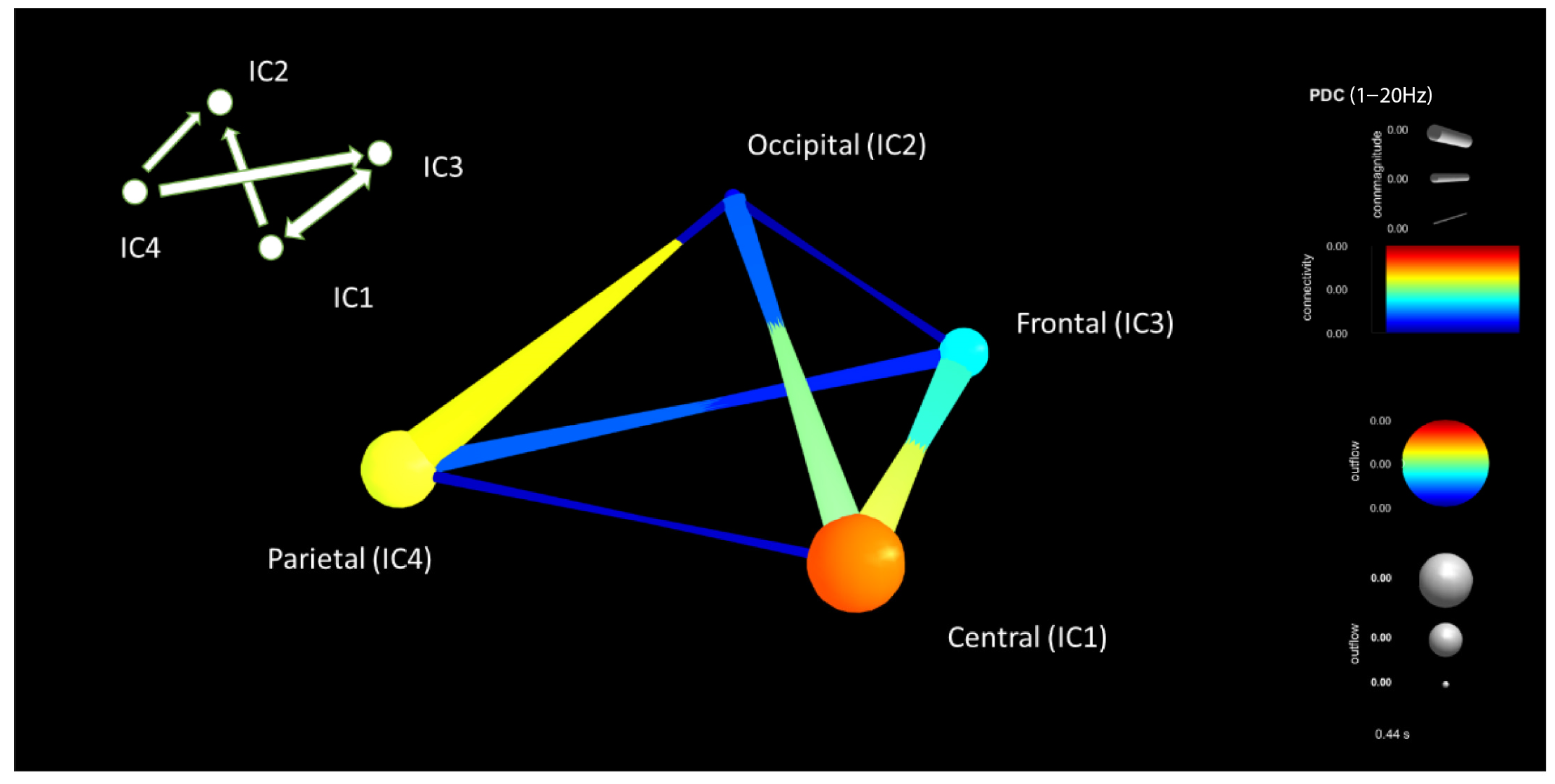
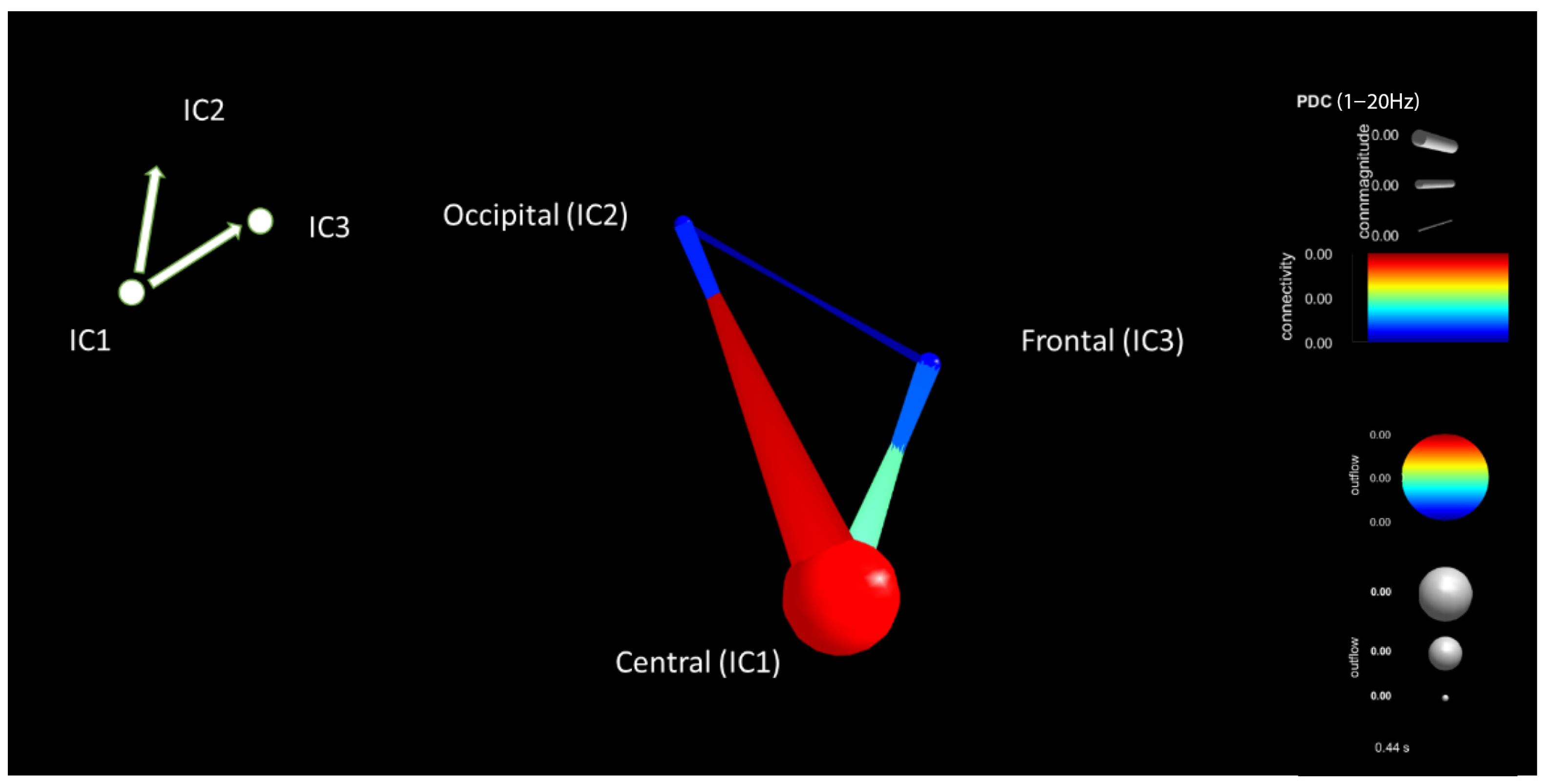
| Criteria | Distracted Driving | Non-Distracted Driving | ||
|---|---|---|---|---|
| Elbow | Min | Elbow | Min | |
| SBC | 5 | 5 | 5 | 5 |
| AIC | 5 | 9 | 5 | 9 |
| FPE | 5 | 9 | 5 | 9 |
| HQ | 5 | 8 | 5 | 8 |
| Features for the Classification | Classification Accuracy |
|---|---|
| Power Spectral Analysis | 74.05% |
| DTF | 70.02% |
| GGC | 82.27% |
| PDC | 86.19% |
| GPDC | 80.95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, D.; Wang, Y.-K.; Lin, C.-T.; Nguyen, H.; Chai, R. Improving EEG-Based Driver Distraction Classification Using Brain Connectivity Estimators. Sensors 2022, 22, 6230. https://doi.org/10.3390/s22166230
Perera D, Wang Y-K, Lin C-T, Nguyen H, Chai R. Improving EEG-Based Driver Distraction Classification Using Brain Connectivity Estimators. Sensors. 2022; 22(16):6230. https://doi.org/10.3390/s22166230
Chicago/Turabian StylePerera, Dulan, Yu-Kai Wang, Chin-Teng Lin, Hung Nguyen, and Rifai Chai. 2022. "Improving EEG-Based Driver Distraction Classification Using Brain Connectivity Estimators" Sensors 22, no. 16: 6230. https://doi.org/10.3390/s22166230
APA StylePerera, D., Wang, Y.-K., Lin, C.-T., Nguyen, H., & Chai, R. (2022). Improving EEG-Based Driver Distraction Classification Using Brain Connectivity Estimators. Sensors, 22(16), 6230. https://doi.org/10.3390/s22166230